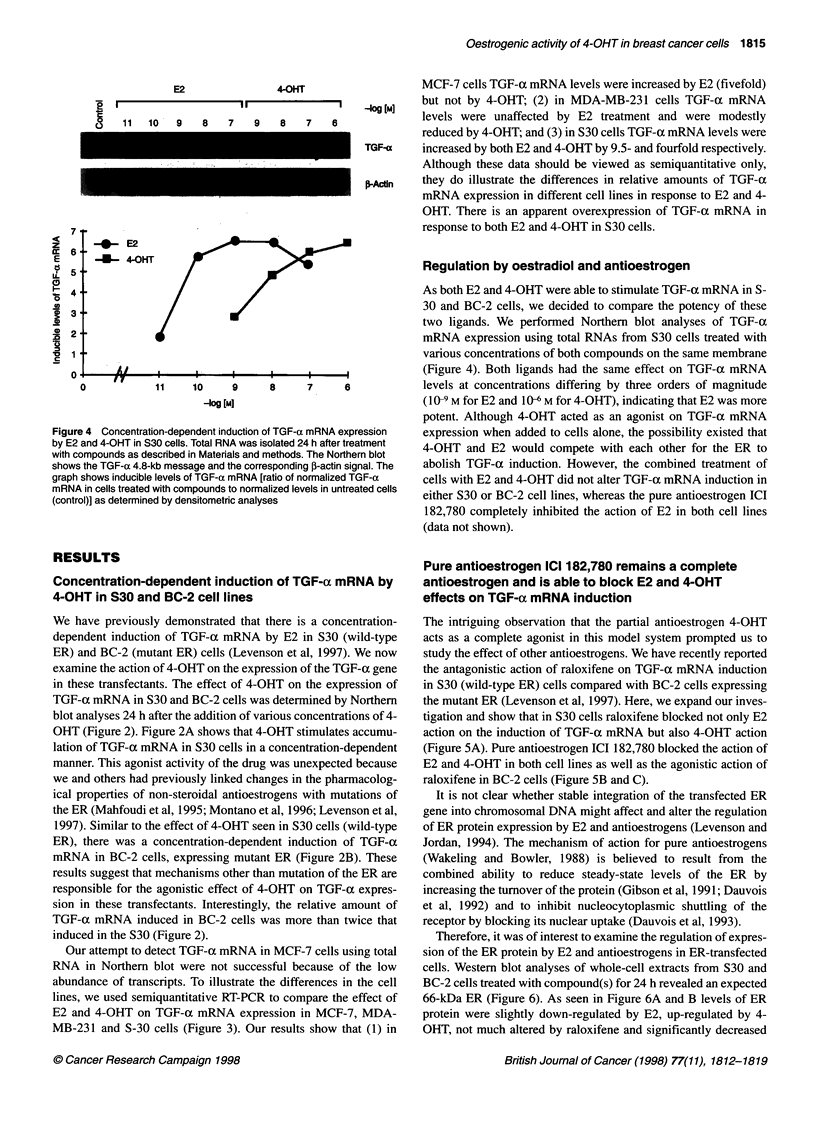
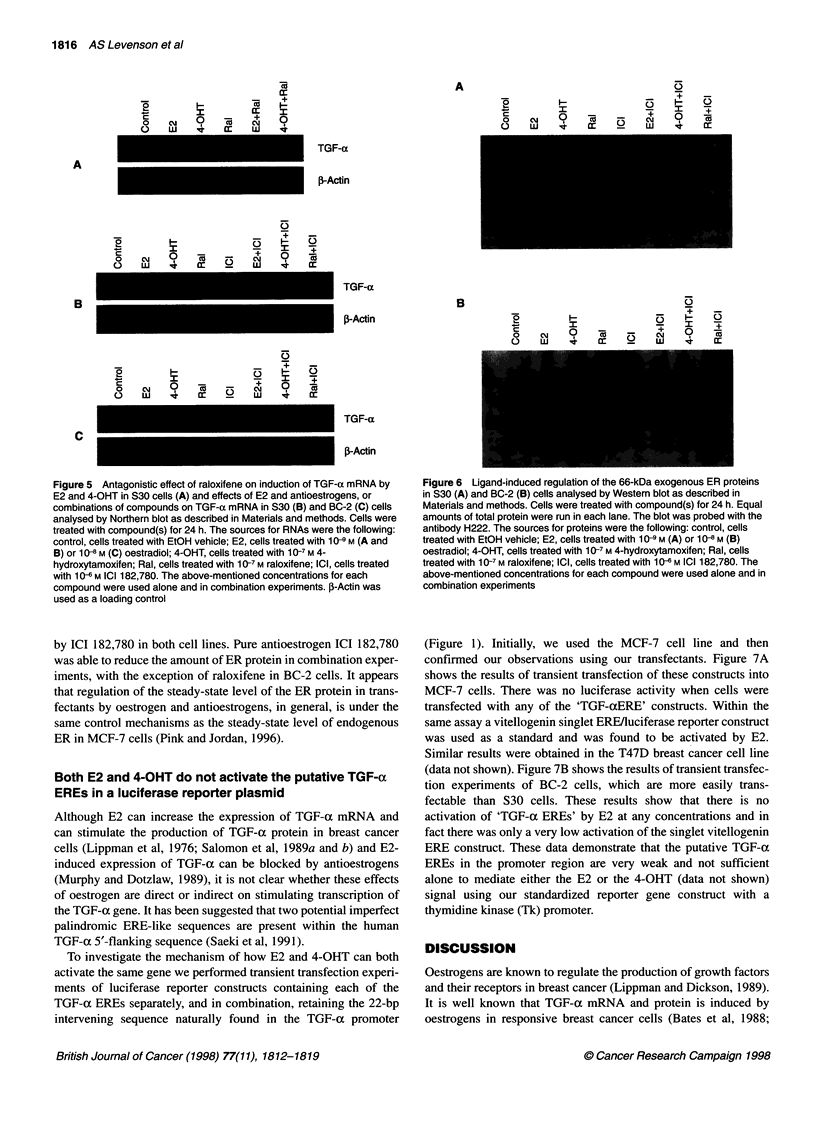
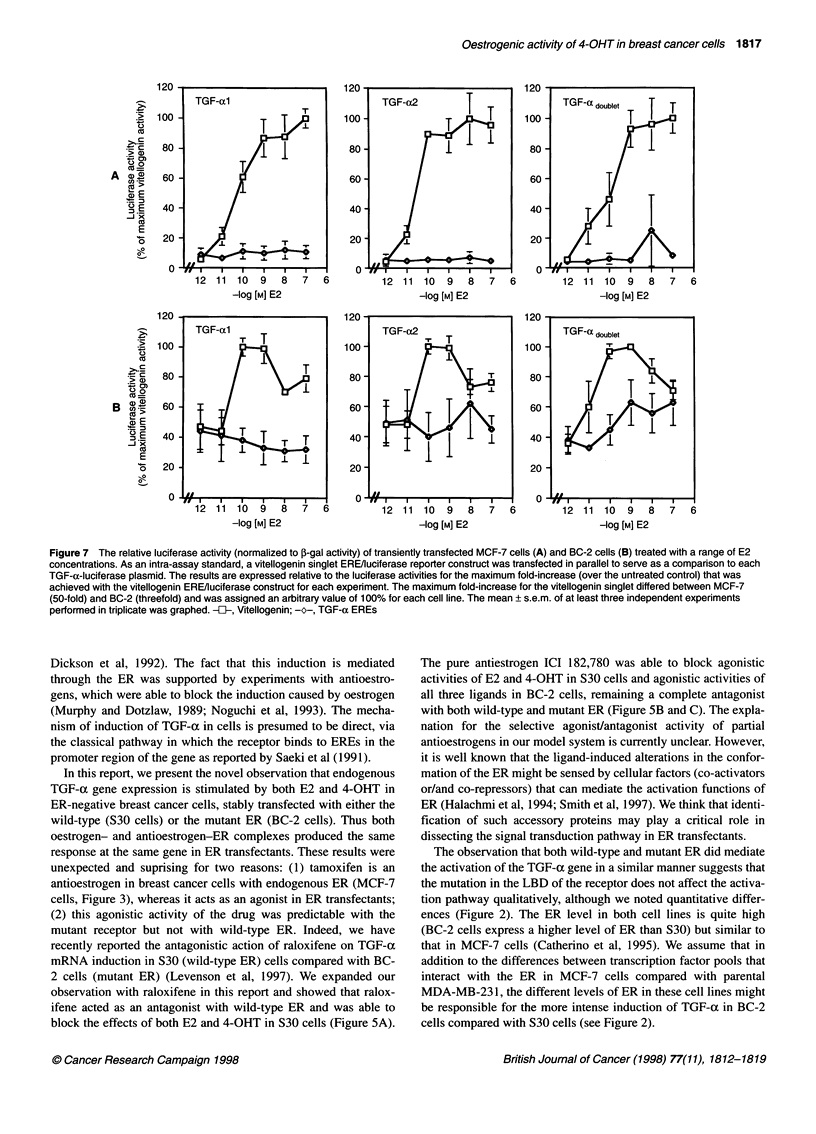
Abstract
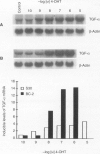
Oestrogens and antioestrogens modulate the synthesis of transforming growth factor alpha (TGF-alpha) in breast cancer cells. The purpose of the present report was to examine regulation of TGF-alpha gene expression by oestradiol (E2) and antioestrogens in MDA-MB-231 breast cancer cells transfected with either the wild-type or mutant oestrogen receptor (ER). We recently reported the concentration-dependent E2 stimulation of TGF-alpha mRNA in MDA-MB-231 ER transfectants (Levenson et al, 1997). We now report that 4-hydroxytamoxifen (4-OHT) shows oestrogen-like effects on the induction of TGF-alpha gene expression in our transfectants. Accumulation of TGF-alpha mRNA in response to both E2 and 4-OHT but not in response to the pure antioestrogen ICI 182,780 suggests that E2-ER and 4-OHT-ER complexes can bind to an oestrogen response element (ERE), located in the promoter region of the TGF-alpha gene and can activate transcription of the gene. Surprisingly, no activation of luciferase expression was observed after transient transfection of the TGF-alpha ERE/luciferase reporter constructs. Possible activation of an alternative ER-mediated pathway responsible for the regulation of TGF-alpha gene expression in the ER transfectants is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J. L., Sundstrom S. A., Lyttle C. R. Estrogen regulation of placental alkaline phosphatase gene expression in a human endometrial adenocarcinoma cell line. Cancer Res. 1990 Jun 1;50(11):3306–3310. [PubMed] [Google Scholar]
- Astruc M. E., Chabret C., Bali P., Gagne D., Pons M. Prolonged treatment of breast cancer cells with antiestrogens increases the activating protein-1-mediated response: involvement of the estrogen receptor. Endocrinology. 1995 Mar;136(3):824–832. doi: 10.1210/endo.136.3.7867590. [DOI] [PubMed] [Google Scholar]
- Bates S. E., Davidson N. E., Valverius E. M., Freter C. E., Dickson R. B., Tam J. P., Kudlow J. E., Lippman M. E., Salomon D. S. Expression of transforming growth factor alpha and its messenger ribonucleic acid in human breast cancer: its regulation by estrogen and its possible functional significance. Mol Endocrinol. 1988 Jun;2(6):543–555. doi: 10.1210/mend-2-6-543. [DOI] [PubMed] [Google Scholar]
- Butler W. B., Berlinski P. J., Hillman R. M., Kelsey W. H., Toenniges M. M. Relation of in vitro properties to tumorigenicity for a series of sublines of the human breast cancer cell line MCF-7. Cancer Res. 1986 Dec;46(12 Pt 1):6339–6348. [PubMed] [Google Scholar]
- Campbell M. J. Lipofection reagents prepared by a simple ethanol injection technique. Biotechniques. 1995 Jun;18(6):1027–1032. [PubMed] [Google Scholar]
- Catherino W. H., Jordan V. C. Increasing the number of tandem estrogen response elements increases the estrogenic activity of a tamoxifen analogue. Cancer Lett. 1995 May 25;92(1):39–47. doi: 10.1016/0304-3835(95)03755-l. [DOI] [PubMed] [Google Scholar]
- Catherino W. H., Wolf D. M., Jordan V. C. A naturally occurring estrogen receptor mutation results in increased estrogenicity of a tamoxifen analog. Mol Endocrinol. 1995 Aug;9(8):1053–1063. doi: 10.1210/mend.9.8.7476979. [DOI] [PubMed] [Google Scholar]
- Dauvois S., Danielian P. S., White R., Parker M. G. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4037–4041. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvois S., White R., Parker M. G. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993 Dec;106(Pt 4):1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Johnson M. D., Bano M., Shi E., Kurebayashi J., Ziff B., Martinez-Lacaci I., Amundadottir L. T., Lippman M. E. Growth factors in breast cancer: mitogenesis to transformation. J Steroid Biochem Mol Biol. 1992 Sep;43(1-3):69–78. doi: 10.1016/0960-0760(92)90189-p. [DOI] [PubMed] [Google Scholar]
- El-Ashry D., Chrysogelos S. A., Lippman M. E., Kern F. G. Estrogen induction of TGF-alpha is mediated by an estrogen response element composed of two imperfect palindromes. J Steroid Biochem Mol Biol. 1996 Nov;59(3-4):261–269. doi: 10.1016/s0960-0760(96)00118-5. [DOI] [PubMed] [Google Scholar]
- Furr B. J., Jordan V. C. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25(2):127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- Gaub M. P., Bellard M., Scheuer I., Chambon P., Sassone-Corsi P. Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell. 1990 Dec 21;63(6):1267–1276. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]
- Gibson M. K., Nemmers L. A., Beckman W. C., Jr, Davis V. L., Curtis S. W., Korach K. S. The mechanism of ICI 164,384 antiestrogenicity involves rapid loss of estrogen receptor in uterine tissue. Endocrinology. 1991 Oct;129(4):2000–2010. doi: 10.1210/endo-129-4-2000. [DOI] [PubMed] [Google Scholar]
- Gong Y., Ballejo G., Murphy L. C., Murphy L. J. Differential effects of estrogen and antiestrogen on transforming growth factor gene expression in endometrial adenocarcinoma cells. Cancer Res. 1992 Apr 1;52(7):1704–1709. [PubMed] [Google Scholar]
- Gottardis M. M., Robinson S. P., Satyaswaroop P. G., Jordan V. C. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988 Feb 15;48(4):812–815. [PubMed] [Google Scholar]
- Halachmi S., Marden E., Martin G., MacKay H., Abbondanza C., Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994 Jun 3;264(5164):1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- Huynh H. T., Pollak M. Insulin-like growth factor I gene expression in the uterus is stimulated by tamoxifen and inhibited by the pure antiestrogen ICI 182780. Cancer Res. 1993 Dec 1;53(23):5585–5588. [PubMed] [Google Scholar]
- Jamil A., Croxtall J. D., White J. O. The effect of anti-oestrogens on cell growth and progesterone receptor concentration in human endometrial cancer cells (Ishikawa). J Mol Endocrinol. 1991 Jun;6(3):215–221. doi: 10.1677/jme.0.0060215. [DOI] [PubMed] [Google Scholar]
- Jeng M. H., Jiang S. Y., Jordan V. C. Paradoxical regulation of estrogen-dependent growth factor gene expression in estrogen receptor (ER)-negative human breast cancer cells stably expressing ER. Cancer Lett. 1994 Jul 29;82(2):123–128. doi: 10.1016/0304-3835(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Jiang S. Y., Jordan V. C. Growth regulation of estrogen receptor-negative breast cancer cells transfected with complementary DNAs for estrogen receptor. J Natl Cancer Inst. 1992 Apr 15;84(8):580–591. doi: 10.1093/jnci/84.8.580. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Collins M. M., Rowsby L., Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977 Nov;75(2):305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Phelps E., Lindgren J. U. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987 Oct;10(1):31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- Levenson A. S., Catherino W. H., Jordan V. C. Estrogenic activity is increased for an antiestrogen by a natural mutation of the estrogen receptor. J Steroid Biochem Mol Biol. 1997 Mar;60(5-6):261–268. doi: 10.1016/s0960-0760(96)00184-7. [DOI] [PubMed] [Google Scholar]
- Levenson A. S., Jordan V. C. Transfection of human estrogen receptor (ER) cDNA into ER-negative mammalian cell lines. J Steroid Biochem Mol Biol. 1994 Dec;51(5-6):229–239. doi: 10.1016/0960-0760(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Dickson R. B. Mechanisms of growth control in normal and malignant breast epithelium. Recent Prog Horm Res. 1989;45:383–440. doi: 10.1016/b978-0-12-571145-6.50012-1. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- Love R. R., Mazess R. B., Barden H. S., Epstein S., Newcomb P. A., Jordan V. C., Carbone P. P., DeMets D. L. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992 Mar 26;326(13):852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- Love R. R., Wiebe D. A., Newcomb P. A., Cameron L., Leventhal H., Jordan V. C., Feyzi J., DeMets D. L. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann Intern Med. 1991 Dec 1;115(11):860–864. doi: 10.7326/0003-4819-115-11-860. [DOI] [PubMed] [Google Scholar]
- Luyten G. P., Hoogeveen A. T., Galjaard H. A fluorescence staining method for the demonstration and measurement of lysosomal enzyme activities in single cells. J Histochem Cytochem. 1985 Sep;33(9):965–968. doi: 10.1177/33.9.3926869. [DOI] [PubMed] [Google Scholar]
- Mahfoudi A., Roulet E., Dauvois S., Parker M. G., Wahli W. Specific mutations in the estrogen receptor change the properties of antiestrogens to full agonists. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4206–4210. doi: 10.1073/pnas.92.10.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano M. M., Ekena K., Krueger K. D., Keller A. L., Katzenellenbogen B. S. Human estrogen receptor ligand activity inversion mutants: receptors that interpret antiestrogens as estrogens and estrogens as antiestrogens and discriminate among different antiestrogens. Mol Endocrinol. 1996 Mar;10(3):230–242. doi: 10.1210/mend.10.3.8833652. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Dotzlaw H. Regulation of transforming growth factor alpha and transforming growth factor beta messenger ribonucleic acid abundance in T-47D, human breast cancer cells. Mol Endocrinol. 1989 Apr;3(4):611–617. doi: 10.1210/mend-3-4-611. [DOI] [PubMed] [Google Scholar]
- Noguchi S., Motomura K., Inaji H., Imaoka S., Koyama H. Down-regulation of transforming growth factor-alpha by tamoxifen in human breast cancer. Cancer. 1993 Jul 1;72(1):131–136. doi: 10.1002/1097-0142(19930701)72:1<131::aid-cncr2820720125>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Philips A., Chalbos D., Rochefort H. Estradiol increases and anti-estrogens antagonize the growth factor-induced activator protein-1 activity in MCF7 breast cancer cells without affecting c-fos and c-jun synthesis. J Biol Chem. 1993 Jul 5;268(19):14103–14108. [PubMed] [Google Scholar]
- Pink J. J., Jordan V. C. Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res. 1996 May 15;56(10):2321–2330. [PubMed] [Google Scholar]
- Saeki T., Cristiano A., Lynch M. J., Brattain M., Kim N., Normanno N., Kenney N., Ciardiello F., Salomon D. S. Regulation by estrogen through the 5'-flanking region of the transforming growth factor alpha gene. Mol Endocrinol. 1991 Dec;5(12):1955–1963. doi: 10.1210/mend-5-12-1955. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Kidwell W. R., Kim N., Ciardiello F., Bates S. E., Valverius E., Lippman M. E., Dickson R. B., Stampfer M. Modulation by estrogen and growth factors of transforming growth factor-alpha and epidermal growth factor receptor expression in normal and malignant human mammary epithelial cells. Recent Results Cancer Res. 1989;113:57–69. doi: 10.1007/978-3-642-83638-1_8. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Nawaz Z., O'Malley B. W. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997 Jun;11(6):657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- Sundstrom S. A., Komm B. S., Xu Q., Boundy V., Lyttle C. R. The stimulation of uterine complement component C3 gene expression by antiestrogens. Endocrinology. 1990 Mar;126(3):1449–1456. doi: 10.1210/endo-126-3-1449. [DOI] [PubMed] [Google Scholar]
- Tahara M., Tasaka K., Masumoto N., Adachi K., Adachi H., Ikebuchi Y., Kurachi H., Miyake A. Expression of messenger ribonucleic acid for epidermal growth factor (EGF), transforming growth factor-alpha (TGF alpha), and EGF receptor in human amnion cells: possible role of TGF alpha in prostaglandin E2 synthesis and cell proliferation. J Clin Endocrinol Metab. 1995 Jan;80(1):138–146. doi: 10.1210/jcem.80.1.7829602. [DOI] [PubMed] [Google Scholar]
- Tonetti D. A., Jordan V. C. Possible mechanisms in the emergence of tamoxifen-resistant breast cancer. Anticancer Drugs. 1995 Aug;6(4):498–507. doi: 10.1097/00001813-199508000-00002. [DOI] [PubMed] [Google Scholar]
- Umayahara Y., Kawamori R., Watada H., Imano E., Iwama N., Morishima T., Yamasaki Y., Kajimoto Y., Kamada T. Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. J Biol Chem. 1994 Jun 10;269(23):16433–16442. [PubMed] [Google Scholar]
- Wakeling A. E., Bowler J. Novel antioestrogens without partial agonist activity. J Steroid Biochem. 1988 Oct;31(4B):645–653. doi: 10.1016/0022-4731(88)90014-3. [DOI] [PubMed] [Google Scholar]
- Wakeling A. E., Dukes M., Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991 Aug 1;51(15):3867–3873. [PubMed] [Google Scholar]
- Webb P., Lopez G. N., Uht R. M., Kushner P. J. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995 Apr;9(4):443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- Wolf D. M., Jordan V. C. The estrogen receptor from a tamoxifen stimulated MCF-7 tumor variant contains a point mutation in the ligand binding domain. Breast Cancer Res Treat. 1994;31(1):129–138. doi: 10.1007/BF00689683. [DOI] [PubMed] [Google Scholar]
- Yang N. N., Venugopalan M., Hardikar S., Glasebrook A. Identification of an estrogen response element activated by metabolites of 17beta-estradiol and raloxifene. Science. 1996 Aug 30;273(5279):1222–1225. doi: 10.1126/science.273.5279.1222. [DOI] [PubMed] [Google Scholar]