Abstract
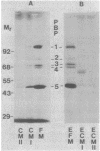
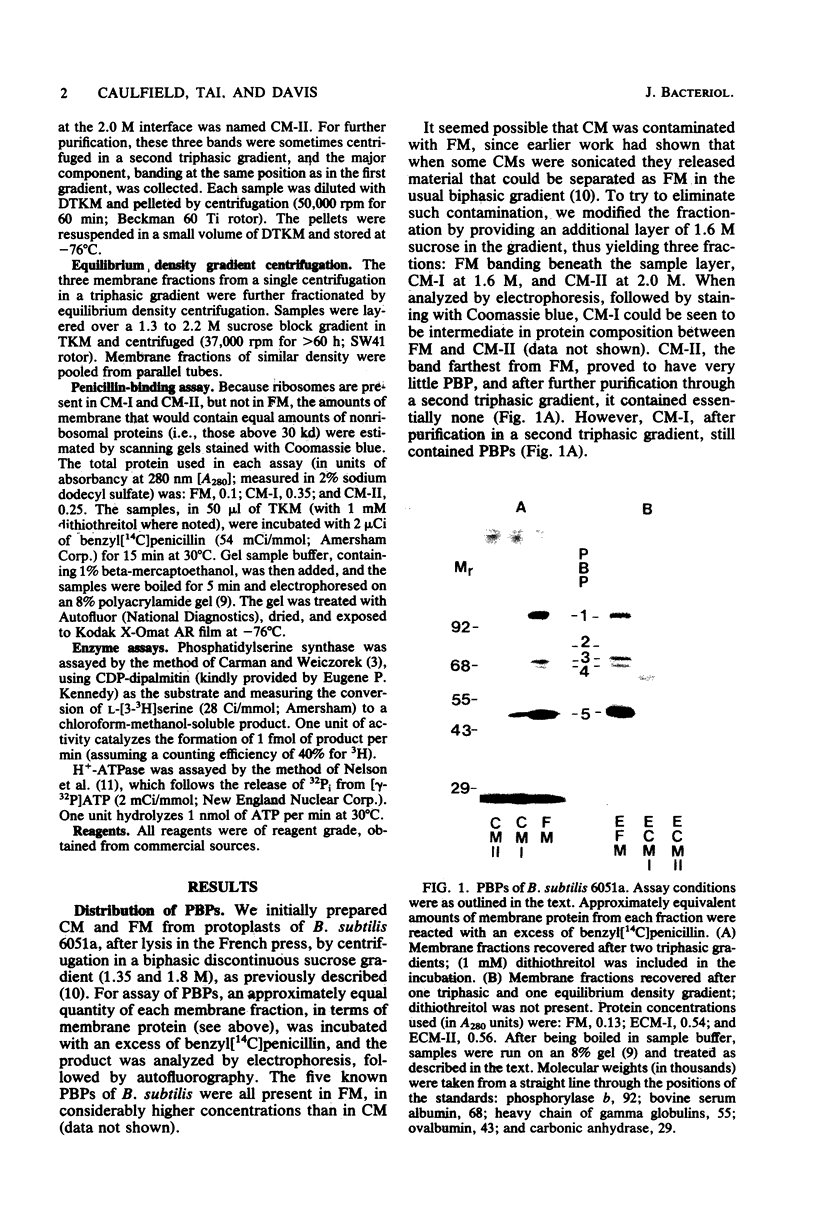
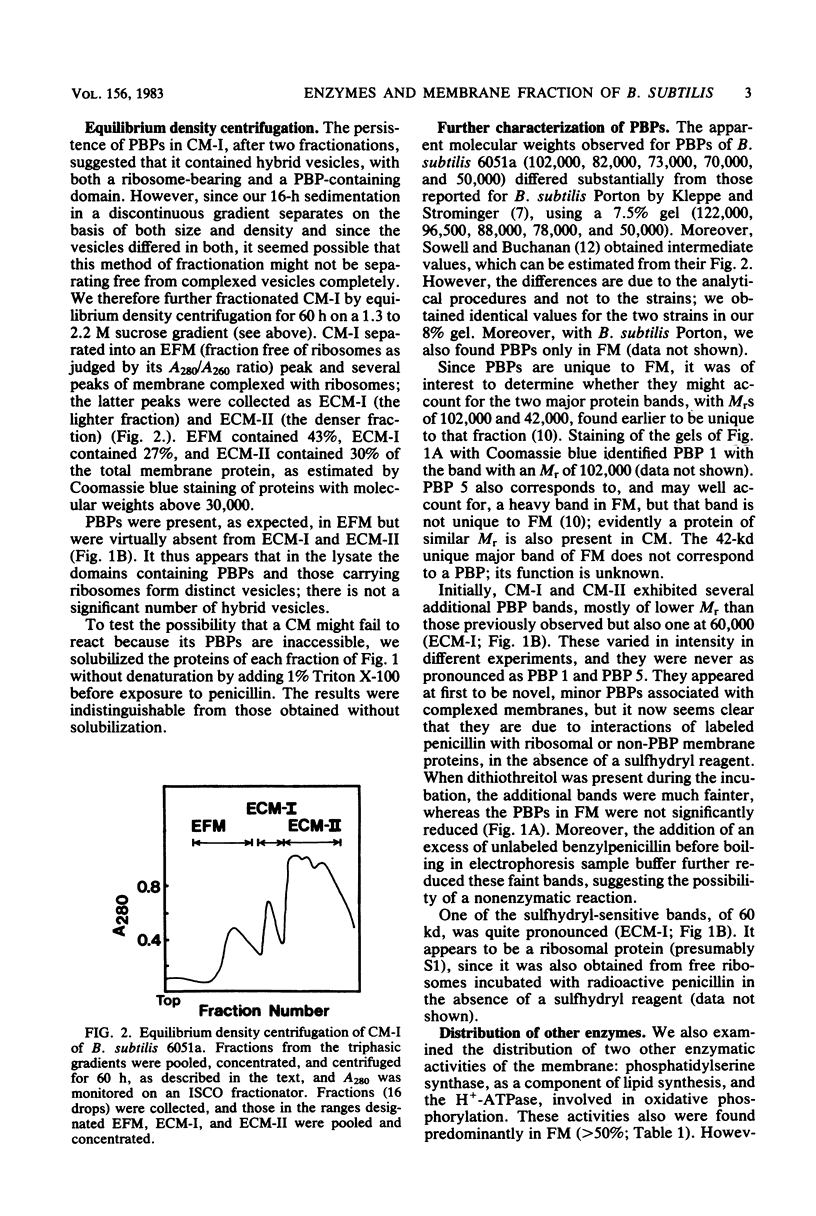
We had previously separated the ribosome-complexed and -free membrane fractions of Bacillus subtilis by sedimentation in a biphasic sucrose gradient. We now have found that the complexed fraction is contaminated with ribosome-free vesicles and that these can be removed by equilibrium density centrifugation. With this improved preparation, it could be shown that the penicillin-binding proteins are present almost exclusively in the ribosome-free membrane fraction. It thus appears that the fragmentation of the membrane in the lysing protoplast yields separate vesicles for the domains involved in protein translocation and for those involved in the synthesis and reshaping of the peptidoglycan. An enzyme of lipid synthesis (phosphatidylserine synthase) and also H+-ATPase were similarly found to be concentrated, but less exclusively, in the ribosome-free membrane fraction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan C. E. Altered membrane proteins in a minicell-producing mutant of Bacillus subtilis. J Bacteriol. 1979 Jul;139(1):305–307. doi: 10.1128/jb.139.1.305-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E. Topographical distribution of penicillin-binding proteins in the Escherichia coli membrane. J Bacteriol. 1981 Mar;145(3):1293–1298. doi: 10.1128/jb.145.3.1293-1298.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman G. M., Wieczorek D. S. Phosphatidylglycerophosphate synthease and phosphatidylserine synthase activites in Clostridium perfringens. J Bacteriol. 1980 Apr;142(1):262–267. doi: 10.1128/jb.142.1.262-267.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Marty-Mazars D., Tai P. C., Davis B. D. Localization and quantitation of proteins characteristic of the complexed membrane of Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1215–1221. doi: 10.1128/jb.154.3.1215-1221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Tai P. C., Davis B. D. A 64-kilodalton membrane protein of Bacillus subtilis covered by secreting ribosomes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3287–3291. doi: 10.1073/pnas.80.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe G., Strominger J. L. Studies of the high molecular weight penicillin-binding proteins of Bacillus subtilis. J Biol Chem. 1979 Jun 10;254(11):4856–4862. [PubMed] [Google Scholar]
- Kreibich G., Ulrich B. L., Sabatini D. D. Proteins of rough microsomal membranes related to ribosome binding. I. Identification of ribophorins I and II, membrane proteins characteristics of rough microsomes. J Cell Biol. 1978 May;77(2):464–487. doi: 10.1083/jcb.77.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty-Mazars D., Horiuchi S., Tai P. C., Davis B. D. Proteins of ribosome-bearing and free-membrane domains in Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1381–1388. doi: 10.1128/jb.154.3.1381-1388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. XI. Magnesium-adenosine triphosphatase properties of heat-activated coupling factor I from chloroplasts. J Biol Chem. 1972 Oct 25;247(20):6506–6510. [PubMed] [Google Scholar]
- Sowell M. O., Buchanan C. E. Changes in penicillin-binding proteins during sporulation of Bacillus subtilis. J Bacteriol. 1983 Mar;153(3):1331–1337. doi: 10.1128/jb.153.3.1331-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]