Abstract
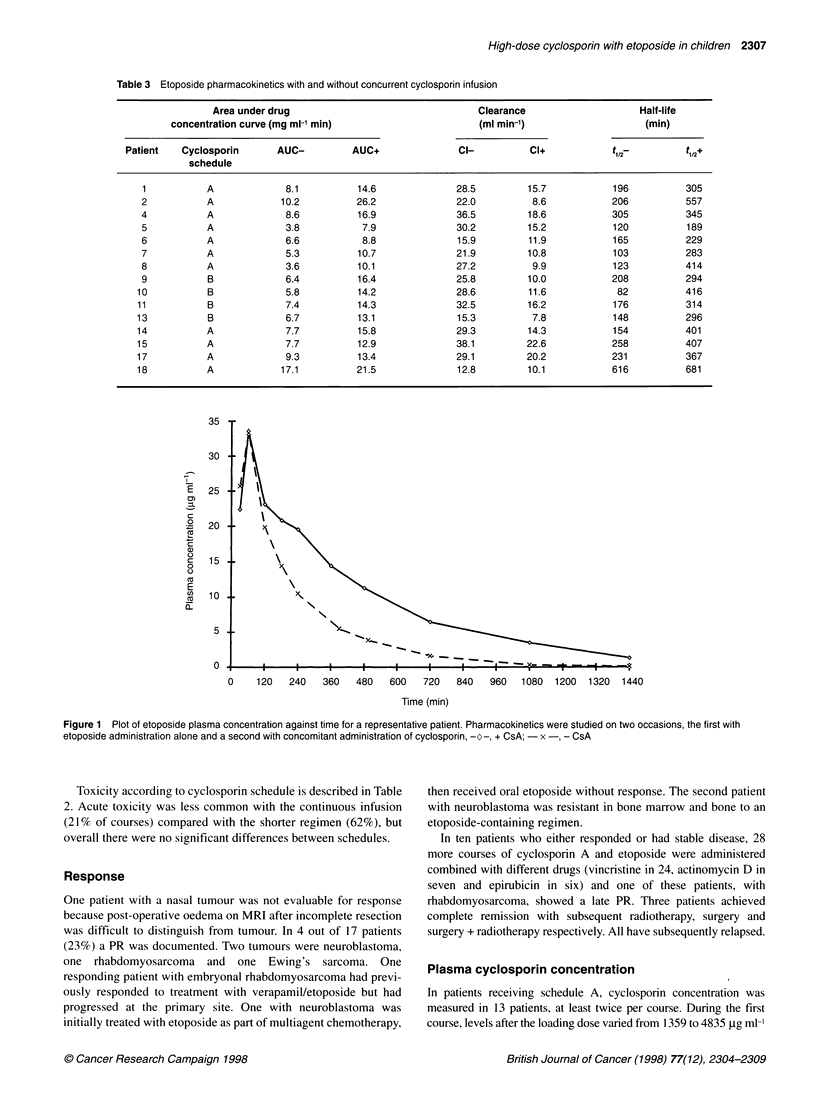
The tolerability, anti-tumour activity and pharmacokinetic interaction of high-dose intravenous cyclosporin combined with intravenous etoposide was evaluated in children. Eighteen patients with recurrent or refractory tumours, all of whom had previously received etoposide, were treated with a combination of high-dose cyclosporin and etoposide. In 13, cyclosporin was given as a continuous infusion (15 mg kg(-1) per 24 h for 60 h) and in five a short 3-hour infusion of 30 mg kg(-1) day(-1) on three consecutive days. Pharmacokinetic profiles of etoposide were determined with and without cyclosporin. Cyclosporin levels ranged from 1359 to 4835 ng ml(-1) and cyclosporin increased the median area under the concentration time for etoposide curve from 7.2 to 12.5 mg ml(-1) min. The major toxicity was acute with varying forms of hypersensitivity reactions. In four cases this was severe. Hyperbilirubinaemia was present in 25 of 32 courses but was of short duration. In 14 courses, creatinine and/or urea was elevated, but was also transient. Significant hypertension was seen in six courses. Four of 17 patients evaluable for response obtained a partial response and one showed stable disease. It is concluded that in children given the combination of high-dose cyclosporin and etoposide, the etoposide dose should be halved in order to achieve an area under the drug concentration-time curve similar to that with etoposide alone. A continuous infusion schedule of cyclosporin is better tolerated during the period of administration but is associated with similar hepatic and renal dysfunction to a short schedule. The 24% response rate in children who had previously received etoposide suggests that this may be an effective method of enhancing drug sensitivity and further phase II evaluation is justified.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett N. L., Lum B. L., Fisher G. A., Brophy N. A., Ehsan M. N., Halsey J., Sikic B. I. Phase I trial of doxorubicin with cyclosporine as a modulator of multidrug resistance. J Clin Oncol. 1994 Apr;12(4):835–842. doi: 10.1200/JCO.1994.12.4.835. [DOI] [PubMed] [Google Scholar]
- Boote D. J., Dennis I. F., Twentyman P. R., Osborne R. J., Laburte C., Hensel S., Smyth J. F., Brampton M. H., Bleehen N. M. Phase I study of etoposide with SDZ PSC 833 as a modulator of multidrug resistance in patients with cancer. J Clin Oncol. 1996 Feb;14(2):610–618. doi: 10.1200/JCO.1996.14.2.610. [DOI] [PubMed] [Google Scholar]
- Bourhis J., Bénard J., Hartmann O., Boccon-Gibod L., Lemerle J., Riou G. Correlation of MDR1 gene expression with chemotherapy in neuroblastoma. J Natl Cancer Inst. 1989 Sep 20;81(18):1401–1405. doi: 10.1093/jnci/81.18.1401. [DOI] [PubMed] [Google Scholar]
- Bourhis J., Hartmann O., DeVathaire F., Terrier-Lacombe M. J., Boccon-Gibod L., Lemerle J., Riou G., Bénard J. Expression of MDR1 and GST pi genes in 35 advanced neuroblastomas. Prog Clin Biol Res. 1991;366:127–134. [PubMed] [Google Scholar]
- Brophy N. A., Marie J. P., Rojas V. A., Warnke R. A., McFall P. J., Smith S. D., Sikic B. I. Mdr1 gene expression in childhood acute lymphoblastic leukemias and lymphomas: a critical evaluation by four techniques. Leukemia. 1994 Feb;8(2):327–335. [PubMed] [Google Scholar]
- Cairo M. S., Siegel S., Anas N., Sender L. Clinical trial of continuous infusion verapamil, bolus vinblastine, and continuous infusion VP-16 in drug-resistant pediatric tumors. Cancer Res. 1989 Feb 15;49(4):1063–1066. [PubMed] [Google Scholar]
- Chan H. S., DeBoer G., Thorner P. S., Haddad G., Gallie B. L., Ling V. Multidrug resistance. Clinical opportunities in diagnosis and circumvention. Hematol Oncol Clin North Am. 1994 Apr;8(2):383–410. [PubMed] [Google Scholar]
- Chan H. S., Haddad G., Thorner P. S., DeBoer G., Lin Y. P., Ondrusek N., Yeger H., Ling V. P-glycoprotein expression as a predictor of the outcome of therapy for neuroblastoma. N Engl J Med. 1991 Dec 5;325(23):1608–1614. doi: 10.1056/NEJM199112053252304. [DOI] [PubMed] [Google Scholar]
- Chan H. S., Thorner P. S., Haddad G., Ling V. Immunohistochemical detection of P-glycoprotein: prognostic correlation in soft tissue sarcoma of childhood. J Clin Oncol. 1990 Apr;8(4):689–704. doi: 10.1200/JCO.1990.8.4.689. [DOI] [PubMed] [Google Scholar]
- Corrias M. V., Cornaglia-Ferraris P., Di Martino D., Stenger A. M., Lanino E., Boni L., Tonini G. P. Expression of multiple drug resistance gene, MDR1, and N-myc oncogene in an Italian population of human neuroblastoma patients. Anticancer Res. 1990 Jul-Aug;10(4):897–902. [PubMed] [Google Scholar]
- Cowie F. J., Pinkerton C. R., Phillips M., Dick G., Judson I., McCarthy P. T., Flanagan R. J. Continuous-infusion verapamil with etoposide in relapsed or resistant paediatric cancers. Br J Cancer. 1995 Apr;71(4):877–881. doi: 10.1038/bjc.1995.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie F., Pinkerton C. R. Enhanced toxicity of dactinomycin and vincristine by cyclosporine given to reverse multidrug resistance. J Clin Oncol. 1994 Sep;12(9):1998–1999. doi: 10.1200/JCO.1994.12.9.1998. [DOI] [PubMed] [Google Scholar]
- Erlichman C., Moore M., Thiessen J. J., Kerr I. G., Walker S., Goodman P., Bjarnason G., DeAngelis C., Bunting P. Phase I pharmacokinetic study of cyclosporin A combined with doxorubicin. Cancer Res. 1993 Oct 15;53(20):4837–4842. [PubMed] [Google Scholar]
- Fridborg H., Jonsson B., Nygren P., Csoka K., Nilsson K., Oberg G., Kristensen J., Bergh J., Tholander B., Olsen L. Activity of cyclosporins as resistance modifiers in primary cultures of human haematological and solid tumours. Br J Cancer. 1994 Jul;70(1):11–17. doi: 10.1038/bjc.1994.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann U. A., Shlyakhter D., Mason V. S., Zelle R. E., Duffy J. P., Galullo V., Armistead D. M., Saunders J. O., Boger J., Harding M. W. Cellular and biochemical characterization of VX-710 as a chemosensitizer: reversal of P-glycoprotein-mediated multidrug resistance in vitro. Anticancer Drugs. 1997 Feb;8(2):125–140. doi: 10.1097/00001813-199702000-00004. [DOI] [PubMed] [Google Scholar]
- Guerci A., Merlin J. L., Missoum N., Feldmann L., Marchal S., Witz F., Rose C., Guerci O. Predictive value for treatment outcome in acute myeloid leukemia of cellular daunorubicin accumulation and P-glycoprotein expression simultaneously determined by flow cytometry. Blood. 1995 Apr 15;85(8):2147–2153. [PubMed] [Google Scholar]
- Kuttesch J. F., Parham D. M., Luo X., Meyer W. H., Bowman L., Shapiro D. N., Pappo A. S., Crist W. M., Beck W. T., Houghton P. J. P-glycoprotein expression at diagnosis may not be a primary mechanism of therapeutic failure in childhood rhabdomyosarcoma. J Clin Oncol. 1996 Mar;14(3):886–900. doi: 10.1200/JCO.1996.14.3.886. [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B., Dalton W., Fisher G. A., Sikic B. I. Reversal of multidrug resistance to cancer chemotherapy. Cancer. 1993 Dec 1;72(11 Suppl):3484–3488. doi: 10.1002/1097-0142(19931201)72:11+<3484::aid-cncr2820721615>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- List A. F., Spier C., Greer J., Wolff S., Hutter J., Dorr R., Salmon S., Futscher B., Baier M., Dalton W. Phase I/II trial of cyclosporine as a chemotherapy-resistance modifier in acute leukemia. J Clin Oncol. 1993 Sep;11(9):1652–1660. doi: 10.1200/JCO.1993.11.9.1652. [DOI] [PubMed] [Google Scholar]
- Lum B. L., Fisher G. A., Brophy N. A., Yahanda A. M., Adler K. M., Kaubisch S., Halsey J., Sikic B. I. Clinical trials of modulation of multidrug resistance. Pharmacokinetic and pharmacodynamic considerations. Cancer. 1993 Dec 1;72(11 Suppl):3502–3514. doi: 10.1002/1097-0142(19931201)72:11+<3502::aid-cncr2820721618>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Lum B. L., Kaubisch S., Yahanda A. M., Adler K. M., Jew L., Ehsan M. N., Brophy N. A., Halsey J., Gosland M. P., Sikic B. I. Alteration of etoposide pharmacokinetics and pharmacodynamics by cyclosporine in a phase I trial to modulate multidrug resistance. J Clin Oncol. 1992 Oct;10(10):1635–1642. doi: 10.1200/JCO.1992.10.10.1635. [DOI] [PubMed] [Google Scholar]
- McLeod H. L. Clinical reversal of the multidrug resistance phenotype: true tumour modulation or pharmacokinetic interaction? Eur J Cancer. 1994;30A(14):2039–2041. doi: 10.1016/0959-8049(94)00423-3. [DOI] [PubMed] [Google Scholar]
- Merlin J. L., Guerci A., Marchal S., Missoum N., Ramacci C., Humbert J. C., Tsuruo T., Guerci O. Comparative evaluation of S9788, verapamil, and cyclosporine A in K562 human leukemia cell lines and in P-glycoprotein-expressing samples from patients with hematologic malignancies. Blood. 1994 Jul 1;84(1):262–269. [PubMed] [Google Scholar]
- Rushing D. A., Raber S. R., Rodvold K. A., Piscitelli S. C., Plank G. S., Tewksbury D. A. The effects of cyclosporine on the pharmacokinetics of doxorubicin in patients with small cell lung cancer. Cancer. 1994 Aug 1;74(3):834–841. doi: 10.1002/1097-0142(19940801)74:3<834::aid-cncr2820740308>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Serra M., Scotlandi K., Manara M. C., Maurici D., Benini S., Sarti M., Campanacci M., Baldini N. Analysis of P-glycoprotein expression in osteosarcoma. Eur J Cancer. 1995 Nov;31A(12):1998–2002. doi: 10.1016/0959-8049(95)00335-5. [DOI] [PubMed] [Google Scholar]
- Stein U., Shoemaker R. H., Schlag P. M. MDR1 gene expression: evaluation of its use as a molecular marker for prognosis and chemotherapy of bone and soft tissue sarcomas. Eur J Cancer. 1996 Jan;32A(1):86–92. doi: 10.1016/0959-8049(95)00478-5. [DOI] [PubMed] [Google Scholar]
- Theis J. G., Liau-Chu M., Chan H. S., Doyle J., Greenberg M. L., Koren G. Anaphylactoid reactions in children receiving high-dose intravenous cyclosporine for reversal of tumor resistance: the causative role of improper dissolution of Cremophor EL. J Clin Oncol. 1995 Oct;13(10):2508–2516. doi: 10.1200/JCO.1995.13.10.2508. [DOI] [PubMed] [Google Scholar]
- Vergier B., Cany L., Bonnet F., Robert J., de Mascarel A., Coindre J. M. Expression of MDR1/P glycoprotein in human sarcomas. Br J Cancer. 1993 Dec;68(6):1221–1226. doi: 10.1038/bjc.1993.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahanda A. M., Alder K. M., Fisher G. A., Brophy N. A., Halsey J., Hardy R. I., Gosland M. P., Lum B. L., Sikic B. I. Phase I trial of etoposide with cyclosporine as a modulator of multidrug resistance. J Clin Oncol. 1992 Oct;10(10):1624–1634. doi: 10.1200/JCO.1992.10.10.1624. [DOI] [PubMed] [Google Scholar]


