Abstract
The Arabidopsis genes EDS1 and NDR1 were shown previously by mutational analysis to encode essential components of race-specific disease resistance. Here, we examined the relative requirements for EDS1 and NDR1 by a broad spectrum of Resistance (R) genes present in three Arabidopsis accessions (Columbia, Landsberg-erecta, and Wassilewskija). We show that there is a strong requirement for EDS1 by a subset of R loci (RPP2, RPP4, RPP5, RPP21, and RPS4), conferring resistance to the biotrophic oomycete Peronospora parasitica, and to Pseudomonas bacteria expressing the avirulence gene avrRps4. The requirement for NDR1 by these EDS1-dependent R loci is either weak or not measurable. Conversely, three NDR1-dependent R loci, RPS2, RPM1, and RPS5, operate independently of EDS1. Another RPP locus, RPP8, exhibits no strong exclusive requirement for EDS1 or NDR1 in isolate-specific resistance to P. parasitica, although resistance is compromised weakly by eds1. Similarly, resistance conditioned by two EDS1-dependent RPP genes, RPP4 and RPP5, is impaired partially by ndr1, implicating a degree of pathway cross-talk. Our results provide compelling evidence for the preferential utilization of either signaling component by particular R genes and thus define at least two disease resistance pathways. The data also suggest that strong dependence on EDS1 or NDR1 is governed by R protein structural type rather than pathogen class.
Disease resistance in plants commonly is specified by genetically paired products that are encoded by plant resistance (R) genes and pathogen avirulence (avr) genes (1). Susceptibility to a particular pathogen race only can occur when either one of the genetic components is missing or inactivated. In many plant-pathogen combinations, this type of resistance is associated with localized cell necrosis at the site of attempted pathogen entry (the hypersensitive response). However, the precise mechanisms that lead to pathogen containment are not understood.
The cloning of avr genes from prokaryotic and eukaryotic plant pathogens and, more recently, corresponding R genes from several different plant species has provided some important insights to the process of pathogen recognition. For example, the tomato Pto gene specifying resistance to a bacterial pathogen encodes a functional serine/threonine protein kinase that specifically phosphorylates a second protein kinase, Pti1 (2). Pto also interacts with the corresponding bacterial Avr protein AvrPto in a yeast two-hybrid assay (3, 4), strongly supporting its role as the physiological receptor for the bacterial ligand. Other R proteins that are predicted to be localized either extracellularly or within the cytoplasm are structurally different to Pto and contain leucine-rich repeats (LRRs) (5), implicating protein–protein interactions as part of the recognition process. In the putatively cytoplasmic R protein members, the LRRs lie adjacent to sequences that constitute a nucleotide binding site (NBS) (5). The NBS/LRR class of R proteins can be subdivided into members that possess an amino-terminal leucine zipper (LZ) motif or those that have amino-terminal similarity to the cytoplasmic domains of the Drosophila Toll and mammalian interleukin 1 transmembrane receptors (referred to as the “TIR” domain) (1, 6).
The representation of a limited number of common structural motifs in the R proteins identified so far raises the possibility that they may function, at least in part, by similar signaling mechanisms. Indeed, the high level of sequence conservation between the tobacco N gene conferring resistance to a virus (7), the flax L6 gene specifying rust fungus resistance (8), and the Arabidopsis RPP5 gene for resistance to an oomycete pathogen (9) suggests conservation of resistance pathways between different plant species and differing pathogen types. A major goal now is to understand how early R protein-mediated recognition events lead to effective resistance.
The model crucifer Arabidopsis has been exploited as a host for bacterial, viral, fungal, and oomycete pathogens (10), and analysis of natural genetic variation between different plant accessions and pathogen isolates has led to the identification of distinct R loci. The cloned RPS2 and RPM1 genes, controlling race-specific resistance to the bacterial pathogen Pseudomonas syringae, encode proteins of the LZ-NBS/LRR class (11–13). In contrast, the RPP5 gene mediating isolate-specific resistance to the biotrophic oomycete Peronospora parasitica encodes a protein of the TIR-NBS/LRR class (9).
Mutational analyses in Arabidopsis have identified other wild-type genes that are required for R gene-mediated resistance (14). A mutation in NDR1 (nonrace-specific disease resistance) abolished resistance conferred by RPS2 and RPS5 to P. syringae expressing avrRpt2 and avrPph3, respectively, as well as a dual specificity resistance encoded by RPM1 to the bacterial genes avrB and avrRpm1 (15). ndr1 plants also were compromised in RPP gene-mediated resistance to several incompatible P. parasitica isolates, suggesting that the wild-type NDR1 protein may function at a common point downstream from the perception of these prokaryotic and eukaryotic pathogens (15). Another resistance signaling component is encoded by EDS1 (enhanced disease susceptibility) (16). A mutation in EDS1 abolished resistance conferred by several RPP loci but had no effect on RPM1-specified resistance, suggesting a function before the convergence of downstream pathways (16).
We thought it possible that the ndr1 and eds1 mutations may reflect the operation of distinct disease resistance signaling pathways and that particular R proteins exhibit a preference for one signaling mode. To address this possibility, we extended our analysis of the relative requirements for EDS1 and NDR1 to a wide spectrum of Arabidopsis R genes in three different genetic backgrounds (accessions). Our results show that the requirements for EDS1 or NDR1 appear to be mutually exclusive. Furthermore, the data suggest that preferential utilization of either EDS1 or NDR1 is determined more by R protein structure than pathogen type.
METHODS
Maintenance of Pathogen Isolates.
Pseudomonas syringae pv. tomato DC3000 containing the avirulence genes avrRps4 (17), avrRpm1 (13), avrRpt2 (11), or avrPph3 (18) in the broad host range vector pVSP61 or DC3000 containing empty pVSP61 were cultured as described. The Peronospora parasitica isolates from Arabidopsis have been described before (16, 19–21). These were cultured on their corresponding compatible hosts as described (21).
Plant Material, Cultivation, and Pathogenicity Tests.
Seeds of the Arabidopsis accession Wassilewskija (Ws-0) originally were obtained from K. Feldman (University of Arizona, Tucson, AZ). Columbia (Col-0 or Col-gl, containing the recessive mutation gl1) were obtained from J. Dangl (University of North Carolina, Chapel Hill, NC). Landsberg-erecta (Ler) seed were obtained from the Nottingham Arabidopsis Stock Centre (Nottingham, UK). The Ws eds1-1 mutation has been described (16). The Ler eds1-2, eds1-3, and eds1-4 mutant lines were isolated from fast neutron-bombarded M2 Ler seed obtained from Lehle Seeds (Tucson, AZ). The Col ndr1-1 mutant line has been described (15). Mutant lines eds1-3 and eds1-4 were backcrossed once, and eds1-1 and eds1-2 were backcrossed twice, to their respective wild-type parent.
Cultivation of plants for bacterial inoculations, infiltration of leaves with bacterial suspensions, and bacterial growth assays were as described (16, 17). Plant cultivation for P. parasitica inoculations was as before (16). Seedlings were either sprayed with conidiospores (9) or individual cotyledons inoculated with droplets of conidiospore inoculum (20). Development of P. parasitica inside leaves or cotyledons was monitored by staining with lactophenol–trypan blue and examining under a light microscope (16).
Selection of Plant Genotypes.
Plant genomic DNA was isolated as described (9). Ler RPP5 and Col RPP4 that are tightly linked on chromosome 4 were selected in F2 progeny (see Table 1) by using codominant cleaved amplified polymorphic sequence primers for g4539 (http://genome-www.stanford.edu). Ler RPS4 (17) and Ler RPP8 (21) lie on chromosome 5 in a ≈15-cM interval between the cleaved amplified polymorphic sequence marker DFR and the microsatellite marker nga129. These two markers were used to select plants homozygous for Ler DNA in Col ndr1-1 × Ler F2 plants. Col RPP2 was selected by using cleaved amplified polymorphic sequence primers for AG (≈1 cM centromeric to RPP2; Jim Beynon, University of London, Wye College, Kent, UK, personal communication). EDS1 maps ≈12 cM telomeric to the RFLP marker m249 (16). Col-gl × Ws eds1-1 F2 plants were genotyped for EDS1 or eds1-1 by using m249-specific primers that detect a simple sequence length polymorphism (16). Genotypes subsequently were confirmed by using EDS1-specific cleaved amplified polymorphic sequence primers designed to detect a MseI polymorphism between EDS1 and eds1-1 (A. Falk, B. Feys, and J.E.P., unpublished data). EDS1 was distinguished from eds1-2 in F2 plants initially by using the m249-specific primers, as above. Genotypes were later confirmed by using EDS1-specific primers to detect a 900-bp deletion in eds1-2 (A. Falk, B. Feys, and J.E.P., unpublished data). Homozygous NDR1 or ndr1-1 plants from Col ndr1-1 × Ler F3 families that had been phenotypically selected kindly were provided by K. Century (University of California, Berkeley). The NDR1/ndr1-1 genotypes were confirmed by using NDR1-specific primers that detected a 1-kb deletion in ndr1-1 (22).
Table 1.
Phenotypes of different RPP loci in combination with wild-type or mutant eds1 or ndr1 after inoculation of selected F3 families with P. parasitica
| RPP locus, P. parasitica isolate | Cross | F3 families, n | Genotype* | Phenotype† |
|---|---|---|---|---|
| RPP2 (Cala2) | Col-gl × Ler-eds1-2 | 5 | RPP2, EDS1 | R |
| 5 | RPP2, eds1-2 | S | ||
| Col-ndr1-1‡ | – | RPP2, ndr1-1 | R | |
| RPP4 (Emwa1) | Col-gl × Ws-eds1-1 | 1 | RPP4, EDS1 | R |
| 2 | RPP4, eds1-1 | S | ||
| Col-ndr1-1‡ | – | RPP4, ndr1-1 | R | |
| RPP5 (Noco2) | Col-ndr1-1 × Ler | 5 | RPP5, NDR1 | R |
| 5 | RPP5, ndr1-1 | R | ||
| 2 | rpp5, NDR1 | S | ||
| RPP8 (Emco5) | Col-ndr1-1 × Ler | 5 | RPP8, NDR1 | R |
| 5 | RPP8, ndr1-1 | R | ||
| Ws-0‡ | – | rpp8, NDR1 | S |
Each F3 family was homozygous for the respective RPP locus and wild-type or mutant allele.
Twenty to 30 9-day-old seedlings were spray-inoculated with P. parasitica conidiospores. Resistance (R, no sporulation) or susceptibility (S, medium to profuse sporulation) was scored with a hand lens on the first true leaves 7 days after inoculation.
Selfed Col ndr1, wild-type Colgl, or Ws-0 seedlings.
RESULTS
Identification of Three New eds1 Alleles.
The eds1 mutation in the Arabidopsis accession Wassilewskija (Ws-0) abolished resistance to several P. parasitica isolates mediated by RPP1, RPP10, and RPP14 on chromosome 3 and RPP12 on chromosomes 4 (16). The Ws eds1 allele hereafter is denoted eds1-1. Three further defective eds1 alleles were identified in two different screens of fast neutron-mutagenized seedlings of accession Landsberg-erecta (Ler). In the first screen, a loss of RPP5-mediated resistance to isolate Noco2 revealed two independent mutant alleles, eds1-2 and eds1-3. In the second screen, a loss of RPS4-specified resistance to the compatible Pseudomonas syringae pv. tomato strain DC3000 expressing avrRps4 (17) identified a fourth allele, eds1-4. It was significant that the requirement for EDS1 in RPS4-specified resistance to a bacterial pathogen suggested a necessity for EDS1 beyond RPP gene-mediated responses.
Resistance to Noco2 in wild-type Ler is conferred by a single gene, RPP5 (9), and in Ws-0 by an unlinked gene, RPP14 (19). Allelism between the new Ler eds1 mutations and Ws eds1-1 was tested by inoculating with Noco2 300 F2 seedlings derived from crosses between each Ler mutant line and eds1-1. All F2 progeny were Noco2-susceptible. Similarly, all F2 seedlings from an eds1-2 × eds1-1 cross were susceptible to Cala2, a Ler-compatible P. parasitica isolate that is recognized by a single R locus, RPP10, in Ws-0 (20, 21). As expected, resistance and susceptibility to Noco2 in wild-type Ler × Ws-0 F2 progeny segregated as a 15:1 ratio (χ2 = 0.089; P < 0.01). Resistance and susceptibility to Cala2 segregated as a 3:1 ratio (χ2 = 0.314; P < 0.05). These tests proved allelism and indicated that the four defective eds1 alleles abolished the function of RPP5, RPP10, and RPP14.
EDS1 Is Required by a Subset of RPP Loci.
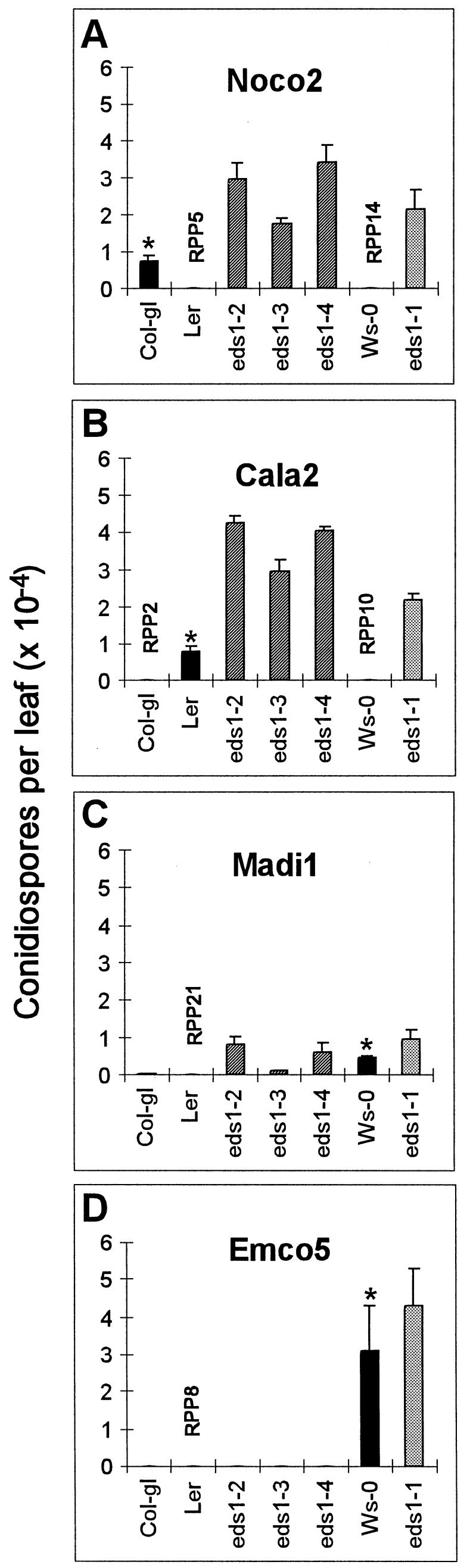
To provide a quantitative assessment of the extent of colonization of Ws eds1-1 and the three Ler eds1 mutant lines by P. parasitica isolates Noco2 and Cala2, asexual conidiospore production was measured. This was compared with sporulation of each isolate on the naturally resistant and susceptible wild-type accessions, as indicated in Fig. 1. All eds1 lines permitted high sporulation levels of Noco2 and Cala2 (Fig. 1 A and B).
Figure 1.
Sporulation levels of different P. parasitica isolates on eds1 mutant lines and wild-type Arabidopsis accessions. Conidiospore suspensions (4 × 104/ml) of each isolate were sprayed onto 2-week-old seedlings. Spores were harvested from leaves and counted after 7 days of incubation (see Methods). For each P. parasitica isolate tested, the genetically susceptible accession is marked with an asterisk and the resistant accession with the corresponding RPP gene. The experiment was repeated on 1-week-old seedlings with similar results.
We extended the analysis to two other P. parasitica isolates that are incompatible on Ler and for which corresponding RPP loci were identified previously (21). P. parasitica isolate Madi1 (corresponding to RPP21) and Emco5 (RPP8) are both compatible on Ws-0. As shown in Fig. 1C, resistance to Madi1 also was suppressed in the Ler eds1 lines, indicating dependence of RPP21 on EDS1 function. Overall, Madi1 spore production was low, but levels were significantly higher in the mutant lines than in the naturally susceptible accession Ws-0. In all three Arabidopsis–pathogen combinations, eds1-3 exhibited a less severe suppression phenotype than either eds1-2 or eds1-4 (Fig. 1 A–C), suggesting an incomplete loss of EDS1 function in the eds1-3 mutant background. In contrast to the clear suppression of resistance to Noco2, Cala2, and Madi1 by eds1, no detectable sporulation was observed in either eds1-2, eds1-3, or eds1-4 after inoculation with Emco5 (Fig. 1D) even though this isolate was fully pathogenic on Ws-0.
The results in Fig. 1 showed that asexual reproduction of each P. parasitica isolate on eds1 plants derived from a susceptible accession was significantly greater than on the corresponding compatible wild-type parent. This is consistent with an “enhanced disease susceptibility” phenotype previously observed on Ws eds1-1 seedlings inoculated with the Ws-compatible isolate Emwa1 (16).
RPP8-Specified Resistance Is Weakly Suppressed by eds1.
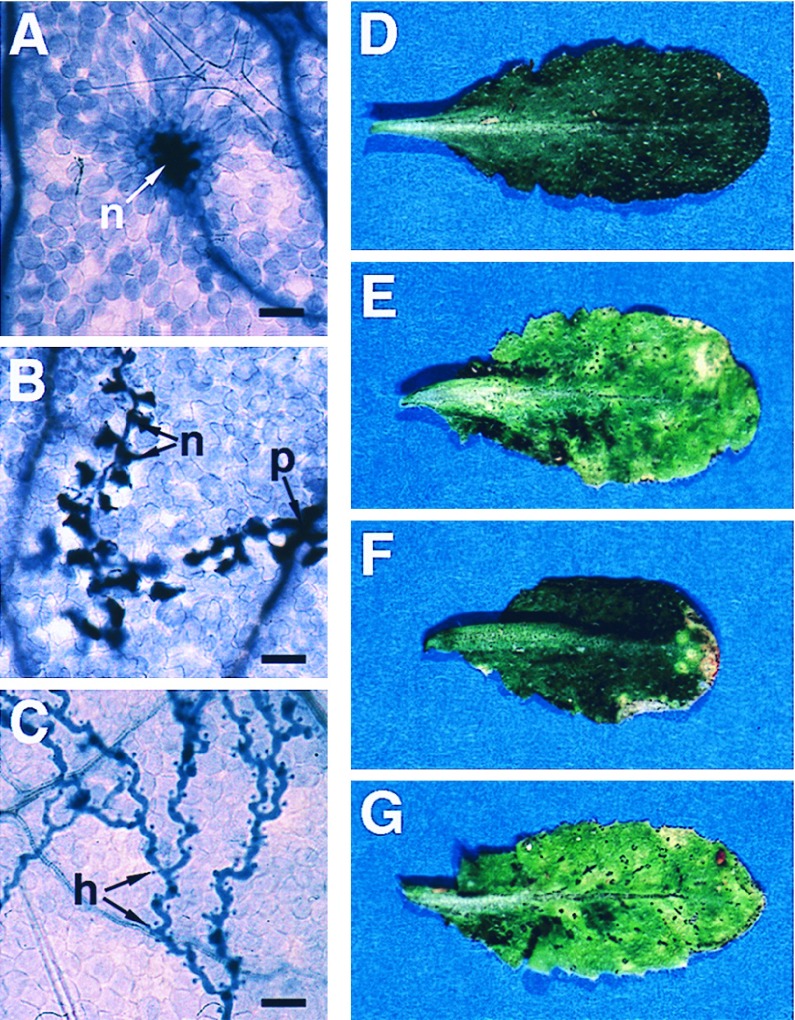
The RPP8-specified response of Ler eds1 seedlings to Emco5 appeared macroscopically to be as resistant as wild-type Ler seedlings (Fig. 1D). We therefore examined the inoculated plants for evidence of vegetative pathogen development. The extent of mycelium growth and plant cell necrosis was observed on a light microscope after staining seedlings with lactophenol–trypan blue (16). As shown in Fig. 2A, wild-type Ler leaves produced discrete necrotic lesions consisting of a few plant cells in response to attempted Emco5 penetration. In contrast, mycelial growth was more extensive in leaves of eds1-2 seedlings, although the mycelium typically was surrounded by a trail of necrotic plant cells (Fig. 2B). Growth of Emco5 in eds1-2 was very restricted compared with development in leaves of the natural Emco5-susceptible accession Ws-0 (Fig. 2C). A similar increase in pathogen growth was observed in eds1-2 cotyledons and in leaves and cotyledons of eds1-3 seedlings (data not shown). We concluded that RPP8-mediated recognition of Emco5 is impaired partially by the eds1 mutation.
Figure 2.
Pathogen development and disease symptom expression in wild-type and eds1 plants inoculated with P. parasitica or P. syringae. Development of P. parasitica isolate Emco5 is shown in A–C. Leaves were stained with lactophenol–trypan blue 5 days after inoculation and viewed under a light microscope to reveal pathogen mycelium and necrotic plant cells. (Bar = 50 μm.) Disease phenotypes of wild-type Ws-0 and Ws eds1-1 leaves inoculated with P. syringae strain DC3000 or DC3000 expressing avrRps4 are shown in D–G. Leaves were photographed 5 days after infiltration with suspensions of 1 × 105 bacteria/ml. (A) Ler wild-type, showing a discrete necrotic lesion (n) of plant cells surrounding an Emco5 penetration site. Resistance is conferred by RPP8. (B) Ler eds1-2, showing mycelium growing beyond the penetration site (p) but surrounded by a trail of necrotic plant cells (n). (C) Ws-0, lacking RPP8, is fully susceptible to Emco5. The mycelium forms haustoria (h) and grows systemically without associated plant cell death. (D) DC3000 expressing avrRps4 causes no disease symptoms in Ws-0 due to resistance conferred by RPS4. (E) DC3000 expressing avrRps4 causes severe disease symptoms in leaves of eds1-1. (F) DC3000 containing no functional avr gene elicits mild disease symptoms in Ws-0 leaves. (G) Disease symptom development in eds1-1 leaves inoculated with DC3000 is more rapid than in Ws-0 leaves.
Requirements for EDS1 or NDR1 by Different R Genes Are Mutually Exclusive.
The screen for mutations affecting RPS4-specified recognition of avrRPS4 from P. syringae identified one defective eds1 allele, eds1-4, demonstrating a requirement for EDS1 beyond RPP gene-mediated disease resistance. Previous studies had shown that other Arabidopsis R genes, RPM1, RPS2, and RPS5, controlling recognition of bacterial avr determinants were suppressed by the ndr1 mutation (15) and that RPM1 functioned independently of EDS1 (16), so we examined the relative requirements of these R loci for EDS1 and NDR1.
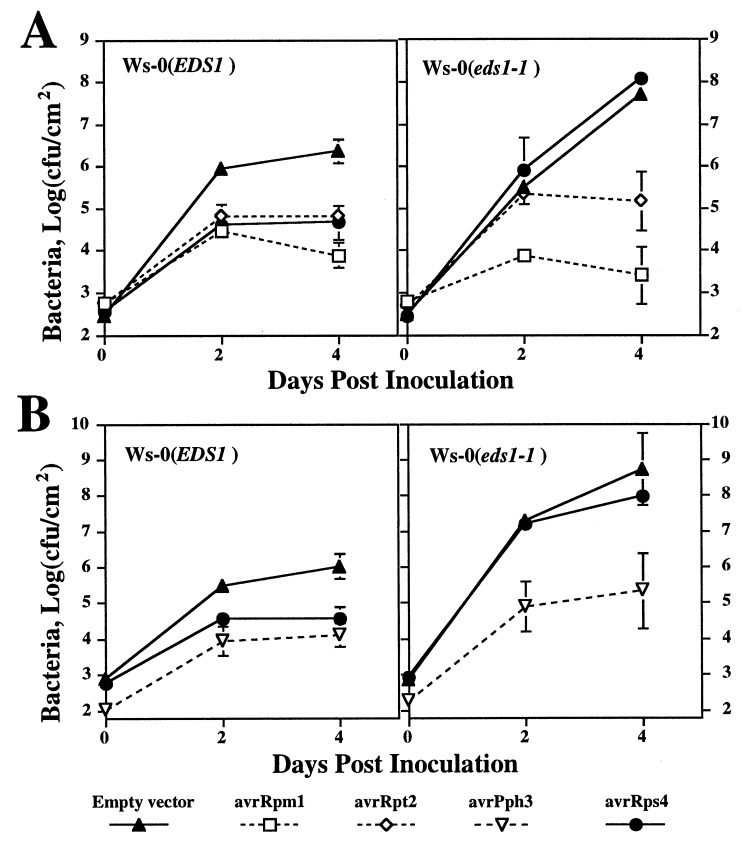
In planta growth of P. syringae pv. tomato, strain DC3000 carrying different avr genes or an empty vector was used as an assay for function of the different R genes: in wild-type plants, a complementary R gene–avr gene combination leads to a significant reduction in bacterial growth (11, 17, 18). Growth of these bacterial strains in Ws-0 and Ws eds1-1 plants, shown in Fig. 3A, was representative of results in all eds1 mutant lines. Results showed that suppression of DC3000 carrying either avrRpm1 or avrRpt2 (corresponding to RPM1 and RPS2, respectively) was the same in Ws-0 and eds1-1 plants, whereas DC3000 carrying avrRps4 (corresponding to RPS4) showed an increase in growth in eds1-1 plants that was three orders of magnitude greater than in Ws-0 plants. The enhanced growth of DC3000 carrying avrRps4 correlated with the formation of severe disease lesions in eds1-1 leaves, whereas inoculated Ws-0 leaves remained symptomless (Fig. 2 D and E). Thus, although RPM1 and RPS2 do not require EDS1, RPS4 has a strong dependence on EDS1. Growth of DC3000 carrying avrPph3, corresponding to RPS5, also was measured in Ws-0 and Ws eds1-1 plants. In this experiment, the Ler eds1 alleles were not tested because wild-type Ler does not contain the RPS5 gene (18). Results (Fig. 3B) showed that EDS1 is not required for RPS5-specified resistance.
Figure 3.
Growth of different P. syringae strains in leaves of Ws-0 or Ws eds1-1. Leaves were infiltrated with suspensions of DC3000 containing an empty vector or expressing different avr genes, as indicated, and bacteria were recovered from leaves at various times after inoculation (see Methods). Results in A and B represent data from two separate experiments. These tests were repeated twice with similar results.
The above experiments confirmed earlier observations (16) that eds1 plants permit significantly more growth of the compatible strain DC3000 than wild-type EDS1 plants. After 4 days, bacterial numbers in eds1-1 leaves were reproducibly one to two orders of magnitude higher than in Ws-0 (Fig. 3). Disease symptom development after DC3000 inoculation was also more severe on eds1-1 leaves (Fig. 2 F and G).
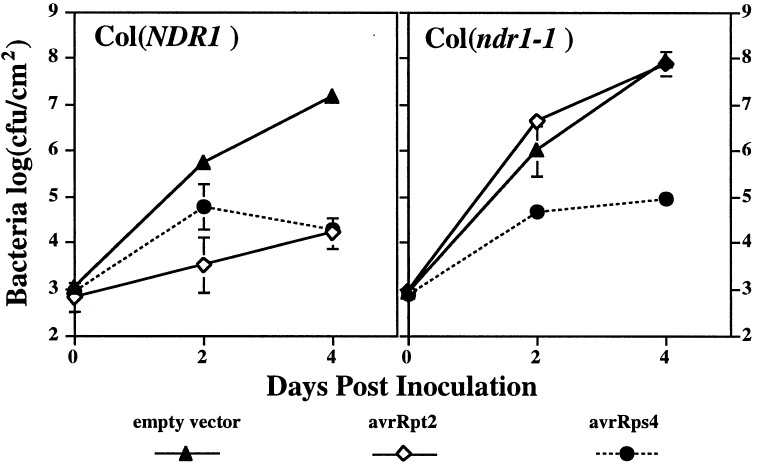
Previous experiments showed that the ndr1-1 mutation abolished RPM1, RPS2, and RPS5 functions in the accession Columbia (Col-0) (15). We therefore tested the effect of ndr1-1 on Col-0 RPS4-specified resistance. Results from the growth assays (Fig. 4) showed that RPS4 function was not significantly compromised by ndr1-1. We also tested the requirement of Ler-RPS4 for NDR1 in selected ndr1 F3 families derived from a Col ndr1-1 × Ler cross. This analysis (results not shown) confirmed that both the Ler RPS4 and Col RPS4 wild-type alleles operate independently of NDR1.
Figure 4.
Growth of different P. syringae strains in leaves of Col-0 or Col ndr1-1. Leaves were inoculated with DC3000 containing an empty vector, DC3000 expressing avrRpt2, or DC3000 expressing avrRps4, as indicated, and growth of bacteria was measured as in Fig. 3. Tests were repeated twice with similar results.
Analysis of the requirements for EDS1 or NDR1 was extended to several mapped RPP loci that, if not present in the available mutant backgrounds, could be selected by using PCR-based polymorphic DNA markers (see Methods for details). Mutant lines or F3 families, homozygous for the respective RPP locus and either the wild-type or mutant eds1 or ndr1 allele, were inoculated with the corresponding P. parasitica isolate. Leaves then were examined for evidence of pathogen asexual sporulation. As shown in Table 1, RPP2-specified resistance to P. parasitica isolate Cala2 and RPP4-specified resistance to Emwa1 in Col-0 (20, 21) were suppressed strongly by eds1. In contrast, Col ndr1-1 seedlings inoculated with Cala2 (RPP2) or Emwa1 (RPP4) under the same conditions showed no increase in pathogen sporulation. Microscopic examination of lactophenol–trypan blue-stained, Cala2-inoculated leaves revealed no significant differences in pathogen vegetative growth between wild-type Col-0 and Col ndr1-1 (not shown). RPP5-mediated resistance to Noco2 and RPP8-specified resistance to Emco5 in Ler were similarly not visibly affected by the ndr1-1 mutation.
The results suggested that impairment of RPP2- and RPP4-mediated resistance that had been demonstrated previously in cotyledons of Col ndr1-1 seedlings (15) may be an effect that is not measurable in leaves. We therefore inoculated individual ndr1-1 cotyledons with Emwa1 conidiospore suspensions, as performed previously (15), and quantified sporangiophore and conidiospore numbers. The results in Table 2 show that there was a small but significant increase in sporangiophore production in ndr1-1 cotyledons, consistent with the earlier observations (15). Spore numbers extracted from ndr1-1 cotyledons increased ≈10-fold over Col-0 wild-type levels. However, Emwa1 spore numbers on ndr1-1 cotyledons were <10% of those harvested from cotyledons of the naturally susceptible accession, Ws-0. A comparably low increase in Noco2 sporulation was observed in cotyledons of RPP5/ndr1-1 F3 seedlings compared with wild-type RPP5/NDR1 F3 seedlings selected from a Col ndr1-1 × Ler cross (not shown). In contrast, no increased sporulation of Emco5 was observed in cotyledons of ndr1-1-selected seedlings (not shown). We concluded that ndr1 has a minor but significant effect on resistance specified by strongly EDS1-dependent RPP genes.
Table 2.
Asexual sporulation on cotyledons of different Arabidopsis lines inoculated with P. parasitica isolate Emwa1
| Plant line | Sporangiophores/cotyledon | Conidiospores/ cotyledon |
|---|---|---|
| Col-0 | 2.9 ± 0.2 | 70 ± 4 |
| Col ndr1-1 | 6.5 ± 0.6 | 644 ± 18 |
| Ws-0 | >20 | 8236 ± 684 |
| Ler | 0 | 0 |
Individual cotyledons of 20–30 7-day-old seedlings were inoculated with 2-μl droplets containing ≈100 conidiospores. Spores were counted 7 days after inoculation.
Altogether, the data are consistent with the preferential utilization of either EDS1 or NDR1 by particular R genes. RPP8-specified resistance to P. parasitica was not compromised strongly by either eds1 or ndr1.
DISCUSSION
We present evidence for the operation of at least two R gene-specified signaling pathways that preferentially require either a functional EDS1 or NDR1 protein. R gene-mediated responses exhibiting a strong requirement for EDS1 showed weak or no dependence on NDR1. Significantly, RPS4 that recognizes a bacterial avirulence determinant, avrRps4, belongs to the EDS1-dependent class, revealing EDS1 signaling function beyond RPP gene-mediated disease resistance. Although P. parasitica inoculations were performed on young (9-day- and 2-week-old) seedlings and bacterial assays were performed on older (4-week-old) plants, the lack of NDR1 dependence by RPS4 showed that the observed signaling trends are unlikely to be due to plant developmental effects.
NDR1 recently was cloned, and the ndr1-1 mutation used in the present study was defined as a null allele due to an extensive deletion of the ORF (22). Similarly, cloning of EDS1 has established that both eds1-2 and eds1-3 have extensive deletions of the EDS1 ORF (A. Falk, B. Feys, and J.E.P., unpublished data). Because eds1-3 lacks the 5′ promoter and amino-terminal amino acids, it was surprising that this allele appeared to have a slightly weaker suppression phenotype than eds1-2 or eds1-4 (Fig. 1). Successive eds1-3 backcrosses to wild-type Ler should enable us to assess whether this is a feature of the mutant background. Overall, however, we conclude that the observed eds1 and ndr1 phenotypes are not due to leakiness of weak mutant alleles.
RPP5, a strongly EDS1-dependent gene, encodes a protein of the TIR-NBS-LRR class that has amino-terminal similarity to the cytoplasmic domains of the Drosophila Toll and mammalian interleukin 1 transmembrane receptors (6, 9). In contrast, RPM1 and RPS2, both exhibiting NDR1 dependence, have been assigned to the LZ-NBS-LRR class that possesses an amino-terminal domain containing a putative leucine zipper (1, 11-13). The functional significance of these domains is not understood, but a pattern emerges suggesting that pathogen recognition through an EDS1- or NDR1-dependent pathway may be directed by a particular R protein type. The structures of several recently cloned Arabidopsis R genes are consistent with this idea. RPS4 (M. Hinsch, W. Gassmann & B.J.S., unpublished data) and a cluster of RPP genes comprising the RPP1, RPP10, and RPP14 specificities (refs. 18 and 19; M. Botella, L. Frost, E.H., J. Beynon, J.E.P. & J. Jones, unpublished data) that are all strongly EDS1-dependent, encode proteins of the TIR-NBS-LRR class, whereas RPS5, an NDR1-requiring gene, encodes a LZ-NBS-LRR protein with strongest homology to RPS2 (23). The recently cloned RPP8 gene that has no exclusive requirement for either EDS1 or NDR1 function encodes an NBS-LRR protein with a putative amino-terminal LZ motif (J. McDowell, M. Dhandaydham, and J. Dangl, personal communication). Thus, a strict separation of EDS1 or NDR1- pathway utilization based simply on the presence of the TIR or LZ domains is not valid. The partial attenuation of RPP8-specified resistance exhibited by eds1 plants implicates minor EDS1 activity in this resistance response even though its loss ultimately can be compensated for to prevent successful pathogen colonization. Double eds1/ndr1 mutant combinations should establish whether RPP8 has a redundant requirement for EDS1 and NDR1 or mediates resistance through a different signaling pathway. It is notable that overall amino-terminal similarity between the “LZ” R protein members is less than that between the “TIR” R proteins, suggesting further mechanistic differences between the LZ class. In this respect, it is interesting that ndr1 suppressed a hypersensitive response incited by high doses of DC3000 expressing avrRpt2 but not avrRpm1, avrB, or avrPph3 (15). Also, different early gene induction profiles were observed in the resistance responses specified by RPS2 and RPM1 (24, 25), implicating other levels of pathway discrimination beyond the separation of events described here.
A weak but significant impairment of resistance specified by the EDS1-dependent genes RPP4 (Table 2) and RPP5 was observed in cotyledons of ndr1 plants. This points to a degree of cross-talk between the proposed EDS1- and NDR1-mediated pathways. We also noted that there was a slight (maximally 5- to 10-fold) but consistent trend toward increased growth of DC3000 expressing avrRpt2 or avrPph3 but not avrRpm1 in leaves of eds1 (Fig. 3). Likewise, ndr1 plants supported 5- to 10-fold greater numbers of DC3000 expressing avrRps4 than wild-type plants (Fig. 4). Although these differences were not statistically significant, it is possible that minor pathway cross-utilization also occurs between these strongly EDS1- and NDR1-mediated processes, as was clearly demonstrated in the RPP4-specified resistance response (Table 2). In the light of possible signaling variations associated with different “LZ” R proteins discussed above, it is notable that EDS1 appears not to impinge at all on RPM1-controlled resistance.
In all compatible P. parasitica–eds1 combinations tested, pathogen sporulation was significantly higher than in the corresponding wild-type susceptible plant (Fig. 1), revealing an “enhanced disease susceptibility” (eds) phenotype. This was also apparent in the interaction between the compatible bacterial strain DC3000 and eds1 plants (Fig. 3). Therefore, EDS1 has a role in limiting pathogen growth both in certain R gene-mediated and compatible interactions. In our studies, ndr1 plants were not significantly more susceptible to DC3000 than wild-type Col-0 (Fig. 4), suggesting a preferential requirement for EDS1, and not NDR1, in restriction of pathogen growth to this bacterial strain. Analyses with another compatible bacterial pathogen, Xanthomonas campestris pv. campestris strain 8004, showed that eds1 plants were not significantly more susceptible than wild-type plants (data not shown). This is consistent with the notion that the “eds” phenotype exhibited by eds1 may be limited to certain plant–pathogen combinations. It will be important to establish whether the biochemical role of EDS1 is the same in the maintenance of R gene-specified disease resistance and the growth limitation of a compatible pathogen.
Other “eds” loci have been identified by using various mutational screens, including assays for increased growth of a P. syringae isolate, PsmES4326 (26, 27). Phenotypic and genetic analyses of the different eds mutations point to the existence of complex, multiple pathways limiting growth of virulent pathogens. Significantly, a number of the eds mutations that have been characterized cause enhanced susceptibility to DC3000, like eds1 (28). Also, they have no appreciable effect on RPS2-specified resistance and are not altered in their capacity to respond to inducers of systemic acquired resistance. This distinguishes them and eds1 from mutations, such as npr1 (29, 30), that compromise systemic resistance responses. One eds mutation, pad4, causes enhanced growth of PsmES4326 and also suppresses RPP2- and RPP4-mediated resistance (31), implicating a role for PAD4 in these EDS1-dependent R gene responses. In that study, RPP7 functioned independently of PAD4, suggesting a specificity in the requirement for PAD4 activity. Further analysis of these mutations and their corresponding wild-type gene products should help to unravel the complexity of particular R gene-specified pathways and how these interact with other processes limiting or promoting pathogen growth in plants.
Acknowledgments
We thank K. Century for providing Col-ndr1-1 × Ler F3 families and M. Hinsch for isolation of eds1-4. We are also grateful to J. Jones and P. Schulze-Lefert for helpful discussions and to colleagues who provided unpublished data. Work at The Sainsbury Laboratory is funded by The Gatsby Charitable Foundation and an European Economic Community Training and Mobility of Researchers fellowship to N.A. M.M. was supported by National Science Foundation Graduate Research Traineeship in Plant Biology 9256469.
ABBREVIATIONS
- LRR
leucine-rich repeats
- NBS
nucleotide binding site
- LZ
leucine zipper
- Ler
Landsberg-erecta
- Ws
Wassilewskija
- Col
Columbia
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Baker B, Zambryski P, Staskawicz B, Dinesh-Kumar S P. Science. 1997;276:726–733. doi: 10.1126/science.276.5313.726. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Loh Y-T, Bressan R A, Martin G B. Cell. 1995;83:925–935. doi: 10.1016/0092-8674(95)90208-2. [DOI] [PubMed] [Google Scholar]
- 3.Tang X, Frederick R D, Zhou J, Halterman D A, Jia Y, Martin G B. Science. 1996;274:2060–2063. doi: 10.1126/science.274.5295.2060. [DOI] [PubMed] [Google Scholar]
- 4.Scofield S R, Tobias C M, Rathjen J P, Chang J H, Lavelle D T, Michelmore R W, Staskawicz B J. Science. 1996;274:2063–2065. doi: 10.1126/science.274.5295.2063. [DOI] [PubMed] [Google Scholar]
- 5.Jones D A, Jones J D G. Adv Bot Res Inc Adv Plant Pathol. 1996;24:89–167. [Google Scholar]
- 6.Rock F L, Hardman G, Timans J C, Kastelein R A, Bazan F. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitham S, Dinesh-Kumar S P, Choi D, Hehl R, Corr C, Baker B. Cell. 1994;78:1011–1115. doi: 10.1016/0092-8674(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence G J, Finnegan E J, Ayliffe M A, Ellis J G. Plant Cell. 1995;7:1195–1206. doi: 10.1105/tpc.7.8.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker J E, Coleman M J, Szabò V, Frost L N, Schmidt R, van der Biezen E A, Moores T, Dean C, Daniels M J, Jones J D G. Plant Cell. 1997;9:879–894. doi: 10.1105/tpc.9.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dangl J. Adv Plant Pathol. 1993;10:127–156. [Google Scholar]
- 11.Bent A F, Kunkel B N, Dahlbeck D, Brown K L, Schmidt R, Giraudat J, Leung J, Staskawicz B J. Science. 1994;265:1856–1860. doi: 10.1126/science.8091210. [DOI] [PubMed] [Google Scholar]
- 12.Mindrinos M, Katagiri F, Yu G-L, Ausubel F M. Cell. 1994;78:1089–1099. doi: 10.1016/0092-8674(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 13.Grant M R, Godiard L, Straube E, Ashfield T, Lewald J, Sattler A, Innes R W, Dangl J L. Science. 1995;269:843–846. doi: 10.1126/science.7638602. [DOI] [PubMed] [Google Scholar]
- 14.Glazebrook J, Rogers E E, Ausubel F M. Annu Rev Genet. 1997;31:547–569. doi: 10.1146/annurev.genet.31.1.547. [DOI] [PubMed] [Google Scholar]
- 15.Century K, Holub E B, Staskawicz B J. Proc Natl Acad Sci USA. 1995;92:6597–6601. doi: 10.1073/pnas.92.14.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker J E, Holub E B, Frost L N, Falk A, Gunn N D, Daniels M J. Plant Cell. 1996;8:2033–2046. doi: 10.1105/tpc.8.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinsch M, Staskawicz B. Mol Plant-Microbe Interact. 1996;9:55–61. doi: 10.1094/mpmi-9-0055. [DOI] [PubMed] [Google Scholar]
- 18.Simonich M T, Innes R W. Mol Plant-Microbe Interact. 1995;8:637–640. doi: 10.1094/mpmi-8-0637. [DOI] [PubMed] [Google Scholar]
- 19.Reignault P, Frost L N, Richardson H, Daniels M J, Jones J D G, Parker J. Mol Plant-Microbe Interact. 1996;9:464–473. doi: 10.1094/mpmi-9-0464. [DOI] [PubMed] [Google Scholar]
- 20.Holub E B, Beynon J L, Crute I R. Mol Plant-Microbe Interact. 1994;7:223–239. doi: 10.1094/mpmi-8-0916. [DOI] [PubMed] [Google Scholar]
- 21.Holub E B, Beynon J L. Adv Bot Res. 1997;24:228–273. [Google Scholar]
- 22.Century K S, Shapiro A D, Repetti P P, Dahlbeck D, Holub E, Staskawicz B J. Science. 1997;278:1963–1965. doi: 10.1126/science.278.5345.1963. [DOI] [PubMed] [Google Scholar]
- 23.Warren, R. F., Henk, A., Mowery, P., Holub, E. & Innes, R. W. (1998) Plant Cell, in press. [DOI] [PMC free article] [PubMed]
- 24.Reuber T L, Ausubel F M. Plant Cell. 1996;8:241–249. doi: 10.1105/tpc.8.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritter C, Dangl J L. Plant Cell. 1996;8:251–257. doi: 10.1105/tpc.8.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glazebrook J, Ausubel F M. Proc Natl Acad Sci USA. 1994;91:8955–8959. doi: 10.1073/pnas.91.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glazebrook J, Rogers E E, Ausubel F M. Genetics. 1996;143:973–982. doi: 10.1093/genetics/143.2.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers E E, Ausubel F M. Plant Cell. 1997;9:305–316. doi: 10.1105/tpc.9.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao H, Bowling S A, Gordon S, Dong X. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao H, Glazebrook J, Clarke J D, Volko S, Dong X. Cell. 1997;88:57–63. doi: 10.1016/s0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- 31.Glazebrook J, Zook M, Mert F, Kagan I, Rogers E E, Crute I R, Holub E B, Hammerschmidt R, Ausubel F M. Genetics. 1997;146:381–392. doi: 10.1093/genetics/146.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]