Abstract
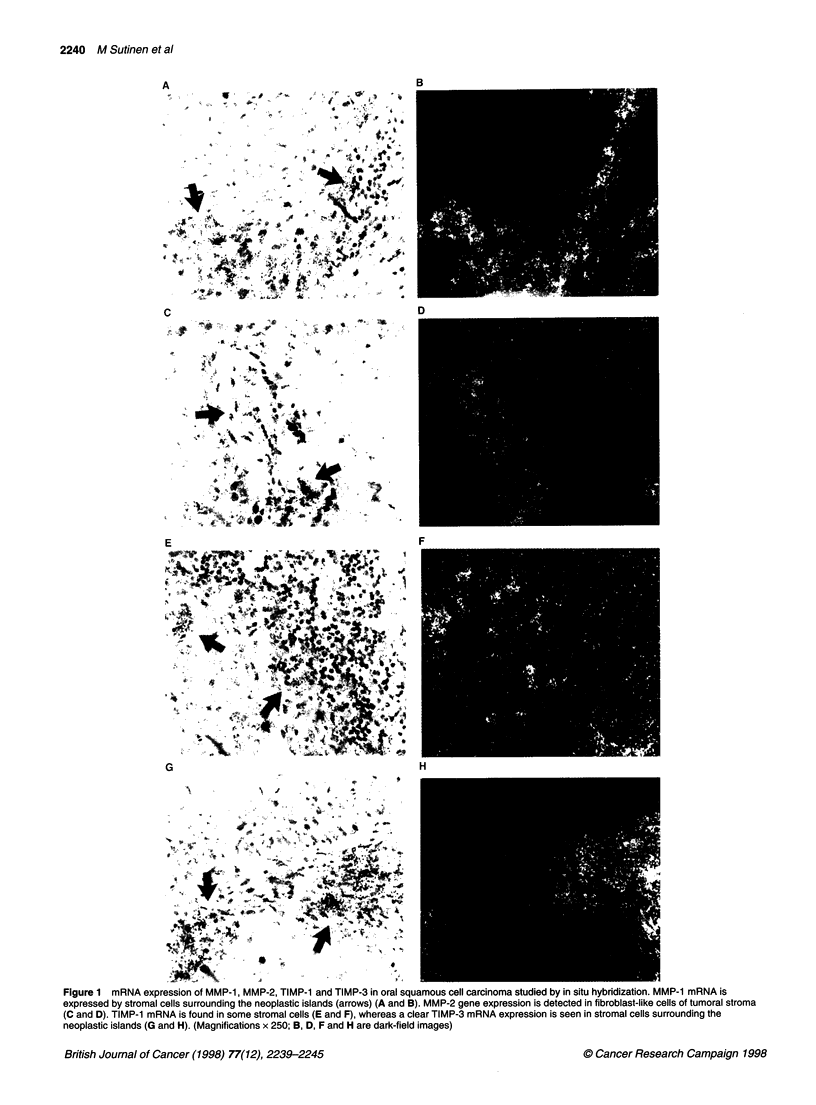
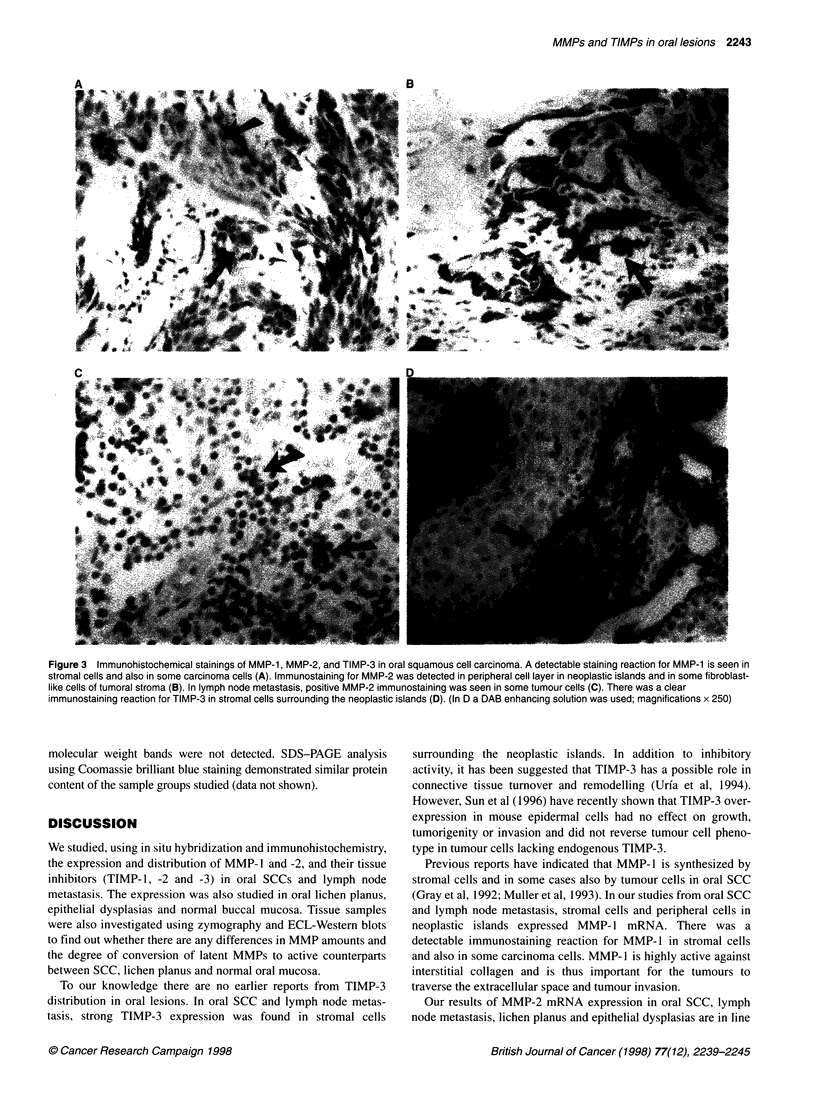
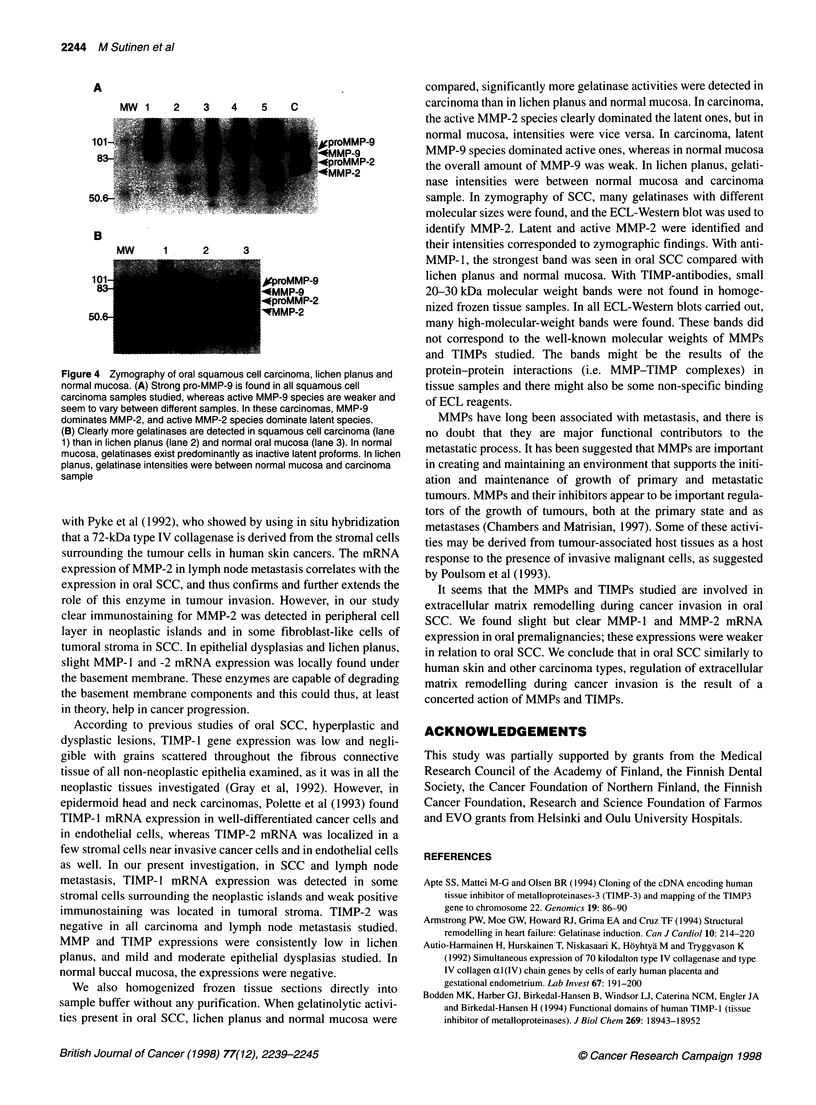
Although matrix metalloproteinases (MMPs) are among the potential key mediators of cancer invasion, their involvement in premalignant lesions and conditions is not clarified. Therefore, we studied, using in situ hybridization, immunohistochemistry and zymography the expression and distribution of MMP-1 and -2, and their tissue inhibitors (TIMPs -1, -2 and -3) in oral squamous cell carcinomas (SCC) and lymph node metastases as well as in oral lichen planus, epithelial dysplasias and normal buccal mucosa. In oral SCC and lymph node metastasis, MMP-1 mRNA was detected in fibroblastic cells of tumoral stroma. In two out of ten carcinomas studied, the peripheral cells of neoplastic islands were also positive. MMP-2 mRNA expression was noted in fibroblasts surrounding the carcinoma cells, and no signal in carcinoma cells was detected. A clear TIMP-3 mRNA expression was seen in stromal cells surrounding the neoplastic islands in all SCCs and lymph node metastases studied. TIMP-1 mRNA was detected in some stromal cells surrounding the neoplastic islands, whereas the mRNA expression for TIMP-2 was negligible. On the other hand, expression of MMPs and TIMPs was consistently low in oral epithelial dysplasias, lichen planus and normal mucosa. In certain epithelial dysplasias and lichen planus, MMP-1 and -2 mRNA expressions were detected in few fibroblasts under the basement membrane zone, but normal mucosa was completely negative. In SCC and lymph node metastasis, a detectable immunostaining for MMP-1 in stromal cells and in some carcinoma cells was observed. MMP-2 immunoreactivity was detected in the peripheral cell layer in neoplastic islands and in some fibroblast-like cells of tumoral stroma. Immunostaining for TIMP-3 was detected in stromal cells surrounding the neoplastic islands. A weak positive staining for TIMP-1 was located in tumoral stroma, whereas the immunostaining for TIMP-2 was negative. Using zymography, elevated levels of MMP-2 and MMP-9 were observed in carcinoma samples in comparison with lichen planus or normal oral mucosa. Our results indicate that the studied MMPs and TIMPs are clearly up-regulated during invasion in oral SCC. However, there was also a clear, although weak, up-regulation of the expression of the MMPs but not TIMPs in some of the lichen planus and dysplastic lesions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte S. S., Mattei M. G., Olsen B. R. Cloning of the cDNA encoding human tissue inhibitor of metalloproteinases-3 (TIMP-3) and mapping of the TIMP3 gene to chromosome 22. Genomics. 1994 Jan 1;19(1):86–90. doi: 10.1006/geno.1994.1016. [DOI] [PubMed] [Google Scholar]
- Armstrong P. W., Moe G. W., Howard R. J., Grima E. A., Cruz T. F. Structural remodelling in heart failure: gelatinase induction. Can J Cardiol. 1994 Mar;10(2):214–220. [PubMed] [Google Scholar]
- Autio-Harmainen H., Hurskainen T., Niskasaari K., Höyhtyä M., Tryggvason K. Simultaneous expression of 70 kilodalton type IV collagenase and type IV collagen alpha 1 (IV) chain genes by cells of early human placenta and gestational endometrium. Lab Invest. 1992 Aug;67(2):191–200. [PubMed] [Google Scholar]
- Bodden M. K., Harber G. J., Birkedal-Hansen B., Windsor L. J., Caterina N. C., Engler J. A., Birkedal-Hansen H. Functional domains of human TIMP-1 (tissue inhibitor of metalloproteinases). J Biol Chem. 1994 Jul 22;269(29):18943–18952. [PubMed] [Google Scholar]
- Brinckerhoff C. E. Regulation of metalloproteinase gene expression: implications for osteoarthritis. Crit Rev Eukaryot Gene Expr. 1992;2(2):145–164. [PubMed] [Google Scholar]
- Carmichael D. F., Sommer A., Thompson R. C., Anderson D. C., Smith C. G., Welgus H. G., Stricklin G. P. Primary structure and cDNA cloning of human fibroblast collagenase inhibitor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2407–2411. doi: 10.1073/pnas.83.8.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A. F., Matrisian L. M. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997 Sep 3;89(17):1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- DeClerck Y. A., Perez N., Shimada H., Boone T. C., Langley K. E., Taylor S. M. Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res. 1992 Feb 1;52(3):701–708. [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Docherty A. J., Murphy G. The tissue metalloproteinase family and the inhibitor TIMP: a study using cDNAs and recombinant proteins. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):469–479. [PubMed] [Google Scholar]
- Fessler L. I., Duncan K. G., Fessler J. H., Salo T., Tryggvason K. Characterization of the procollagen IV cleavage products produced by a specific tumor collagenase. J Biol Chem. 1984 Aug 10;259(15):9783–9789. [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Gray S. T., Wilkins R. J., Yun K. Interstitial collagenase gene expression in oral squamous cell carcinoma. Am J Pathol. 1992 Aug;141(2):301–306. [PMC free article] [PubMed] [Google Scholar]
- Huhtala P., Chow L. T., Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990 Jul 5;265(19):11077–11082. [PubMed] [Google Scholar]
- Höyhtyä M., Fridman R., Komarek D., Porter-Jordan K., Stetler-Stevenson W. G., Liotta L. A., Liang C. M. Immunohistochemical localization of matrix metalloproteinase 2 and its specific inhibitor TIMP-2 in neoplastic tissues with monoclonal antibodies. Int J Cancer. 1994 Feb 15;56(4):500–505. doi: 10.1002/ijc.2910560408. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Monteagudo C., Merino M. J., San-Juan J., Liotta L. A., Stetler-Stevenson W. G. Immunohistochemical distribution of type IV collagenase in normal, benign, and malignant breast tissue. Am J Pathol. 1990 Mar;136(3):585–592. [PMC free article] [PubMed] [Google Scholar]
- Muller D., Wolf C., Abecassis J., Millon R., Engelmann A., Bronner G., Rouyer N., Rio M. C., Eber M., Methlin G. Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer Res. 1993 Jan 1;53(1):165–169. [PubMed] [Google Scholar]
- Murphy G., Ward R., Hembry R. M., Reynolds J. J., Kühn K., Tryggvason K. Characterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem J. 1989 Mar 1;258(2):463–472. doi: 10.1042/bj2580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polette M., Clavel C., Birembaut P., De Clerck Y. A. Localization by in situ hybridization of mRNAs encoding stromelysin 3 and tissue inhibitors of metallo-proteinases TIMP-1 and TIMP-2 in human head and neck carcinomas. Pathol Res Pract. 1993 Nov;189(9):1052–1057. doi: 10.1016/S0344-0338(11)80679-5. [DOI] [PubMed] [Google Scholar]
- Polette M., Clavel C., Muller D., Abecassis J., Binninger I., Birembaut P. Detection of mRNAs encoding collagenase I and stromelysin 2 in carcinomas of the head and neck by in situ hybridization. Invasion Metastasis. 1991;11(2):76–83. [PubMed] [Google Scholar]
- Poulsom R., Hanby A. M., Pignatelli M., Jeffery R. E., Longcroft J. M., Rogers L., Stamp G. W. Expression of gelatinase A and TIMP-2 mRNAs in desmoplastic fibroblasts in both mammary carcinomas and basal cell carcinomas of the skin. J Clin Pathol. 1993 May;46(5):429–436. doi: 10.1136/jcp.46.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke C., Ralfkiaer E., Huhtala P., Hurskainen T., Danø K., Tryggvason K. Localization of messenger RNA for Mr 72,000 and 92,000 type IV collagenases in human skin cancers by in situ hybridization. Cancer Res. 1992 Mar 1;52(5):1336–1341. [PubMed] [Google Scholar]
- Salo T., Lyons J. G., Rahemtulla F., Birkedal-Hansen H., Larjava H. Transforming growth factor-beta 1 up-regulates type IV collagenase expression in cultured human keratinocytes. J Biol Chem. 1991 Jun 25;266(18):11436–11441. [PubMed] [Google Scholar]
- Seltzer J. L., Weingarten H., Akers K. T., Eschbach M. L., Grant G. A., Eisen A. Z. Cleavage specificity of type IV collagenase (gelatinase) from human skin. Use of synthetic peptides as model substrates. J Biol Chem. 1989 Nov 25;264(33):19583–19586. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Brown P. D., Onisto M., Levy A. T., Liotta L. A. Tissue inhibitor of metalloproteinases-2 (TIMP-2) mRNA expression in tumor cell lines and human tumor tissues. J Biol Chem. 1990 Aug 15;265(23):13933–13938. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Liotta L. A. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989 Oct 15;264(29):17374–17378. [PubMed] [Google Scholar]
- Sun Y., Kim H., Parker M., Stetler-Stevenson W. G., Colburn N. H. Lack of suppression of tumor cell phenotype by overexpression of TIMP-3 in mouse JB6 tumor cells identification of a transfectant with increased tumorigenicity and invasiveness. Anticancer Res. 1996 Jan-Feb;16(1):1–7. [PubMed] [Google Scholar]
- Tryggvason K., Höyhtyä M., Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987 Nov 25;907(3):191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- Uría J. A., Ferrando A. A., Velasco G., Freije J. M., López-Otín C. Structure and expression in breast tumors of human TIMP-3, a new member of the metalloproteinase inhibitor family. Cancer Res. 1994 Apr 15;54(8):2091–2094. [PubMed] [Google Scholar]
- Wallon U. M., Overall C. M. The hemopexin-like domain (C domain) of human gelatinase A (matrix metalloproteinase-2) requires Ca2+ for fibronectin and heparin binding. Binding properties of recombinant gelatinase A C domain to extracellular matrix and basement membrane components. J Biol Chem. 1997 Mar 14;272(11):7473–7481. doi: 10.1074/jbc.272.11.7473. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P. Human skin fibroblast collagenase inhibitor. Comparative studies in human connective tissues, serum, and amniotic fluid. J Biol Chem. 1983 Oct 25;258(20):12259–12264. [PubMed] [Google Scholar]