Abstract
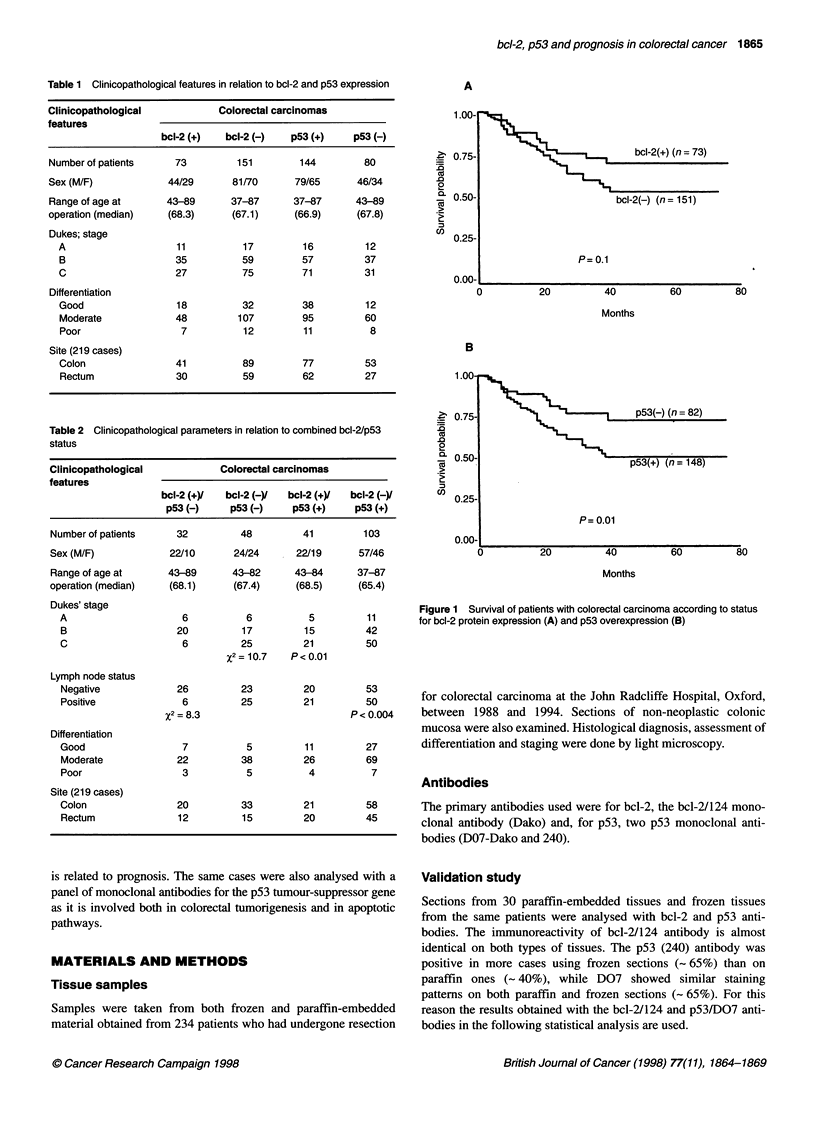
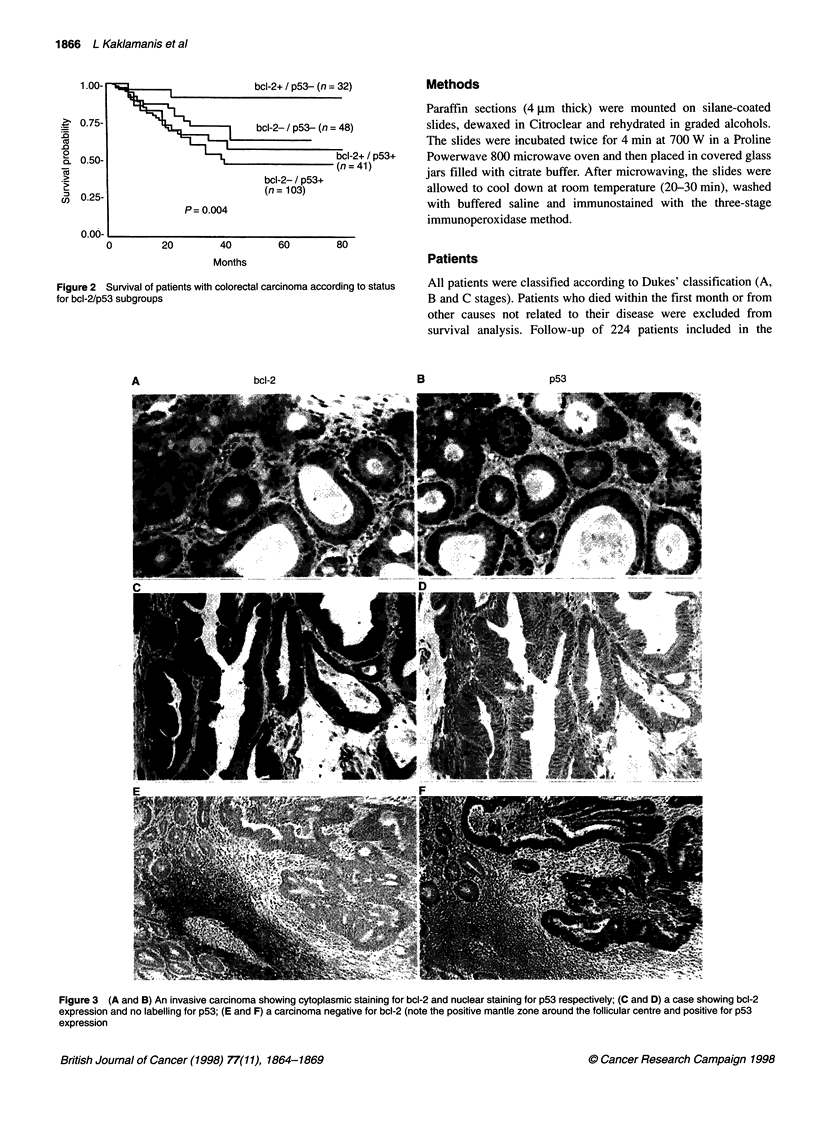
Bcl-2 expression in colorectal carcinomas was studied in a series of 224 patients and the relation to p53 expression, stage and survival assessed. Bcl-2 expression was down-regulated compared with normal mucosa in 67% (151) of the cases. In 144 cases staining was positive for p53 (MAB DO7), and 41 of these 144 p53-positive cases were also bcl-2 positive (28%) compared with 32 of the remaining 80 p53-negative cases (40%). Survival was significantly worse (P = 0.01) in the p53-positive cases. Bcl-2-positive cases, including patients in all Dukes' stages, had a slightly better prognosis which was not statistically significant. However, cases at an early stage (Dukes' stages A and B) and with negative p53 status, had a much better prognosis if they showed bcl-2 protein expression, suggesting that the bcl-2 status itself has an effect on prognosis (P = 0.01). Neither bcl-2 nor p53 alone was correlated with stage, but when examined by both p53 and bcl-2 status a group [bcl-2(+)/p53(-)] with better prognosis was defined. The last group was significantly lower Dukes' stage, with 26 out of 32 cases (81%) being A or B compared with 22 (11%) of the 202 remaining cases (P = 0.004). Thus, either loss of bcl-2 expression or gain of abnormal p53 expression is associated with high stage and poor prognosis. The bcl-2(+)/p53(-) phenotype is similar to that of normal mucosa, and these results suggest that such cases represent an indolent group at an early stage in the progression of colorectal cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas I. O., Mulder J. W., Offerhaus G. J., Vogelstein B., Hamilton S. R. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994 Jan;172(1):5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Baretton G. B., Diebold J., Christoforis G., Vogt M., Müller C., Dopfer K., Schneiderbanger K., Schmidt M., Löhrs U. Apoptosis and immunohistochemical bcl-2 expression in colorectal adenomas and carcinomas. Aspects of carcinogenesis and prognostic significance. Cancer. 1996 Jan 15;77(2):255–264. doi: 10.1002/(SICI)1097-0142(19960115)77:2<255::AID-CNCR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Bosari S., Moneghini L., Graziani D., Lee A. K., Murray J. J., Coggi G., Viale G. bcl-2 oncoprotein in colorectal hyperplastic polyps, adenomas, and adenocarcinomas. Hum Pathol. 1995 May;26(5):534–540. doi: 10.1016/0046-8177(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Castle V. P., Heidelberger K. P., Bromberg J., Ou X., Dole M., Nuñez G. Expression of the apoptosis-suppressing protein bcl-2, in neuroblastoma is associated with unfavorable histology and N-myc amplification. Am J Pathol. 1993 Dec;143(6):1543–1550. [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Rowley J. D., Variakojis D., Golomb H. M. Chromosome abnormalities in poorly differentiated lymphocytic lymphoma. Cancer Res. 1979 Aug;39(8):3119–3128. [PubMed] [Google Scholar]
- Furihata M., Sonobe H., Ohtsuki Y., Yamashita M., Morioka M., Yamamoto A., Terao N., Kuwahara M., Fujisaki N. Detection of p53 and bcl-2 protein in carcinoma of the renal pelvis and ureter including dysplasia. J Pathol. 1996 Feb;178(2):133–139. doi: 10.1002/(SICI)1096-9896(199602)178:2<133::AID-PATH455>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Goh H. S., Yao J., Smith D. R. p53 point mutation and survival in colorectal cancer patients. Cancer Res. 1995 Nov 15;55(22):5217–5221. [PubMed] [Google Scholar]
- Hague A., Moorghen M., Hicks D., Chapman M., Paraskeva C. BCL-2 expression in human colorectal adenomas and carcinomas. Oncogene. 1994 Nov;9(11):3367–3370. [PubMed] [Google Scholar]
- Haldar S., Negrini M., Monne M., Sabbioni S., Croce C. M. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994 Apr 15;54(8):2095–2097. [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Kaklamanis L., Savage A., Mortensen N., Tsiotos P., Doussis-Anagnostopoulou I., Biddolph S., Whitehouse R., Harris A. L., Gatter K. C. Early expression of bcl-2 protein in the adenoma-carcinoma sequence of colorectal neoplasia. J Pathol. 1996 May;179(1):10–14. doi: 10.1002/(SICI)1096-9896(199605)179:1<10::AID-PATH540>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- LeBrun D. P., Warnke R. A., Cleary M. L. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol. 1993 Mar;142(3):743–753. [PMC free article] [PubMed] [Google Scholar]
- Leek R. D., Kaklamanis L., Pezzella F., Gatter K. C., Harris A. L. bcl-2 in normal human breast and carcinoma, association with oestrogen receptor-positive, epidermal growth factor receptor-negative tumours and in situ cancer. Br J Cancer. 1994 Jan;69(1):135–139. doi: 10.1038/bjc.1994.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q. L., Poulsom R., Wong L., Hanby A. M. Bcl-2 expression in adult and embryonic non-haematopoietic tissues. J Pathol. 1993 Apr;169(4):431–437. doi: 10.1002/path.1711690408. [DOI] [PubMed] [Google Scholar]
- Manne U., Myers R. B., Moron C., Poczatek R. B., Dillard S., Weiss H., Brown D., Srivastava S., Grizzle W. E. Prognostic significance of Bcl-2 expression and p53 nuclear accumulation in colorectal adenocarcinoma. Int J Cancer. 1997 Jun 20;74(3):346–358. doi: 10.1002/(sici)1097-0215(19970620)74:3<346::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Krajewski S., Krajewska M., Wang H. G., Lin H. K., Liebermann D. A., Hoffman B., Reed J. C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994 Jun;9(6):1799–1805. [PubMed] [Google Scholar]
- Mosnier J. F., Perret A. G., Vindimian M., Dumollard J. M., Balique J. G., Perpoint B., Boucheron S. An immunohistochemical study of the simultaneous expression of bcl-2 and p53 oncoproteins in epithelial tumors of the colon and rectum. Arch Pathol Lab Med. 1996 Jul;120(7):654–659. [PubMed] [Google Scholar]
- Ngan B. Y., Chen-Levy Z., Weiss L. M., Warnke R. A., Cleary M. L. Expression in non-Hodgkin's lymphoma of the bcl-2 protein associated with the t(14;18) chromosomal translocation. N Engl J Med. 1988 Jun 23;318(25):1638–1644. doi: 10.1056/NEJM198806233182502. [DOI] [PubMed] [Google Scholar]
- Ofner D., Riehemann K., Maier H., Riedmann B., Nehoda H., Tötsch M., Böcker W., Jasani B., Schmid K. W. Immunohistochemically detectable bcl-2 expression in colorectal carcinoma: correlation with tumour stage and patient survival. Br J Cancer. 1995 Oct;72(4):981–985. doi: 10.1038/bjc.1995.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Pereira H., Silva S., Julião R., Garcia P., Perpétua F. Prognostic markers for colorectal cancer: expression of P53 and BCL2. World J Surg. 1997 Feb;21(2):210–213. doi: 10.1007/s002689900218. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Turley H., Kuzu I., Tungekar M. F., Dunnill M. S., Pierce C. B., Harris A., Gatter K. C., Mason D. Y. bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993 Sep 2;329(10):690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Papadopoulos N., Markowitz S., Willson J. K., Kinzler K. W., Vogelstein B. Paradoxical inhibition of solid tumor cell growth by bcl2. Cancer Res. 1994 Jul 15;54(14):3714–3717. [PubMed] [Google Scholar]
- Reed J. C., Tsujimoto Y., Alpers J. D., Croce C. M., Nowell P. C. Regulation of bcl-2 proto-oncogene expression during normal human lymphocyte proliferation. Science. 1987 Jun 5;236(4806):1295–1299. doi: 10.1126/science.3495884. [DOI] [PubMed] [Google Scholar]
- Schneider H. J., Sampson S. A., Cunningham D., Norman A. R., Andreyev H. J., Tilsed J. V., Clarke P. A. Bcl-2 expression and response to chemotherapy in colorectal adenocarcinomas. Br J Cancer. 1997;75(3):427–431. doi: 10.1038/bjc.1997.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Bovey R., Tardy S., Sahli R., Sordat B., Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinicrope F. A., Ruan S. B., Cleary K. R., Stephens L. C., Lee J. J., Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995 Jan 15;55(2):237–241. [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ikegaki N., Croce C. M. Characterization of the protein product of bcl-2, the gene involved in human follicular lymphoma. Oncogene. 1987;2(1):3–7. [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Wang Y., Szekely L., Okan I., Klein G., Wiman K. G. Wild-type p53-triggered apoptosis is inhibited by bcl-2 in a v-myc-induced T-cell lymphoma line. Oncogene. 1993 Dec;8(12):3427–3431. [PubMed] [Google Scholar]
- Watson A. J., Merritt A. J., Jones L. S., Askew J. N., Anderson E., Becciolini A., Balzi M., Potten C. S., Hickman J. A. Evidence of reciprocity of bcl-2 and p53 expression in human colorectal adenomas and carcinomas. Br J Cancer. 1996 Apr;73(8):889–895. doi: 10.1038/bjc.1996.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]