Abstract
A treatment regimen that takes advantage of the induction of intracellular porphyrins such as protoporphyrin IX (PPIX) by exposure to exogenous 5-amino-laevulinic acid (ALA) followed by localized exposure to visible light represents a promising new approach to photodynamic therapy (PDT). Acting upon the suggestion that the effectiveness of ALA-dependent PDT may depend upon the state of cellular differentiation, we investigated the effect of terminal differentiation upon ALA-induced synthesis of and the subsequent phototoxicity attributable to PPIX in primary mouse keratinocytes. Induction of keratinocyte differentiation augmented intracellular PPIX accumulation in cells treated with ALA. These elevated PPIX levels resulted in an enhanced lethal photodynamic sensitization of differentiated cells. The differentiation-dependent increase in cellular PPIX levels resulted from several factors including: (a) increased ALA uptake, (b) enhanced PPIX production and (c) decreased PPIX export into the culture media. Simultaneously, steady-state levels of coproporphyrinogen oxidase mRNA increased but aminolaevulinic acid dehydratase mRNA levels remained unchanged. From experiments using 12-o-tetradecanoylphorbol-13-acetate, transforming growth factor beta 1 and calcimycin we demonstrated that the increase in PPIX concentration in terminally differentiating keratinocytes is calcium- and differentiation specific. Stimulation of the haem synthetic capacity is seen in primary keratinocytes, but not in PAM 212 cells that fail to undergo differentiation. Interestingly, increased PPIX formation and elevated coproporphyrinogen oxidase mRNA levels are not limited to differentiating keratinocytes; these were also elevated in the C2C12 myoblast and the PC12 adrenal cell lines upon induction of differentiation. Overall, the therapeutic implications of these results are that the effectiveness of ALA-dependent PDT depends on the differentiation status of the cell and that this may enable selective targeting of several tissue types.
Full text
PDF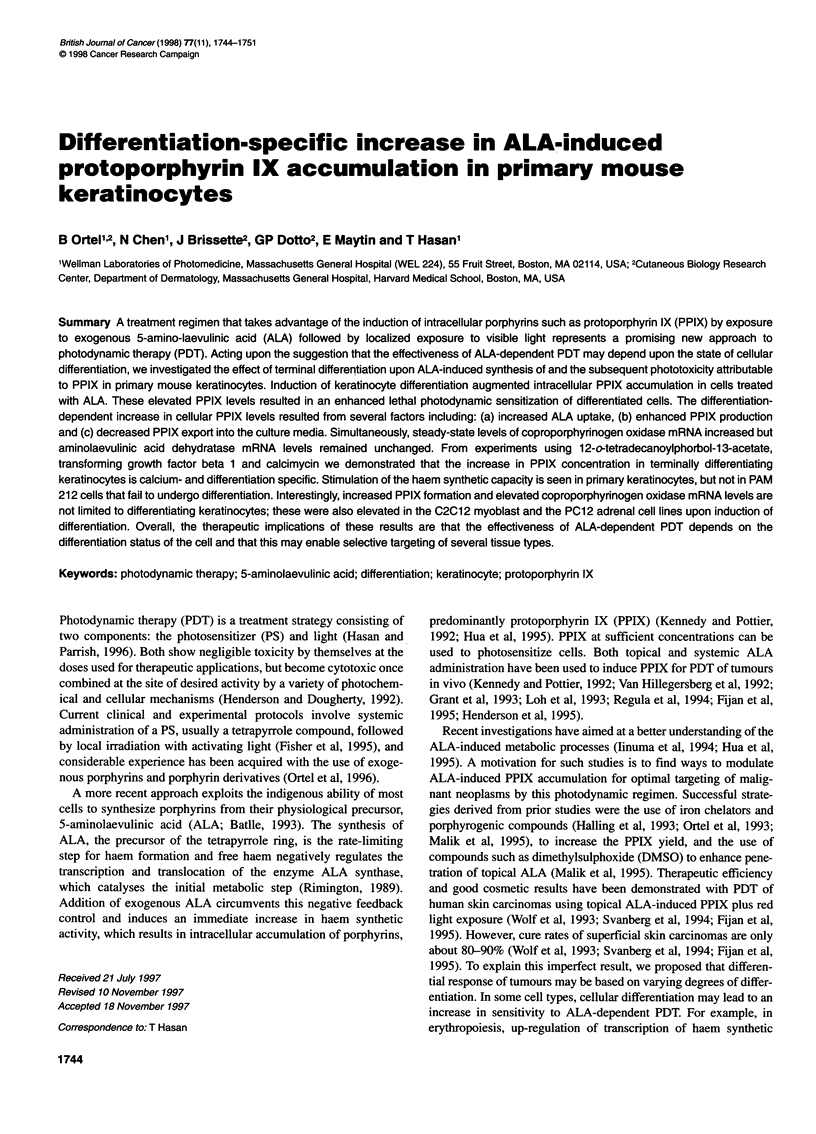
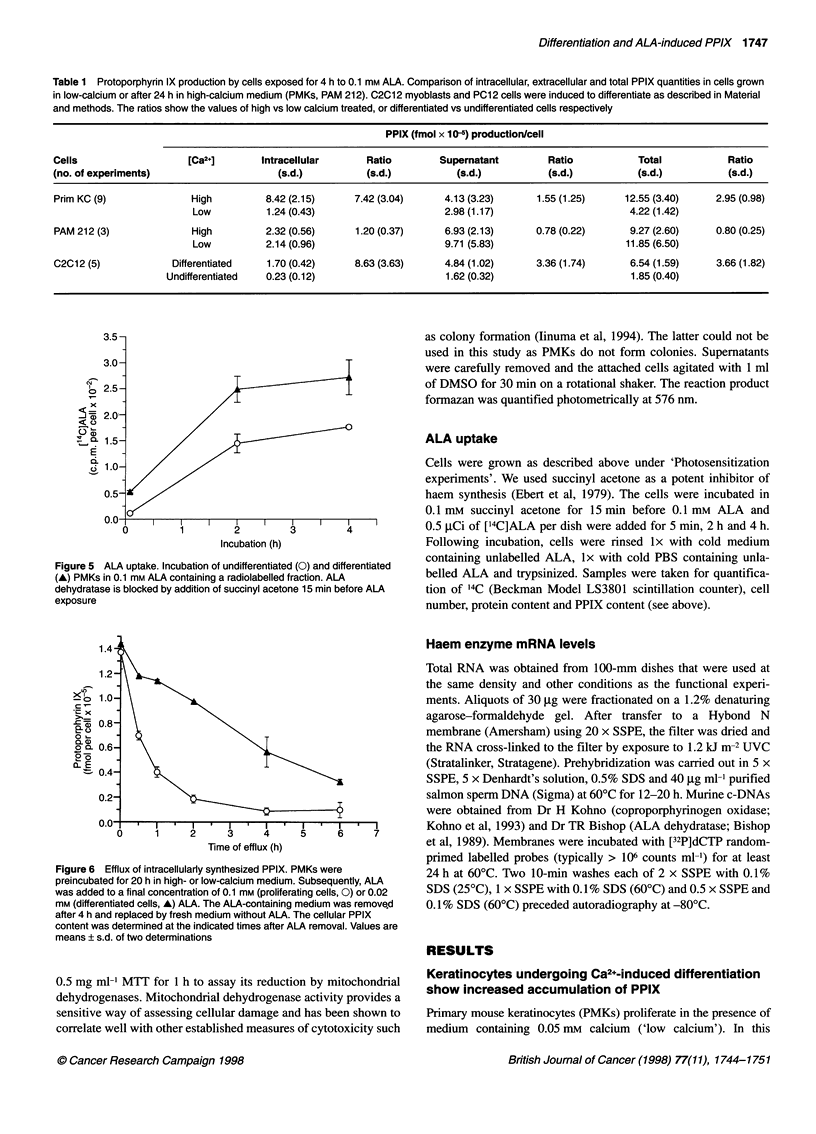
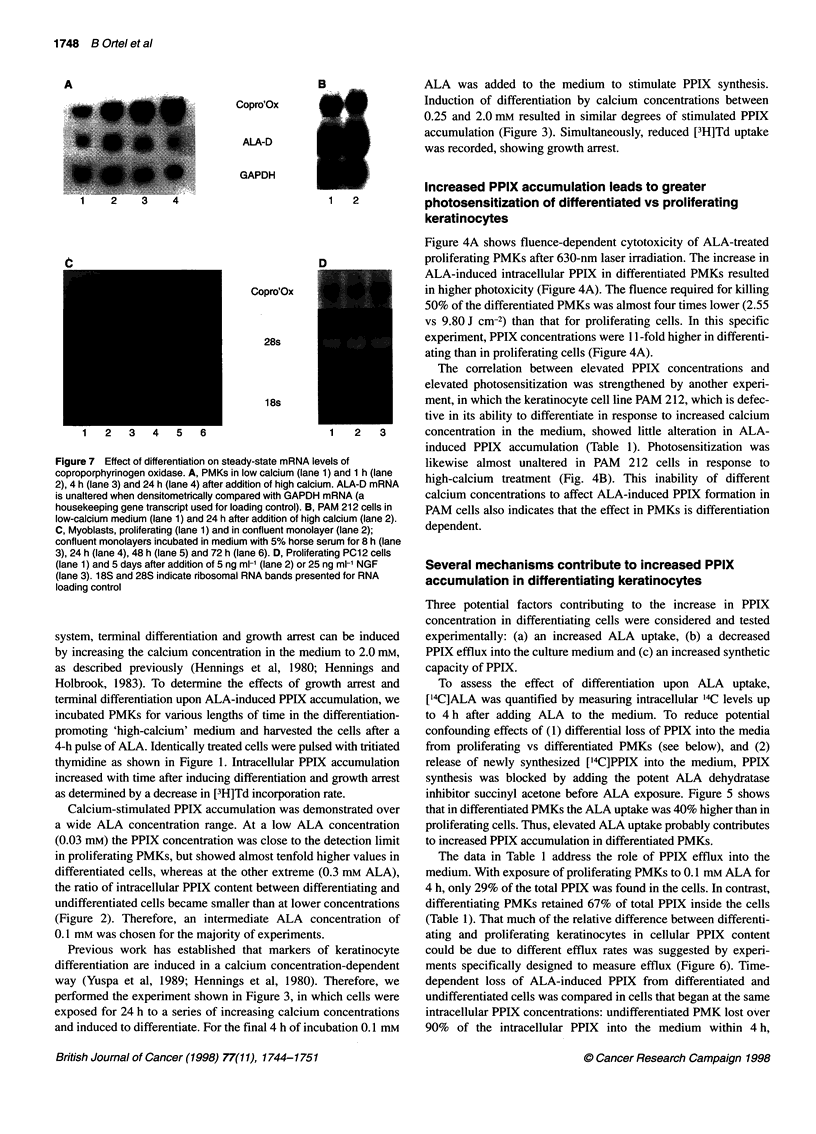
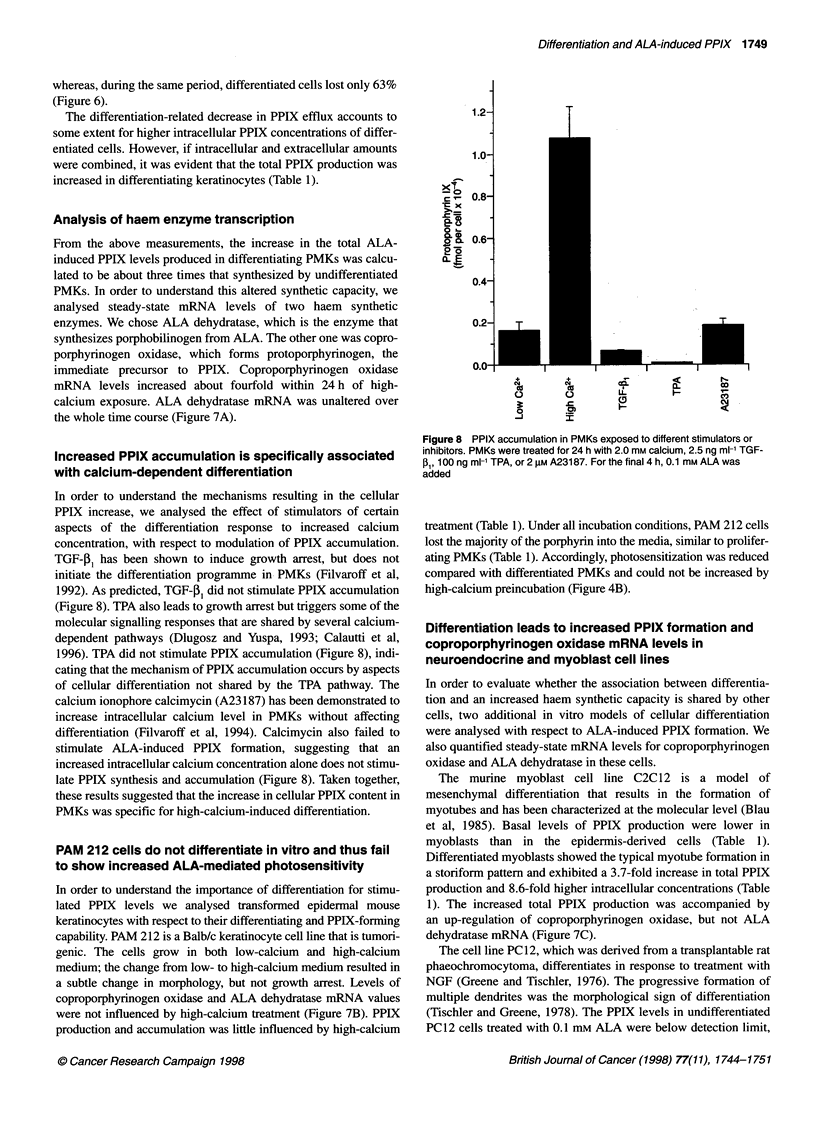


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batlle A. M. Porphyrins, porphyrias, cancer and photodynamic therapy--a model for carcinogenesis. J Photochem Photobiol B. 1993 Sep;20(1):5–22. doi: 10.1016/1011-1344(93)80127-u. [DOI] [PubMed] [Google Scholar]
- Bishop T. R., Hodes Z. I., Frelin L. P., Boyer S. H. Cloning and sequence of mouse erythroid delta-aminolevulinate dehydratase cDNA. Nucleic Acids Res. 1989 Feb 25;17(4):1775–1775. doi: 10.1093/nar/17.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Bottomley S. S., Muller-Eberhard U. Pathophysiology of heme synthesis. Semin Hematol. 1988 Oct;25(4):282–302. [PubMed] [Google Scholar]
- Calautti E., Missero C., Stein P. L., Ezzell R. M., Dotto G. P. fyn tyrosine kinase is involved in keratinocyte differentiation control. Genes Dev. 1995 Sep 15;9(18):2279–2291. doi: 10.1101/gad.9.18.2279. [DOI] [PubMed] [Google Scholar]
- Conder L. H., Woodard S. I., Dailey H. A. Multiple mechanisms for the regulation of haem synthesis during erythroid cell differentiation. Possible role for coproporphyrinogen oxidase. Biochem J. 1991 Apr 15;275(Pt 2):321–326. doi: 10.1042/bj2750321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz A. A., Yuspa S. H. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J Cell Biol. 1993 Jan;120(1):217–225. doi: 10.1083/jcb.120.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert P. S., Hess R. A., Frykholm B. C., Tschudy D. P. Succinylacetone, a potent inhibitor of heme biosynthesis: effect on cell growth, heme content and delta-aminolevulinic acid dehydratase activity of malignant murine erythroleukemia cells. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1382–1390. doi: 10.1016/0006-291x(79)91133-1. [DOI] [PubMed] [Google Scholar]
- Fijan S., Hönigsmann H., Ortel B. Photodynamic therapy of epithelial skin tumours using delta-aminolaevulinic acid and desferrioxamine. Br J Dermatol. 1995 Aug;133(2):282–288. doi: 10.1111/j.1365-2133.1995.tb02630.x. [DOI] [PubMed] [Google Scholar]
- Filvaroff E., Calautti E., McCormick F., Dotto G. P. Specific changes of Ras GTPase-activating protein (GAP) and a GAP-associated p62 protein during calcium-induced keratinocyte differentiation. Mol Cell Biol. 1992 Dec;12(12):5319–5328. doi: 10.1128/mcb.12.12.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filvaroff E., Calautti E., Reiss M., Dotto G. P. Functional evidence for an extracellular calcium receptor mechanism triggering tyrosine kinase activation associated with mouse keratinocyte differentiation. J Biol Chem. 1994 Aug 26;269(34):21735–21740. [PubMed] [Google Scholar]
- Fisher A. M., Murphree A. L., Gomer C. J. Clinical and preclinical photodynamic therapy. Lasers Surg Med. 1995;17(1):2–31. doi: 10.1002/lsm.1900170103. [DOI] [PubMed] [Google Scholar]
- Fujita H., Yamamoto M., Yamagami T., Hayashi N., Bishop T. R., De Verneuil H., Yoshinaga T., Shibahara S., Morimoto R., Sassa S. Sequential activation of genes for heme pathway enzymes during erythroid differentiation of mouse Friend virus-transformed erythroleukemia cells. Biochim Biophys Acta. 1991 Nov 11;1090(3):311–316. doi: 10.1016/0167-4781(91)90195-r. [DOI] [PubMed] [Google Scholar]
- Grant W. E., Hopper C., MacRobert A. J., Speight P. M., Bown S. G. Photodynamic therapy of oral cancer: photosensitisation with systemic aminolaevulinic acid. Lancet. 1993 Jul 17;342(8864):147–148. doi: 10.1016/0140-6736(93)91347-o. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B. W., Dougherty T. J. How does photodynamic therapy work? Photochem Photobiol. 1992 Jan;55(1):145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Vaughan L., Bellnier D. A., van Leengoed H., Johnson P. G., Oseroff A. R. Photosensitization of murine tumor, vasculature and skin by 5-aminolevulinic acid-induced porphyrin. Photochem Photobiol. 1995 Oct;62(4):780–789. doi: 10.1111/j.1751-1097.1995.tb08730.x. [DOI] [PubMed] [Google Scholar]
- Hennings H., Holbrook K. A. Calcium regulation of cell-cell contact and differentiation of epidermal cells in culture. An ultrastructural study. Exp Cell Res. 1983 Jan;143(1):127–142. doi: 10.1016/0014-4827(83)90115-5. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hua Z., Gibson S. L., Foster T. H., Hilf R. Effectiveness of delta-aminolevulinic acid-induced protoporphyrin as a photosensitizer for photodynamic therapy in vivo. Cancer Res. 1995 Apr 15;55(8):1723–1731. [PubMed] [Google Scholar]
- Iinuma S., Farshi S. S., Ortel B., Hasan T. A mechanistic study of cellular photodestruction with 5-aminolaevulinic acid-induced porphyrin. Br J Cancer. 1994 Jul;70(1):21–28. doi: 10.1038/bjc.1994.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. C., Pottier R. H. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992 Jul 30;14(4):275–292. doi: 10.1016/1011-1344(92)85108-7. [DOI] [PubMed] [Google Scholar]
- Kohno H., Furukawa T., Yoshinaga T., Tokunaga R., Taketani S. Coproporphyrinogen oxidase. Purification, molecular cloning, and induction of mRNA during erythroid differentiation. J Biol Chem. 1993 Oct 5;268(28):21359–21363. [PubMed] [Google Scholar]
- Loh C. S., MacRobert A. J., Bedwell J., Regula J., Krasner N., Bown S. G. Oral versus intravenous administration of 5-aminolaevulinic acid for photodynamic therapy. Br J Cancer. 1993 Jul;68(1):41–51. doi: 10.1038/bjc.1993.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik Z., Kostenich G., Roitman L., Ehrenberg B., Orenstein A. Topical application of 5-aminolevulinic acid, DMSO and EDTA: protoporphyrin IX accumulation in skin and tumours of mice. J Photochem Photobiol B. 1995 Jun;28(3):213–218. doi: 10.1016/1011-1344(95)07117-k. [DOI] [PubMed] [Google Scholar]
- Ortel B., Calzavara-Pinton P. G., Szeimies R. M., Hasan T. Perspectives in cutaneous photodynamic sensitization. J Photochem Photobiol B. 1996 Nov;36(2):209–211. doi: 10.1016/s1011-1344(96)07374-5. [DOI] [PubMed] [Google Scholar]
- Ortel B., Tanew A., Hönigsmann H. Lethal photosensitization by endogenous porphyrins of PAM cells--modification by desferrioxamine. J Photochem Photobiol B. 1993 Mar;17(3):273–278. doi: 10.1016/1011-1344(93)80025-5. [DOI] [PubMed] [Google Scholar]
- Regula J., Ravi B., Bedwell J., MacRobert A. J., Bown S. G. Photodynamic therapy using 5-aminolaevulinic acid for experimental pancreatic cancer--prolonged animal survival. Br J Cancer. 1994 Aug;70(2):248–254. doi: 10.1038/bjc.1994.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenberg R., Fuchs C., Kriegmair M. Photodynamic effects of 5-aminolevulinic acid-induced porphyrin on human bladder carcinoma cells in vitro. Eur J Cancer. 1996 Feb;32A(2):328–334. doi: 10.1016/0959-8049(95)00548-x. [DOI] [PubMed] [Google Scholar]
- Rimington C. Haem biosynthesis and porphyrias: 50 years in retrospect. J Clin Chem Clin Biochem. 1989 Aug;27(8):473–486. [PubMed] [Google Scholar]
- Rittenhouse-Diakun K., Van Leengoed H., Morgan J., Hryhorenko E., Paszkiewicz G., Whitaker J. E., Oseroff A. R. The role of transferrin receptor (CD71) in photodynamic therapy of activated and malignant lymphocytes using the heme precursor delta-aminolevulinic acid (ALA). Photochem Photobiol. 1995 May;61(5):523–528. doi: 10.1111/j.1751-1097.1995.tb02356.x. [DOI] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld N., Mamet R., Nordenberg Y., Shafran M., Babushkin T., Malik Z. Protoporphyrin biosynthesis in melanoma B16 cells stimulated by 5-aminolevulinic acid and chemical inducers: characterization of photodynamic inactivation. Int J Cancer. 1994 Jan 2;56(1):106–112. doi: 10.1002/ijc.2910560119. [DOI] [PubMed] [Google Scholar]
- Steinbach P., Weingandt H., Baumgartner R., Kriegmair M., Hofstädter F., Knüchel R. Cellular fluorescence of the endogenous photosensitizer protoporphyrin IX following exposure to 5-aminolevulinic acid. Photochem Photobiol. 1995 Nov;62(5):887–895. doi: 10.1111/j.1751-1097.1995.tb09152.x. [DOI] [PubMed] [Google Scholar]
- Svanberg K., Andersson T., Killander D., Wang I., Stenram U., Andersson-Engels S., Berg R., Johansson J., Svanberg S. Photodynamic therapy of non-melanoma malignant tumours of the skin using topical delta-amino levulinic acid sensitization and laser irradiation. Br J Dermatol. 1994 Jun;130(6):743–751. doi: 10.1111/j.1365-2133.1994.tb03412.x. [DOI] [PubMed] [Google Scholar]
- Taketani S., Kohno H., Okuda M., Furukawa T., Tokunaga R. Induction of peripheral-type benzodiazepine receptors during differentiation of mouse erythroleukemia cells. A possible involvement of these receptors in heme biosynthesis. J Biol Chem. 1994 Mar 11;269(10):7527–7531. [PubMed] [Google Scholar]
- Taketani S., Yoshinaga T., Furukawa T., Kohno H., Tokunaga R., Nishimura K., Inokuchi H. Induction of terminal enzymes for heme biosynthesis during differentiation of mouse erythroleukemia cells. Eur J Biochem. 1995 Jun 1;230(2):760–765. doi: 10.1111/j.1432-1033.1995.0760h.x. [DOI] [PubMed] [Google Scholar]
- Tischler A. S., Greene L. A. Morphologic and cytochemical properties of a clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Lab Invest. 1978 Aug;39(2):77–89. [PubMed] [Google Scholar]
- Van Hillegersberg R., Van den Berg J. W., Kort W. J., Terpstra O. T., Wilson J. H. Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats. Gastroenterology. 1992 Aug;103(2):647–651. doi: 10.1016/0016-5085(92)90860-2. [DOI] [PubMed] [Google Scholar]
- Wang J. K., Morgan J. I., Spector S. Differentiation of Friend erythroleukemia cells induced by benzodiazepines. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3770–3772. doi: 10.1073/pnas.81.12.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P., Rieger E., Kerl H. Topical photodynamic therapy with endogenous porphyrins after application of 5-aminolevulinic acid. An alternative treatment modality for solar keratoses, superficial squamous cell carcinomas, and basal cell carcinomas? J Am Acad Dermatol. 1993 Jan;28(1):17–21. doi: 10.1016/0190-9622(93)70002-b. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7(3):159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Hawley-Nelson P., Koehler B., Stanley J. R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980 Dec;40(12):4694–4703. [PubMed] [Google Scholar]
- Yuspa S. H., Kilkenny A. E., Steinert P. M., Roop D. R. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989 Sep;109(3):1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]