Abstract
Genetic variation in Opisthorchis viverrini adults originating from different locations in northeast Thailand and Laos, People’s Democratic Republic (PDR), was examined using random amplified polymorphic DNA (RAPD) analyses. In an initial analysis, the genomic DNA of one fluke from each of ten localities was amplified using 15 random primers (10-mers); however, genetic variation among O. viverrini specimens was detected reliably for only four primers. A more detailed RAPD analysis using these four primers was conducted on ten individuals from nine localities. Considerable genetic variation was detected among O. viverrini from different geographical areas and among some individuals from the same collecting locality. Comparison of the RAPD profiles revealed that O. viverrini adults from Laos PDR were genetically distinct from those from northeast Thailand. The taxonomic significance of this finding needs to be explored in more detail. The RAPD markers established in the present study provide opportunities to examine the biology and epidemiology of O. viverrini and fish-borne trematodes within the region. Additionally, application of these markers in such studies could have important implications in relation to the prevalence of cholangiocarcinoma in different regions of Asia.
Introduction
The liver fluke, Opisthorchis viverrini, is the most common food-borne trematode infection in Southeast Asia and has posed a serious health threat in Thailand and neighbouring countries (WHO 1995). Because of its close correlation with the incidence of cholangiocarcinoma (CHCA), it is recognised as one of the carcinogenic parasites in addition to Schistosoma haematobium and the bacterium Helicobacter pylori (see IARC 1994). It has been estimated that at least nine million people in Thailand alone are infected by O. viverrini (see WHO 1995; Sithithaworn and Haswell-Elkins 2003). Humans acquire this parasite through the consumption of raw or improperly cooked cyprinid fish containing infective metacercaria. The adult flukes are luminal parasites as they do not undergo systemic migration but reside in the small bile ducts and gall bladder. Eggs exit the biliary tract and are excreted in faeces. The eggs are ingested by snails (first intermediate host), multiply and become free-swimming cercariae that will penetrate the skin of a fish (second intermediate host). Chronic and heavy infection in highly endemic areas induces significant morbidities in the form of hepatobiliary disease, and some of the infected subjects develop CHCA (Elkins et al. 1990; Vatanasapt et al. 1990; Mairiang et al. 1992). The distribution of O. viverrini in Thailand and other endemic areas has marked regional variation in the prevalence and intensity of infection (Jongsuksuntigul and Imsomboon 1997; Sithithaworn and Haswell-Elkins 2003). The north-east region is the major focus of infection, but strong variability in infection occurs at the community, district and provincial levels. Although the causes of this observed variability are probably complex as the parasite has a three-host life cycle (Petney 2001), the role of genetic heterogeneity on infectivity in different hosts, transmission and associated disease is not known.
A number of studies have attempted to determine the cause of carcinogenesis in Opisthorchis-associated CHCA and host factors such as degree of inflammatory response and genetic variability of antioxidant enzymes, which may play roles in pathogenesis (Pinlaor et al. 2003, 2004; Honjo et al. 2005). Currently, limited information is available on the magnitude of genetic variation within and among O. viverrini populations from different geographical areas of Thailand (Ando et al. 2001) and its relevance to transmission and pathogenesis of infection. Furthermore, there is limited information concerning the O. viverrini genome. In the present study, we used random amplified polymorphic DNA (RAPD) analyses to examine the extent of genetic variation of O. viverrini from northeast Thailand and Laos, People’s Democratic Republic (PDR).
Materials and methods
Specimens of O. viverrini were obtained from naturally infected cyprinid fish collected between October 1999 and February 2001 from endemic areas in northeast Thailand (Khon Kaen, Kalasin, Mahasarakham, Chaiya Phum and Nahon Phanom) and Laos PDR (Vientiane). Metacercaria, isolated from fish using a pepsin digestion method (Sithithaworn et al. 1997; Srisawangwong et al. 1997), were used to infect 150 hamsters (50 metacercariae/animal) to produce adult worms. Four months after infection, adult worms were recovered from infected hamsters and stored at -20°C.
Frozen O. viverrini adults were disrupted individually in a glass tissue grinder on ice with 100 μl of extraction buffer (20 mM Tris-HCl, 20 mM ethylenediaminetetraacetic acid [EDTA], 300 mM NaCl, pH 8.3). Sodium dodecyl sulphate and proteinase K were added to the final concentration of 1% and 0.1 mg/ml, respectively. The homogenate was incubated at 55°C for 3 h. Genomic (g) DNA was isolated after the removal of all protein components by extraction with phenol and chloroform. The gDNA was precipitated with absolute ethanol (2x volume) and then chilled at -20°C for 3-12 h. After centrifugation, the pellet was washed with 70% ethanol and air dried. The gDNA was resuspended in 20 μl of Tris-EDTA buffer. RAPD analyses were performed in a DNA thermocycler (Hybrid, Bio-Active Co., Ltd.) using a reaction mixture (30 μl) containing 2 mM of each deoxyribonucleotide triphosphate, 3 μl of buffer (1.5 mM MgCl2, 30 mM KCl and 10 mM Tris, pH 8.3), 2 μmol of primer, 11.25 ng of gDNA template and 0.6 U of Taq DNA polymerase (Pharmacia Biotech, Sweden). The polymerase chain reaction conditions used were as follows: 95°C for 5 min cycle followed by 45 cycles of 95°C for 1 min (denaturation), 36°C for 1 min (annealing) and 72°C for 2 min (extension). Amplicons were subjected to electrophoretic analysis in 2% agarose gels using 1x Tris-borate-EDTA buffer, pH 8.0. Gels were stained with ethidium bromide, and the banding patterns were recorded using an Image Master® VDS (Pharmacia Biotech, USA).
In an initial experiment, 15 10-mer oligonucleotide primers (Table 1) were tested to determine those that produced reproducible RAPD patterns. Each primer was tested several times using a single worm from ten localities. Variation in banding patterns among O. viverrini individuals was only detected reliably using four primers (A2, A17, B5 and B17). These four primers were then used to determine the RAPD profiles of ten O. viverrini adults from nine collecting localities. Only reproducible and distinct bands were recorded; others were considered to be primer-template mismatches or artefacts. The RAPD profiles of specimens generated by primers A2, A17, B5 and B17 were scored separately, such that a distinct band at each defined migration position on an agarose gel was assumed to represent an independent character. Thus, the presence or absence of a band in samples was coded as either 1 or 0, respectively. Pairwise comparisons of the level of genetic difference (%) between individuals were calculated using the formula (I/P)×100, where P was the maximum number of bands for all samples combined and I was the number of bands differing between two individuals. A phenogram was constructed using an unweighted pair group method using arithmetic averages (UPGMA) analysis (Sneath and Sokal 1973).
Table 1.
Characteristics of random oligonucleotide primers used in the RAPD analyses of O. viverrini
| Primer | Sequence (5′-3′) | Molecular weight | Tm | % G+C |
|---|---|---|---|---|
| 3301 | TCGTAGCCAA | 3,013.0 | 32.1 | 50 |
| 3303 | TCACGATGCA | 3,013.0 | 32.6 | 50 |
| IL0525 | CGGACGTCGC | 3,030.0 | 45.9 | 80 |
| 3307 | AGTGCTACGT | 3,044.0 | 22.7 | 50 |
| B5 | TGCGCCCTTC | 2,956.0 | 45.6 | 70 |
| B6 | TGCTCTGCCC | 2,956.0 | 40.2 | 70 |
| B10 | CTGCTGGGAC | 3,045.0 | 33.6 | 70 |
| B17 | AGGGAACGAG | 3,127.0 | 32.3 | 60 |
| C19 | GTTGCCAGCC | 3,005.0 | 39.6 | 70 |
| A2 | TGCCGAGCTG | 3,045.0 | 42.4 | 70 |
| A9 | GGGTAACGCC | 3,054.0 | 38.7 | 70 |
| A10 | GTGATCGCAG | 3,069.0 | 29.8 | 60 |
| C2 | GTGAGGCGTC | 3,085.0 | 33.4 | 70 |
| A17 | GAAACGGGTG | 3,118.1 | 34.5 | 60 |
| A8 | GTGACGTAGG | 3,109.1 | 22.9 | 60 |
Results and discussion
In the initial experiment, no variation in the RAPD profiles was detected among individual O. viverrini adults from ten different populations in northeast Thailand and Laos PDR for the 11 of the 15 random 10-mer oligonucleotide primers. However, amplification of the gDNA of O. viverrini with four primers (A2, A17, B5 and B17) produced variable, yet reproducible, banding patterns. These amplicons contained 3-20 fragments ranging in size from 0.1 to 2.4 kb. Figure 1 is a representative agarose gel depicting the RAPD profiles of ten O. viverrini adults amplified using primer B17. For this primer, each adult worm had a distinct RAPD profile; however, the nine specimens from northeast Thailand shared major bands at 530 and 820 bp. These bands were absent in the specimen from Vientiane, Laos PDR.
Fig. 1.
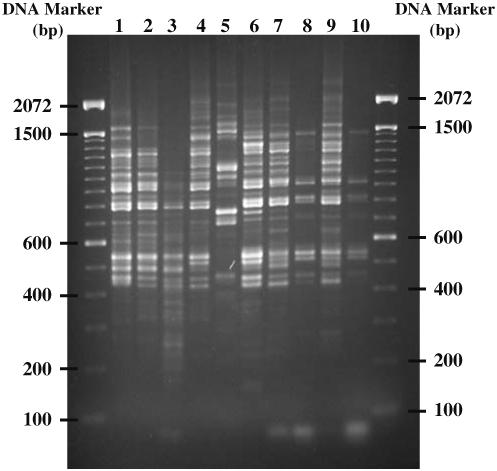
RAPD profiles of O. viverrini from different localities in northeastern Thailand and Laos (1 Nam Pong, Khon Kaen; 2 Mahasarakham; 3 Phuvaing, Khon Kaen; 4 Nakon Phanom; 5 Vientiane, Laos PDR; 6 Chaiya Phum; 7 Ban Lerngpleuy, Khon Kaen; 8 Ban Sa-ard, Khon Kaen; 9 Ban Phai, Khon Kaen and 10 Kalasin) generated by primer B17 (5′-AGGGAACGAG-3′)
In the more comprehensive analyses, based on comparisons of ten individual O. viverrini adults from eight localities in northeast Thailand and one locality in Laos PDR, there was considerable variation in RAPD profiles among trematodes using four primers. A total of 29, 17, 19 and 31 distinct bands at different migration positions were detected among amplicons generated by primers A2, A17, B5 and B17, respectively. The magnitude of the genetic differences among O. viverrini specimens ranged from 3 to 73%. Figure 2 shows the phenogram derived from a UPGMA analysis of the combined data set (i.e. for all four primers). There was some evidence of genetic similarity among samples based on geographical locality. All ten trematodes from Laos PDR formed a distinct cluster. One cluster contained nine of the ten specimens from Nam Pong in the province of Khon Kaen, as well as eight of the ten specimens from Mahasarakham Province. Similarly, eight specimens from Chaiya Phum Province fell within a single cluster. Seven individuals from Ban Pai (Khon Kaen), six from Nakon Phanom, six from Kalasin and five from Ban Lerngpleuy (Khon Kaen) also formed discrete clusters. It remains to be determined whether there is any correlation between different O. viverrini genotypes/clusters and phenotypic properties, such as growth and development, fecundity and response to drug treatment.
Fig. 2.
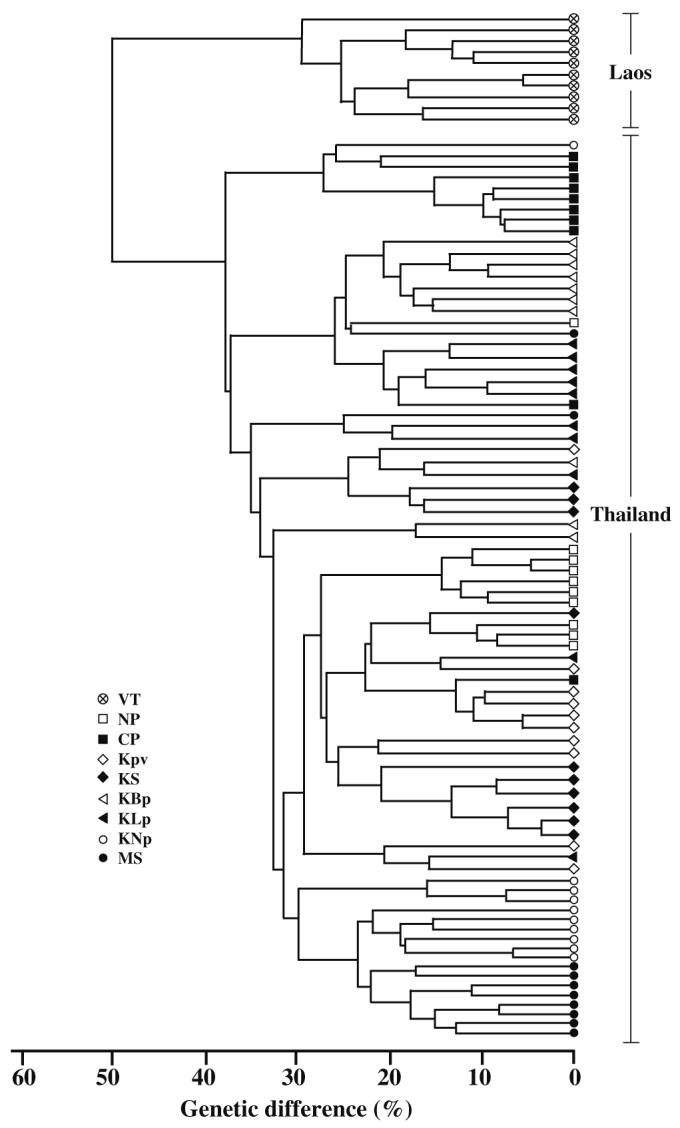
Phenogram depicting the genetic differences of individual O. viverrini adults from sites in northeast Thailand and Laos PDR (KLp Ban Lerngpleuy, Khon Kaen; KBp Ban Phai, Khon Kaen; KNp Nam Pong, Khon Kaen; Kpv Phuvaing, Khon Kaen; MS Mahasarakham; NP Nakon Phanom; CP Chaiya Phum; KS Kalasin; VT Vientiane, Laos PDR) based on the RAPD profiles generated by four 10-mer primers (A2, A17, B5 and B17)
Of significance was the finding that O. viverrini adults from Vientiane (Laos PDR) were genetically distinct from all specimens from northeastern Thailand. These two groups differed in RAPD profiles by an average of 50%. This genetic difference may be related to differences associated with differences in the intermediate host (i.e. subspecies of snail and/or species of cyprinid fish, susceptibility, dispersal ability, etc.) and ecological differences associated with the different geographical regions. For instance, of the eight collection localities in northeastern Thailand, seven were from the Chi River or its associated branches and reservoirs, while the eighth locality in the province of Nakon Phanom was from Songkram River, which is connected to the Mekong River. The specimens from Vientiane in Laos PDR originated from the Nam Ngum Reservoir associated with the Nam Ngum River, which has no connection with the Chi River. Another possibility for the difference in RAPD profiles between the two groups of O. viverrini is that each may represent a different species. However, the biological and taxonomic significance of the genetic difference between O. viverrini from Laos PDR and northeastern Thailand needs to be explored in more detail using a variety of molecular and biochemical techniques (e.g. restriction fragment length polymorphism, single-strand conformational polymorphism, DNA sequencing and multilocus enzyme electrophoresis) and at different genes. For instance, the results of the study by Ando et al. (2001) suggest that variation in the mitochondrial cytochrome oxidase subunit I gene may be useful for examining genetic variation within O. viverrini on a broad geographic scale.
In conclusion, the four new RAPD markers established in the present study provide the foundation to conduct further detailed studies on the genetic variation in O. viverrini and the biological significance of this variation from different geographical regions. Also, the approach employed in this study may be also applied to examine genetic variation in other fish-borne trematodes. The RAPD markers we have established are an important epidemiological tool, which will provide a greater understanding of the biology of O. viverrini and fish-borne trematodes in the region. Furthermore, they can be used to determine whether there is a link between the roles of genetic variation and biological characteristics and virulence particularly in cases presenting with overt clinical manifestation (i.e. CHCA).
Acknowledgments
This research was supported by a research grant from the Faculty of Medicine and financial support from the Graduate School, Khon Kaen University, in 2001 and the European Commission. We thank Dr. Vanla Dittapongpitch for help with the data analyses and wish to acknowledge the support of the Wellcome Trust and the continuing support of Olympus Australia.
Contributor Information
Paiboon Sithithaworn, Department of Parasitology, Liver Fluke and Cholangiocarcinoma Research Centre (LFCRC), Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand. e-mail: paib_sit@kku.ac.th.
Chadaporn Nuchjungreed, Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Tuanchai Srisawangwong, Department of Parasitology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Katsuhiko Ando, Department of Medical Zoology, School of Medicine, Mie University, Tsu 514-8507, Japan.
Trevor N. Petney, Institute of Zoology 1: Ecology and Parasitology, University of Karlsruhe, Kornblumen Strasse 13, Karlsruhe, Germany; School of Pharmacy and Medical Sciences, University of South Australia, GPO Box 2471, Adelaide, SA 5001, Australia
Neil B. Chilton, Department of Biology, University of Saskatchewan, Saskatoon, Saskatchewan S7N 5E2, Canada
Ross H. Andrews, School of Pharmacy and Medical Sciences, University of South Australia, GPO Box 2471, Adelaide, SA 5001, Australia
References
- Ando K, Sithithaworn P, Nuchjungreed C, Tesana S, Srisawangwong T, Limviroj W, Chinzei Y. Nucleotide sequence of mitochondrial CO I and ribosomal ITS II genes of Opisthorchis viverrini in northeast Thailand. Southeast Asian J Trop Med Public Health. 2001;32:17–22. [PubMed] [Google Scholar]
- Elkins DB, Haswell-Elkins MR, Mairiang E, Mairiang P, Sithithaworn P, Kaewkes S, Bhudhisawasdi V, Uttaravichien T. A high frequency of hepatobiliary disease and suspected cholangiocarcinoma associated with heavy Opisthorchis viverrini infection in a small community in north-east Thailand. Trans R Soc Trop Med Hyg. 1990;84:715–719. doi: 10.1016/0035-9203(90)90159-c. [DOI] [PubMed] [Google Scholar]
- Honjo S, Srivatanakul P, Sriplung H, Kikukawa H, Hanai S, Uchida K, Todoroki T, Jedpiyawongse A, Kittiwatanachot P, Sripa B, Deerasamee S, Miwa M. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005;117:854–860. doi: 10.1002/ijc.21146. [DOI] [PubMed] [Google Scholar]
- IARC. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . IARC Monogr Eval Carcinog Risks Hum. Vol. 61. Lyon: Jun 7-14, 1994. 1994. Schistosomes, liver flukes and Helicobacter pylori; pp. 1–241. [PMC free article] [PubMed] [Google Scholar]
- Jongsuksuntigul P, Imsomboon T. The impact of a decade long opisthorchiasis control program in northeastern Thailand. Southeast Asian J Trop Med Public Health. 1997;28:551–557. [PubMed] [Google Scholar]
- Mairiang E, Elkins DB, Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V, Posri S, Sithithaworn P, Haswell-Elkins M. Relationship between intensity of Opisthorchis viverrini infection and hepatobiliary disease detected by ultrasonography. J Gastroenterol Hepatol. 1992;7:17–21. doi: 10.1111/j.1440-1746.1992.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Petney TN. Environmental, cultural and social changes and their influence on parasite infections. Int J Parasitol. 2001;31:919–932. doi: 10.1016/s0020-7519(01)00196-5. [DOI] [PubMed] [Google Scholar]
- Pinlaor S, Yongvanit P, Hiraku Y, Ma N, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. 8-Nitroguanine formation in the liver of hamsters infected with Opisthorchis viverrini. Biochem Biophys Res Commun. 2003;309:567–571. doi: 10.1016/j.bbrc.2003.08.039. [DOI] [PubMed] [Google Scholar]
- Pinlaor S, Hiraku Y, Ma N, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. Mechanism of NO-mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation-mediated carcinogenesis. Nitric Oxide. 2004;11:175–183. doi: 10.1016/j.niox.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Sneath PHRA, Sokal RR. Numerical Taxonomy: The principles and practice of numerical classification. WH Freeman; San Francisco, California: 1973. [Google Scholar]
- Sithithaworn P, Haswell-Elkins M. Epidemiology of Opisthorchis viverrini. Acta Trop. 2003;88:187–194. doi: 10.1016/j.actatropica.2003.02.001. [DOI] [PubMed] [Google Scholar]
- Sithithaworn P, Pipitgool V, Srisawangwong T, Elkins DB, Haswell-Elkins MR. Seasonal variation of Opisthorchis viverrini infection in cyprinoid fish in north-east Thailand: implications for parasite control and food safety. Bull World Health Organ. 1997;75:125–131. [PMC free article] [PubMed] [Google Scholar]
- Srisawangwong T, Sithithaworn P, Tesana S. Metacercariae isolated from cyprinoid fishes in Khon Kaen District by digestion technic. Southeast Asian J Trop Med Public Health. 1997;28:224–226. [PubMed] [Google Scholar]
- Vatanasapt V, Uttaravichien T, Mairiang E, Pairojkul C, Chartbanchachai W, Haswell-Elkins M. Cholangiocarcinoma in north-east Thailand. Lancet. 1990;335:116–117. doi: 10.1016/0140-6736(90)90591-r. [DOI] [PubMed] [Google Scholar]
- World Health Organization Control of Foodborne Trematode Infection. WHO Technical Report Series. 1995;849 [PubMed] [Google Scholar]


