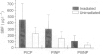Abstract
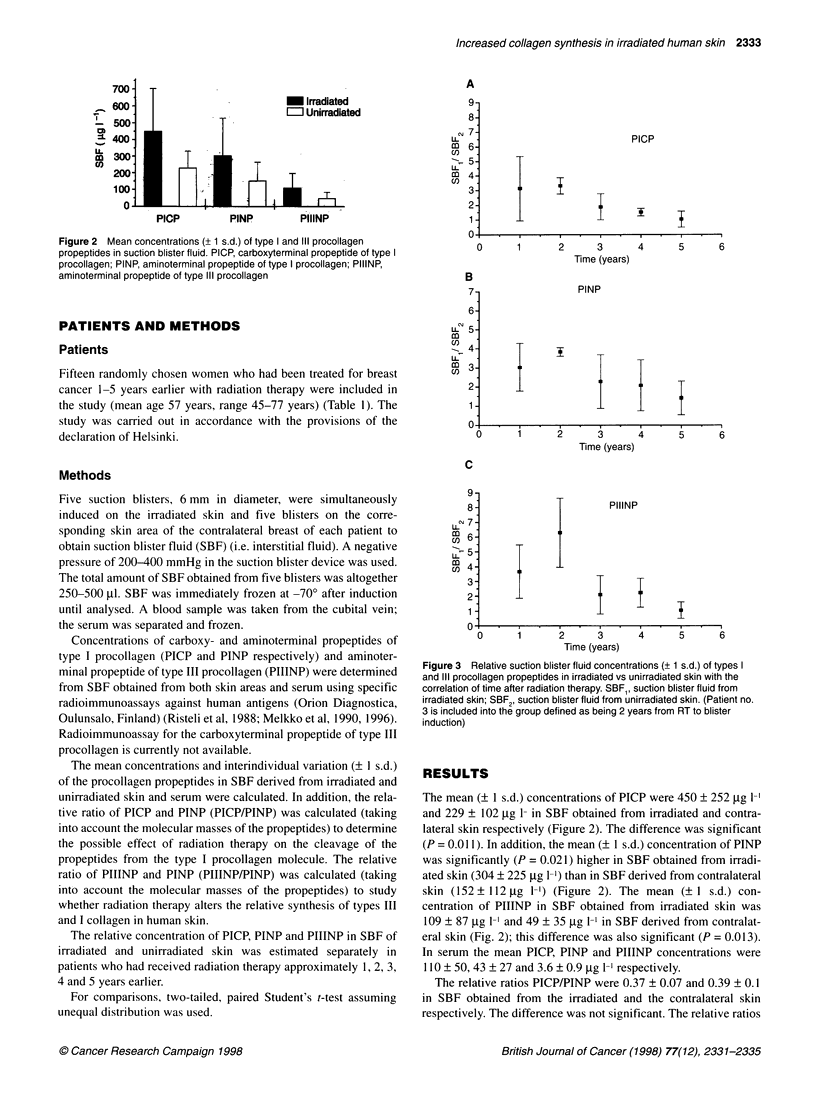
Fibrosis is a common side-effect of radiation therapy. As a complex network of cytokines and other mediators plays a central role in the process leading to fibrosis, we used an in vivo method to measure skin collagen synthesis, taking into account the physiological conditions. We determined suction blister (i.e. interstitial) fluid concentrations of types I and III procollagen propeptides, reflecting types I and III collagen synthesis, in irradiated and unirradiated skin of breast cancer patients 1-5 years after surgery and radiation therapy, hence using the patients as their own controls. The mean concentrations of the measured collagen markers were approximately two times higher in the irradiated skin than in the unirradiated contralateral breast skin. The difference slowly diminishes with time. These results indicate that abundant collagen synthesis in the irradiated skin continues several years after discontinuation of the radiation therapy, leading to fibrosis. The method outlined here offers a new in vivo perspective to study events leading to radiation fibrosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alileche A., Han D., Plaisance S., Assier E., Sahraoui Y., Clemanceau C., Metivier D., Brouty-Boyer D., Jasmin C., Azzarone B. IL-2 production by myofibroblasts from post-radiation fibrosis in breast cancer patients. Int Immunol. 1994 Oct;6(10):1585–1591. doi: 10.1093/intimm/6.10.1585. [DOI] [PubMed] [Google Scholar]
- Autio P., Karjalainen J., Risteli L., Risteli J., Kiistala U., Oikarinen A. Effects of an inhaled steroid (budesonide) on skin collagen synthesis of asthma patients in vivo. Am J Respir Crit Care Med. 1996 Mar;153(3):1172–1175. doi: 10.1164/ajrccm.153.3.8630563. [DOI] [PubMed] [Google Scholar]
- Autio P., Oikarinen A., Melkko J., Risteli J., Risteli L. Systemic glucocorticoids decrease the synthesis of type I and type III collagen in human skin in vivo, whereas isotretinoin treatment has little effect. Br J Dermatol. 1994 Nov;131(5):660–663. doi: 10.1111/j.1365-2133.1994.tb04978.x. [DOI] [PubMed] [Google Scholar]
- Autio P., Risteli J., Kiistala U., Risteli L., Karvonen J., Oikarinen A. Serum markers of collagen synthesis and degradation in skin diseases. Altered levels in diseases with systemic manifestation and during systemic glucocorticoid treatment. Arch Dermatol Res. 1993;285(6):322–327. doi: 10.1007/BF00371831. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Uitto J. Collagen in cutaneous diseases. Int J Dermatol. 1979 May;18(4):251–270. doi: 10.1111/j.1365-4362.1979.tb01926.x. [DOI] [PubMed] [Google Scholar]
- Bentzen S. M., Overgaard M., Overgaard J. Clinical correlations between late normal tissue endpoints after radiotherapy: implications for predictive assays of radiosensitivity. Eur J Cancer. 1993;29A(10):1373–1376. doi: 10.1016/0959-8049(93)90004-y. [DOI] [PubMed] [Google Scholar]
- Bentzen SM, Overgaard J. Patient-to-Patient Variability in the Expression of Radiation-Induced Normal Tissue Injury. Semin Radiat Oncol. 1994 Apr;4(2):68–80. doi: 10.1053/SRAO00400068. [DOI] [PubMed] [Google Scholar]
- Burnet N. G., Nyman J., Turesson I., Wurm R., Yarnold J. R., Peacock J. H. Prediction of normal-tissue tolerance to radiotherapy from in-vitro cellular radiation sensitivity. Lancet. 1992 Jun 27;339(8809):1570–1571. doi: 10.1016/0140-6736(92)91833-t. [DOI] [PubMed] [Google Scholar]
- Burnet N. G., Nyman J., Turesson I., Wurm R., Yarnold J. R., Peacock J. H. The relationship between cellular radiation sensitivity and tissue response may provide the basis for individualising radiotherapy schedules. Radiother Oncol. 1994 Dec;33(3):228–238. doi: 10.1016/0167-8140(94)90358-1. [DOI] [PubMed] [Google Scholar]
- Cooper J. S., Fu K., Marks J., Silverman S. Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys. 1995 Mar 30;31(5):1141–1164. doi: 10.1016/0360-3016(94)00421-G. [DOI] [PubMed] [Google Scholar]
- Cromack D. T., Porras-Reyes B., Purdy J. A., Pierce G. F., Mustoe T. A. Acceleration of tissue repair by transforming growth factor beta 1: identification of in vivo mechanism of action with radiotherapy-induced specific healing deficits. Surgery. 1993 Jan;113(1):36–42. [PubMed] [Google Scholar]
- Geara F. B., Peters L. J., Ang K. K., Garden A. S., Tucker S. L., Levy L. B., Brown B. W. Comparison between normal tissue reactions and local tumor control in head and neck cancer patients treated by definitive radiotherapy. Int J Radiat Oncol Biol Phys. 1996 Jun 1;35(3):455–462. doi: 10.1016/s0360-3016(96)80006-x. [DOI] [PubMed] [Google Scholar]
- Geara F. B., Peters L. J., Ang K. K., Wike J. L., Brock W. A. Prospective comparison of in vitro normal cell radiosensitivity and normal tissue reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys. 1993 Dec 1;27(5):1173–1179. doi: 10.1016/0360-3016(93)90540-c. [DOI] [PubMed] [Google Scholar]
- Haukipuro K., Melkko J., Risteli L., Kairaluoma M. I., Risteli J. Connective tissue response to major surgery and postoperative infection. Eur J Clin Invest. 1992 May;22(5):333–340. doi: 10.1111/j.1365-2362.1992.tb01471.x. [DOI] [PubMed] [Google Scholar]
- Kiistala U. Suction blister device for separation of viable epidermis from dermis. J Invest Dermatol. 1968 Feb;50(2):129–137. doi: 10.1038/jid.1968.15. [DOI] [PubMed] [Google Scholar]
- Melkko J., Kauppila S., Niemi S., Risteli L., Haukipuro K., Jukkola A., Risteli J. Immunoassay for intact amino-terminal propeptide of human type I procollagen. Clin Chem. 1996 Jun;42(6 Pt 1):947–954. [PubMed] [Google Scholar]
- Melkko J., Niemi S., Risteli L., Risteli J. Radioimmunoassay of the carboxyterminal propeptide of human type I procollagen. Clin Chem. 1990 Jul;36(7):1328–1332. [PubMed] [Google Scholar]
- Oikarinen A., Autio P., Kiistala U., Risteli L., Risteli J. A new method to measure type I and III collagen synthesis in human skin in vivo: demonstration of decreased collagen synthesis after topical glucocorticoid treatment. J Invest Dermatol. 1992 Feb;98(2):220–225. doi: 10.1111/1523-1747.ep12555884. [DOI] [PubMed] [Google Scholar]
- Randall K., Coggle J. E. Long-term expression of transforming growth factor TGF beta 1 in mouse skin after localized beta-irradiation. Int J Radiat Biol. 1996 Sep;70(3):351–360. doi: 10.1080/095530096145085. [DOI] [PubMed] [Google Scholar]
- Risteli J., Niemi S., Trivedi P., Mäentausta O., Mowat A. P., Risteli L. Rapid equilibrium radioimmunoassay for the amino-terminal propeptide of human type III procollagen. Clin Chem. 1988 Apr;34(4):715–718. [PubMed] [Google Scholar]
- Rodemann H. P., Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol. 1995 May;35(2):83–90. doi: 10.1016/0167-8140(95)01540-w. [DOI] [PubMed] [Google Scholar]
- Rodemann H. P., Peterson H. P., Schwenke K., von Wangenheim K. H. Terminal differentiation of human fibroblasts is induced by radiation. Scanning Microsc. 1991 Dec;5(4):1135–1143. [PubMed] [Google Scholar]
- Rubin P., Johnston C. J., Williams J. P., McDonald S., Finkelstein J. N. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995 Aug 30;33(1):99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- Søndergaard K., Heickendorff L., Risteli L., Risteli J., Zachariae H., Stengaard-Pedersen K., Deleuran B. Increased levels of type I and III collagen and hyaluronan in scleroderma skin. Br J Dermatol. 1997 Jan;136(1):47–53. [PubMed] [Google Scholar]
- Taylor A. M., Harnden D. G., Arlett C. F., Harcourt S. A., Lehmann A. R., Stevens S., Bridges B. A. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975 Dec 4;258(5534):427–429. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- Thornton S. C., Walsh B. J., Bennett S., Robbins J. M., Foulcher E., Morgan G. W., Penny R., Breit S. N. Both in vitro and in vivo irradiation are associated with induction of macrophage-derived fibroblast growth factors. Clin Exp Immunol. 1996 Jan;103(1):67–73. doi: 10.1046/j.1365-2249.1996.898598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S. L., Turesson I., Thames H. D. Evidence for individual differences in the radiosensitivity of human skin. Eur J Cancer. 1992;28A(11):1783–1791. doi: 10.1016/0959-8049(92)90004-l. [DOI] [PubMed] [Google Scholar]
- Turesson I., Nyman J., Holmberg E., Odén A. Prognostic factors for acute and late skin reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys. 1996 Dec 1;36(5):1065–1075. doi: 10.1016/s0360-3016(96)00426-9. [DOI] [PubMed] [Google Scholar]
- Turesson I., Thames H. D. Repair capacity and kinetics of human skin during fractionated radiotherapy: erythema, desquamation, and telangiectasia after 3 and 5 year's follow-up. Radiother Oncol. 1989 Jun;15(2):169–188. doi: 10.1016/0167-8140(89)90131-x. [DOI] [PubMed] [Google Scholar]
- Turesson I. The progression rate of late radiation effects in normal tissue and its impact on dose-response relationships. Radiother Oncol. 1989 Jul;15(3):217–226. doi: 10.1016/0167-8140(89)90089-3. [DOI] [PubMed] [Google Scholar]
- Varga J., Haustein U. F., Creech R. H., Dwyer J. P., Jimenez S. A. Exaggerated radiation-induced fibrosis in patients with systemic sclerosis. JAMA. 1991 Jun 26;265(24):3292–3295. [PubMed] [Google Scholar]
- Vermeer B. J., Reman F. C., van Gent C. M. The determination of lipids and proteins in suction blister fluid. J Invest Dermatol. 1979 Oct;73(4):303–305. doi: 10.1111/1523-1747.ep12531833. [DOI] [PubMed] [Google Scholar]