Abstract
Occludin is a transmembrane protein of the tight junction that functions in creating both an intercellular permeability barrier and an intramembrane diffusion barrier. Creation of the barrier requires the precise localization of occludin, and a distinct family of transmembrane proteins called claudins, into continuous linear fibrils visible by freeze-fracture microscopy. Conflicting evidence exists regarding the relative importance of the transmembrane and extracellular versus the cytoplasmic domains in localizing occludin in fibrils. To specifically address whether occludin's COOH-terminal cytoplasmic domain is sufficient to target it into tight junction fibrils, we created chimeras with the transmembrane portions of connexin 32. Despite the gap junction targeting information present in their transmembrane and extracellular domains, these connexin-occludin chimeras localized within fibrils when expressed in MDCK cells, as assessed by immunofluorescence and immunogold freeze-fracture imaging. Localization of chimeras at tight junctions depends on the COOH-terminal ZO-binding domain and not on the membrane proximal domain of occludin. Furthermore, neither endogenous occludin nor claudin is required for targeting to ZO-1–containing cell–cell contacts, since in normal rat kidney fibroblasts targeting of chimeras again required only the ZO-binding domain. These results suggest an important role for cytoplasmic proteins, presumably ZO-1, ZO-2, and ZO-3, in localizing occludin in tight junction fibrils. Such a scaffolding and cytoskeletal coupling function for ZO MAGUKs is analogous to that of other members of the MAGUK family.
Keywords: tight junction, occludin, connexin 32, ZO-1, MAGUK
The tight junction provides a continuous band of cell–cell contact that seals the paracellular space, allowing separation of apical and basal fluid compartments. It also defines apical and basal membrane polarity by restricting lipid and protein diffusion in the bilayer (Dragsten et al. 1981; van Meer and Simons 1986). In transmission electron micrographs, the tight junction appears as a series of focal membrane contacts. By freeze-fracture electron microscopic analysis, contacts appear as either continuous fibrils or rows of particles (depending on the fixation conditions) that partition with either the protoplasmic (P-face)1 leaflet or the ectoplasmic leaflet of the membrane fracture plane. These fibrils and particles have been shown to contain occludin and members of the claudin family (Fujimoto 1995; Furuse et al. 1996, Furuse et al. 1998a). It is important to determine the mechanism for targeting and organizing occludin in fibrils, since this intricate architecture is presumably required to form a continuous barrier.
Several proteins have been localized to a cytoplasmic plaque underlying the tight junction fibrils, either by immunohistochemistry or by virtue of binding to known tight junction proteins (for review see Balda et al., 1998). It is likely that, to some extent, the organization of occludin into fibrils depends on this underlying plaque. Specifically, occludin binds (ZO-1) (Stevenson et al. 1986; Willott et al. 1993), ZO-2 (Jesaitis and Goodenough 1994) and ZO-3 (Haskins et al. 1998), members of a family of membrane-associated signaling proteins termed the membrane-associated guanylate kinase (MAGUK) superfamily. Several other MAGUK proteins have been shown to cluster ion channels and receptors at specific membrane domains and link these proteins to the cytoskeleton (for review see Fanning and Anderson 1998). By analogy, a similar role exists for ZO family members at the tight junction.
Both occludin and the claudins, 65 kD and ∼22 kD, respectively, are predicted to span the membrane four times, forming two extracellular loops with cytosolic NH2 and COOH termini (Furuse et al. 1993, Furuse et al. 1998a). This predicted topology is similar to that of connexin and synaptophysin and, in the case of occludin, has been confirmed by immunomicroscopic studies (Furuse et al. 1993; Van Itallie and Anderson 1997). Although occludin is in tight junction fibrils, it is not essential for their formation, as occludin knockout embryonic stem cells still make claudin-containing fibrils and form intercellular diffusion barriers (Saitou et al. 1998). Nonetheless, evidence indicates occludin is normally a functional component of the junction. For example, overexpression of occludin in cultured epithelial cell monolayers results in an electrically tighter junction (Balda et al. 1996), and overexpression of truncated occludin disrupts the electrical barrier (McCarthy et al. 1996; Bamforth et al. 1999). Similarly, brain endothelial cells that form the impermeable blood–brain barrier express high levels of occludin, whereas endothelial cells in nonneural tissues, which have leakier tight junctions, express lower levels of occludin (Hirase et al. 1997).
Recent studies suggest that the structural basis for occludin's sealing properties may arise from the ability of this protein to form adhesive contacts with proteins on adjacent cells. Addition of a peptide comprising occludin's second extracellular loop to a cultured epithelial monolayer results in a loss of occludin at the membrane and subsequent disruption of ion and macromolecule selectivity (Wong and Gumbiner 1997). A second study has shown that occludin can mediate intercellular adhesion, suggesting that adhesion may be required to form the intercellular seal. Transfection of occludin null fibroblasts with occludin confers Ca2+-independent cell adhesion, which can be competed for by peptides corresponding to the extracellular loops (Van Itallie and Anderson 1997). It is likely that occludin mediates this interaction through homophillic interactions (Furuse et al. 1998b).
Recent studies suggest at least two distinct, but not exclusive, mechanisms for occludin targeting and localization at the tight junction. First, transmembrane or extracellular regions of occludin may self-associate and/or associate with claudins and provide an anchor for cytoplasmic proteins, transducing an instructive signal to assemble the plaque. This is similar to the proposed mechanism by which cadherin nucleates the adherens junction or integrins nucleate focal contacts. Alternately, organizational information from a cytoplasmic protein such as ZO-1 may recruit and tether occludin in fibrils at the junction.
In support of the first model, two studies reported that occludin lacking the COOH-terminal cytoplasmic tail localized at the tight junction when expressed in MDCK cells. Balda et al. 1996 demonstrated that chicken occludin constructs lacking the entire COOH-terminal tail localize at tight junctions in MDCK cells, although in a discontinuous pattern. Similarly, a microinjected occludin construct lacking almost the entire COOH terminus localized at tight junctions in Xenopus embryos (Chen et al. 1997). These results suggest the NH2-terminal half of occludin is sufficient for localization. However, tail-less occludin may localize by oligomerizing with a full-length occludin (Chen et al. 1997) that targeted because of an interaction with cytoplasmic proteins. More recently, Furuse et al. 1998b demonstrated that transfection of occludin alone into L-cell fibroblasts is insufficient to form fibrils, but that cotransfection with claudin-1 or -2 results in fibril formation, suggesting that occludin–claudin interactions may be necessary for occludin's incorporation in fibrils. Matter and Balda 1998 recently demonstrated that although the COOH-terminal tail of occludin contains basolateral targeting information, it is not sufficient to localize Fc-occludin chimeras at the tight junction, again suggesting that the NH2 terminus is responsible for tight junction localization. Therefore, putative occludin–occludin or claudin–occludin interactions may occur via occludin's membrane-spanning domains. Thus, several studies support the role of the membrane-spanning, amino half of occludin in localization to tight junctions, and occludin self-oligomerization or occludin–claudin co-oligomerization may provide an explanation for this requirement.
In contrast and in support of the second model, other data have argued that the COOH-terminal tail of occludin mediates tight junction targeting. Structural and functional data suggest that the COOH-terminal tail (human occludin residues 266–522) has at least two subdomains. A domain encompassing the COOH-terminal 150 amino acids (here referred to as the distal or ZO-binding domain) is highly charged, relatively conserved across species, and binds directly to ZO-1 (Furuse et al. 1994), ZO-2 (Itoh et al. 1999), and ZO-3 (Haskins et al. 1998). The second occludin tail domain, encompassing the 150–amino acids proximal to the membrane (here referred to as the proximal domain), is not known to interact with other proteins. This region is less charged and less conserved across species than the distal domain (Ando-Akatsuka et al. 1996). Furuse et al. 1994 reported that transfected chicken occludin constructs lacking the distal domain do not localize at tight junctions in a bovine kidney cell line. Recently, Bamforth et al. 1999 demonstrated that an NH2 terminally truncated occludin missing three of its four transmembrane domains can still localize at tight junctions in a mouse submandibular cell line. Furthermore, full-length occludin localizes at ZO-1 containing sites of cell–cell contact in the normal rat kidney (NRK) fibroblast cell line (Van Itallie and Anderson 1997) NRKs lacks endogenous occludin and, presumably, claudins, thereby implicating cytoplasmic plaque proteins not self-association, in the recruitment of occludin to these sites.
These differing observations raise the following question, what is the contribution of cytoplasmic plaque protein interactions in organizing occludin into the linear, fibrillar structure of tight junctions? To address this question, we constructed chimeric proteins containing the tail of human occludin, with or without the region that binds ZO proteins, fused to the membrane-spanning portions of the gap junction protein rat connexin 32 (cx32). cx32 oligomerizes into connexons, which form extracellular homotypic contacts between adjacent cells. The information that targets connexin 32 to the gap junction resides in the NH2-terminal membrane-spanning half of the protein (Leube 1995), and has been shown in analogous studies to be sufficient to target chimeric proteins to the gap junction (Troyanovsky et al. 1993). We observed that chimeras containing the ZO-binding domain of occludin target to tight junction fibrils, even in the presence of this gap junction targeting information. We also investigated these chimeras in NRK fibroblasts to determine how the absence of endogenous occludin and claudin family proteins affect occludin targeting. MDCK cells contain occludin and at least claudin 1, 2, and 3 and establish functional tight junctions, whereas NRK cells do not contain occludin or claudin 1 or 2 (or, presumably, other claudin family members because of the mesenchymal origins of NRKs) and do not generate tight junctions. Again, we observed that the ZO-binding domain of human occludin was sufficient to incorporate chimeras into ZO-1–containing sites of cell–cell contact in NRK fibroblasts. These results demonstrate that localization does not require occludin–occludin or occludin–claudin interactions. Further, these data suggest that interactions with ZO MAGUK proteins contribute to scaffolding occludin at the tight junction.
Materials and Methods
Plasmid cDNA Constructs
Plasmids containing cDNAs encoding either human occludin (Van Itallie and Anderson 1997) or cx32 (a gift of Dr. David Paul, Harvard Medical School, Cambridge, MA) were used as templates in PCRs to construct chimeric cDNAs. All cloned PCR products were confirmed by dideoxy sequencing in both directions. The vesicular stomatitis virus glycoprotein (VSV-G) epitope–tagged human occludin cDNA (HOC) has been described previously (Van Itallie and Anderson 1997). All constructs were expressed in MDCK or NRK cells using the mammalian expression vector pCB6 (Karl Matter, University of Geneva, Switzerland).
The connexin-occludin chimera (CX/OC) was constructed in three steps. First, primers 19985 (5′-GTACTAGTAGGCAGGATGAACTGGACAGGT-3′) and 19984 (5′-ATCATCCGGGCCTGTGCCCGCGCGGCCGC-3′) were used to amplify the NH2-terminal 219-residues encompassing all four transmembrane regions of connexin 32 using Vent polymerase (New England Biolabs Inc.). The product was digested with Spe1 and Not1 and cloned into pSK-Bluescript (Stratagene). Second, using primers 19983 (5′-cgcggccgcgaaaactcgaagaaagatggac-3′) and 19986 (5′-GACTATGATAGACAGAAAACAGCCTACACCGACATCGAGATGAACAGGCTGGGCAAGTGAGAGCTC-3′), the entire COOH-terminal cytoplasmic tail of occludin was amplified and a VSV-G tag was added. The resulting fragment was digested with NotI and SacI and inserted into pSK-Bluescript. Third, PCR products were subsequently excised and ligated into pCB6.
The distal COOH-terminal residues of occludin (DOC) chimera were made by ligating the transmembrane region of connexin 32 (generated via primers 19985 and 19984 as described above) to the distal 150 amino acids of occludin (residues 373–522), as amplified and VSV-G tagged using primer 19986 combined with 21063 (5′-CGCGGCCGCGAACTTTGAGACAGGTTGAAAAACA-3′). The resulting fragments were digested as above and subcloned as above into pCB6.
The proximal residues 266–372 of the occludin cytoplasmic tail (POC) chimera were made by ligating the transmembrane region of connexin 32 (generated via primers 19985 and 19984 as described above) to the membrane proximal portion of the occludin tail (residues 266–372), which was amplified and VSV-G tagged using primers 19983 and 24008 (5′-CCTCGTTACAGCAGCGGTGGTGCCTACACCGACATCGAGATGAACAGGCTGGGCAAGTGAGGATCC-3′). The resulting fragments were digested with SpeI, NotI and NotI, and BamHI, respectively, and subcloned into pCB6.
Cell Culture and Generation of Stable Lines
MDCK and NRK cells were obtained from the American Type Culture Collection and grown in DME supplemented with 10% FBS (Atlanta Biologicals), 5 mM L-glutamine (GIBCO BRL), 5 mM penicillin, and 5 U/ml streptomycin, hereafter known as DME-complete.
To generate MDCK cells stably expressing the constructs, cells were transfected via lipofection (GIBCO BRL) or calcium-phosphate coprecipitation (Chen and Okayama 1988) and selected using DME-complete containing 0.8 mg/ml G418 (GIBCO BRL). Resistant colonies were isolated and maintained in DME-complete containing 0.25 mg/ml G418.
Transient DNA Transfections and Immunofluorescence
cDNAs were introduced transiently into NRK cells plated on coverslips by calcium phosphate coprecipitation (Chen and Okayama 1988). MDCK clones stably expressing chimeric proteins were plated on coverslips and cultured 2–6 d before being fixed. 12–16 h after transient transfection, NRK cells were fixed and prepared for immunofluorescence as described in Fanning et al. 1998. VSV-G–tagged constructs were detected with a rabbit polyclonal anti–VSV-G antibody (MBL Laboratories) or mouse mAb P5D4 (a gift of Dr. Thomas Kreis, University of Geneva, Switzerland), both used at 1:200. To visualize the endogenous ZO-1, cells were labeled using rat mAb 40.76 (Stevenson et al. 1986) or a rabbit polyclonal antibody 4476 (Zymed Labs, Inc.). Rat connexin 32 was visualized with mouse mAb M12.13 (a gift of Dr. David Paul, Harvard Medical School, Cambridge, MA) or mouse anti–rat connexin 32 mAb (Chemicon International, Inc.). For epifluorescent microscopy, labeling was visualized using a Nikon Microphot FX microscope with a 60× PlanApo objective and images were captured using either T-MAX 400 film or a Sensys cooled CCD camera (Photometrics). These images were processed using Adobe Photoshop 4.0 or Image Pro Plus 2.0 (Media Cybernetics), respectively.
SDS-digested Freeze-Fracture Replica Labeling Electron Microscopy
MDCK cells were plated in 10-cm diameter tissue culture plates and grown to confluence. Cells were fixed in 1% paraformaldehyde in Dulbecco's phosphate-buffered saline (DPBS) for 15 min at 4°C for SDS-digested freeze-fracture replica labeling or in 2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, for 30 min at 4°C for conventional freeze-fracture. The cells were washed in the respective buffers, scraped from the substrate with a plastic cell scraper and infiltrated with graded amounts of glycerol in 0.1 M cacodylate buffer for 50 min at 4°C. Cell pellets were frozen in liquid nitrogen slush and freeze-fractured at −115°C in a Balzers 400 freeze-fracture unit (Balzers, Liechtenstein). SDS digestion and immunolabeling of replicas with polyclonal anti–VSV-G antibody (1:50 or 1:100) followed by protein A gold (10 nm) was performed as described (Fujimoto 1995). Replicas were examined using a Philips 301 electron microscope.
To quantitate gold particle distribution on micrographs, the distance from the center of a gold particle to the center of the nearest fibril was measured on photographic enlargements of micrographs using dial calipers (Small Parts, Inc.) that accurately measure in increments of 0.02 mm. The number of junctions and gold particles analyzed was as follows: 13 junctions and 343 gold particles for HOC, 8 junctions and 735 gold particles for CX/OC, and 2 junctions and 9 gold particles for control cells. The distance of a gold particle from fibril versus the number of occurrences was plotted using Microsoft Excel.
Results
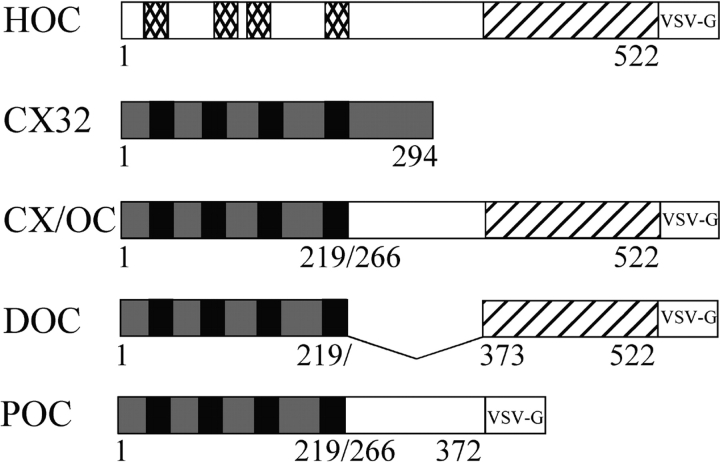
To investigate the role of the cytoplasmic COOH-terminal domain of occludin in targeting occludin, we fused regions of the tail of human occludin to the membrane-spanning portions of rat connexin 32 (cx32). Occludin and cx32 share similar membrane topogenics, with each predicted to form two extracellular loops, four transmembrane helices, and NH2 and COOH cytoplasmic termini. Fig. 1 illustrates constructs used in this study. An 11–amino acid tag from the VSV-G protein was engineered at the COOH terminus of each connexin-occludin construct to facilitate immunodetection.
Figure 1.
Schematic illustration of constructs used. HOC and cx32 contain four membrane-spanning domains (checked and solid boxes) with cytoplasmic NH2 and COOH termini. Chimeric proteins containing the NH2-terminal transmembrane regions of cx32 fused to the entire COOH-terminal tail of occludin (CX/OC), the membrane proximal residues 266–372 of occludin (POC), or the distal COOH-terminal residues 373–522 of occludin (DOC) are indicated. Only CX/OC and DOC include the ZO-binding domain (thick striped box). VSV-G tagged chimeras are indicated.
MDCK and NRK cells do not express detectable amounts of rat cx32, as determined by immunofluorescence labeling and immunoblotting (data not shown), providing null backgrounds in which to introduce chimeric proteins. Plasmids encoding chimeric proteins were transfected into MDCK cells and multiple clones of each construct were isolated by selection for G418 resistance. Stably transfected cell lines used were morphologically indistinguishable from their untransfected counterparts. At least three clones were analyzed for each experiment and each showed results similar to the representative clones reported in this study. NRK experiments reported in this study were performed by transient transfection.
A Connexin-Occludin Chimera Localizes at Tight Junctions in MDCK Cells
We first investigated the membrane targeting characteristics of full-length cx32 transfected into MDCK cells to interpret the results of subsequent chimeric studies. Published studies have shown that the NH2-terminal, membrane-spanning half of cx32 contains information sufficient to target to gap junctions (Leube 1995). Indirect immunofluorescence revealed that cx32 transiently transfected in MDCKs localized in the typical punctate pattern of gap junctions (Fig. 2 a). Protein was concentrated on the lateral surfaces of transfected cells regardless of whether adjacent cells also expressed cx32. Colabeling for ZO-1, a protein highly concentrated at tight junctions, demonstrated typical tight junction staining in a focal plane distinct from cx32 labeling (Fig. 2 b). Thus, rat liver cx32 clusters in gap junctionlike structures on the lateral surface of MDCKs and has no inherent ability to localize with ZO-1 at tight junctions.
Figure 2.
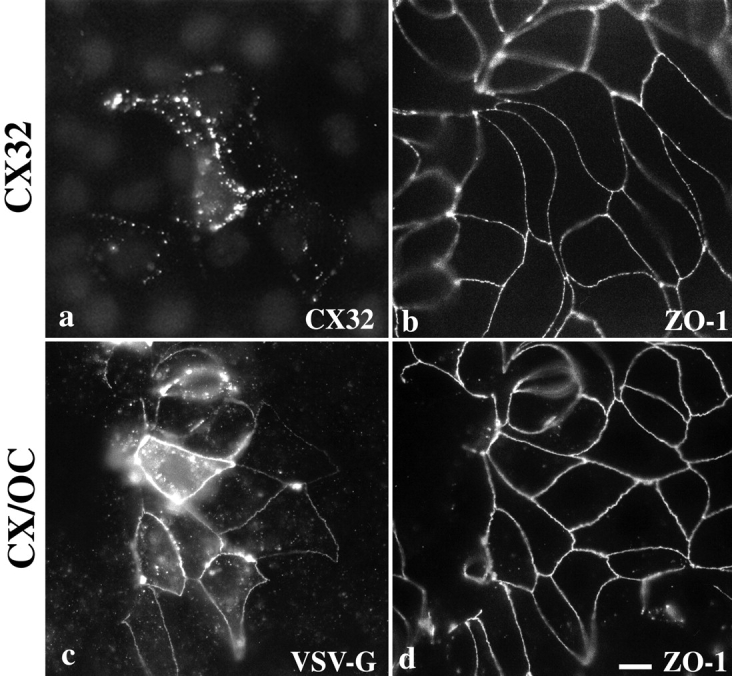
Immunofluorescent localization of cx32 and CX/OC in MDCK cells. cx32 localizes at gap junctionlike puncta around the cell periphery (a) in a focal plane basal to that of ZO-1 (b). Cells stably expressing CX/OC, visualized using polyclonal anti–VSV-G antibody, localized it at tight junctions (c), similar to endogenous ZO-1 (d). Bar, 30 μm.
In contrast, when the COOH-terminal tail of cx32 was replaced with the COOH-terminal tail of occludin to form the CX/OC chimera (Fig. 1), VSV-G epitope immunolabeling revealed that this chimera localized at tight junctions and not solely at gap junctions, as might be expected because of the gap junction targeting information in the NH2 terminus (Fig. 2 c). VSV-G staining for CX/OC paralleled that of endogenous ZO-1 staining (Fig. 2 d), with both colocalized in a reticular pattern around the apical surface of cells. Note that expression of the chimera in adjacent cells is not required for localization, as continuous, circumferential VSV-G labeling is seen in cells adjacent to untransfected neighbors. Interestingly, in clones expressing very low levels of CX/OC, the protein localized primarily at tight junctions (as seen in Fig. 2 c), whereas clones greatly overexpressing CX/OC localized it at both tight and gap junctions (data not shown). These observations may imply that the incorporation of CX/OC into tight junctions is saturable, and that excess chimeric protein follows a default localization to gap junctions. Thus, the human occludin tail is sufficient to target chimeras containing the transmembrane portions of rat cx32 to the tight junction, even though the NH2-terminal half of cx32 possesses gap junction targeting information. In addition, the NH2 -terminal, membrane-spanning half of human occludin is not necessary for occludin's localization at the tight junction.
CX/OC Chimeras Are Incorporated into Tight Junction Fibrils
To ask whether the CX/OC chimera was actually incorporated specifically into fibrils or simply concentrated around them, we employed immunogold labeling of freeze-fracture replicas. Control cells expressing pCB6 vector alone showed no specific labeling at tight junction fibrils (Fig. 3 a). In contrast, cells expressing full-length human occludin (HOC) (Fig. 3 b) or CX/OC (Fig. 3c and Fig. d) both showed specific labeling along fibrils. Note that in CX/OC-containing cells, a two-dimensional crystalline lattice reminiscent of gap junctions is also labeled (Fig. 3 d, arrow), indicating that chimeras expressed at high levels can also assemble into gap junctionlike aggregates. The increased gold labeling in CX/OC cells versus human occludin cells is probably due to higher protein expression in the CX/OC cells, as documented by immunoblotting (data not shown).
Figure 3.
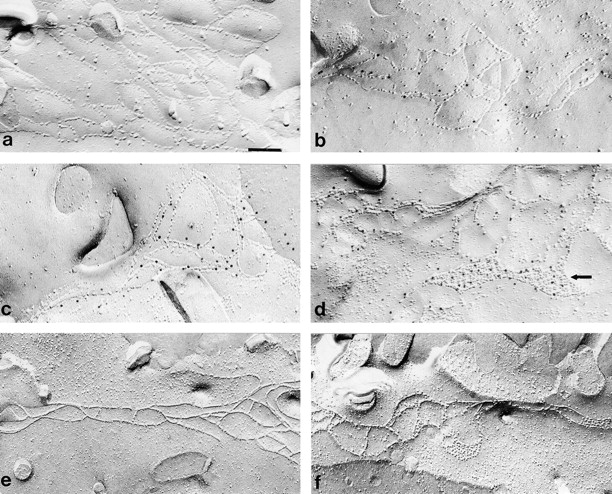
Freeze-fracture immunolabeling of CX/OC in MDCK cells. For SDS-digested freeze-fracture replica labeling electron microscopy, replicas of MDCK cells stably transfected with empty pCB6 vector (a), HOC (b), or CX/OC (c and d) were fixed in 1% paraformaldehyde, reacted with anti–VSV-G polyclonal antibody and labeled with 10 nm protein A gold as described in Materials and Methods. Minimal nonspecific gold labeling occurs at tight junctions in empty pCB6 vector cells (a). Gold label appears at tight junction fibrils in HOC- (b) and CX/OC-transfected cells (c and d). Immunolabeling of CX/OC was also observed at gap junctionlike plaques (d, arrow). Conventional freeze-fracture of CX/OC-expressing cells using 2% glutaraldehyde (f) revealed distinct connexonlike particles within the fused fibrils of the tight junction. Such particles are not visible in glutaraldehyde fixed control (empty pCB6) cells (e). Bar: (a–d) 0.11 μm; (e and f) 0.16 μm.
It has long been known that fixation of cells with glutaraldehyde will transform the linear junction particles into continuously fused fibrils (van Deurs and Luft 1979). Whereas unfixed particles appear on the ectoplasmic face, fixed fibrils appear on the P-face as continuous strands interspersed with occasional hemispherical particles (Staehelin 1973; van Deurs and Luft 1979). The proportion of particles to strands varies depending on cell type. In contrast, gap junctions visualized by freeze-fracture in glutaraldehyde fixed cells never fuse but always appear as large, paracrystalline aggregates of discrete connexon particles on the P-face. Interestingly, in CX/OC-expressing cells fixed in 2% glutaraldehyde and processed for conventional freeze-fracture, distinct particles similar in size and shape to connexons are visible within the fused fibrils of the P-face (Fig. 3 f), but are absent in cells transfected with HOC (data not shown) and the control empty vector (Fig. 3 e). These particles, which presumably represent the connexin portion of CX/OC, occur in perfect register with the rest of the fibril. Similar morphological changes are not apparent in the immunogold-labeled replicas because the relatively brief, gentle fixation of 1% formaldehyde used in the gold labeling was not sufficient to fuse the fibrils. Together, these results strongly suggest that the CX/OC chimera is intercalated directly into junctional fibrils.
To determine whether the CX/OC chimera was within or just near fibrils, we next quantified the orthogonal distance between individual gold particles and the nearest fibril. Transfected, epitope-tagged occludin was assumed to be incorporated into fibrils and was used as a control reference. Both occludin and the CX/OC chimera show similar distance distributions from fibrils, with the most common distance ranging from 6–12 nm (Fig. 4). This distance corresponds roughly to the length of the intervening primary and secondary antibodies used in the labeling procedure (Staehelin 1973). Thus, the CX/OC chimera localized directly within the tight junction fibril, presumably interspersed with endogenous occludin and claudin family molecules, and without obviously disrupting normal junction morphology.
Figure 4.
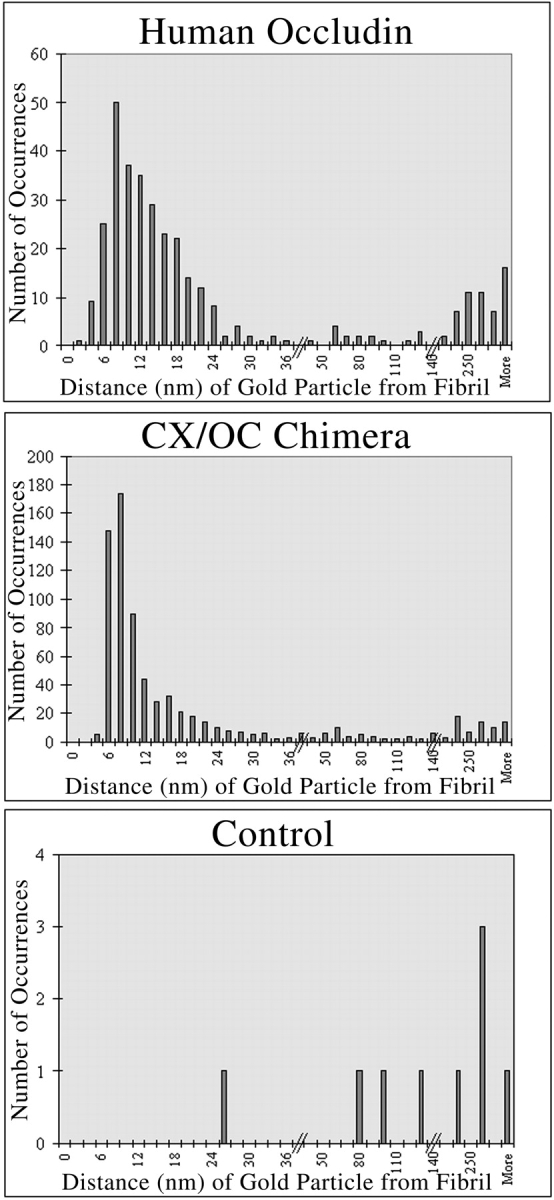
Quantitation of gold label distance from freeze-fracture fibrils. The distance from the center of each gold particle to the center of the nearest fibril was measured using dial calipers accurate to 0.02 mm. The majority of HOC and CX/OC are both located 6–12 nm from a fibril, suggesting that CX/OC is incorporated directly into occludin-containing tight junction fibrils.
The ZO-binding Domain Is Necessary for Connexin Chimeras to Localize at Tight Junctions
We next asked whether the ZO-binding domain is necessary for localization of connexin chimeras at tight junctions. In MDCK cells stably expressing DOC, which contains the ZO-binding domain, VSV-G labeling occurred at the tight junction (Fig. 5 a), as seen by its colocalization with endogenous ZO-1 (Fig. 5 b). However POC, which lacks the ZO-binding domain, localized predominantly in discrete puncta (Fig. 5 c), similar to gap junctionlike structures seen with full-length connexin 32 (Fig. 2 a). ZO-1 at the tight junction (Fig. 5 d) was visible in a more apical focal plane than POC immunoreactivity. These results suggest that potential interactions between endogenous occludin and transfected POC in the juxtamembrane region (amino acids 266–372) do not confer tight junction localization to POC. In addition, these results suggest that only the ZO-binding region of occludin is necessary to localize a connexin chimera at the tight junction.
Figure 5.
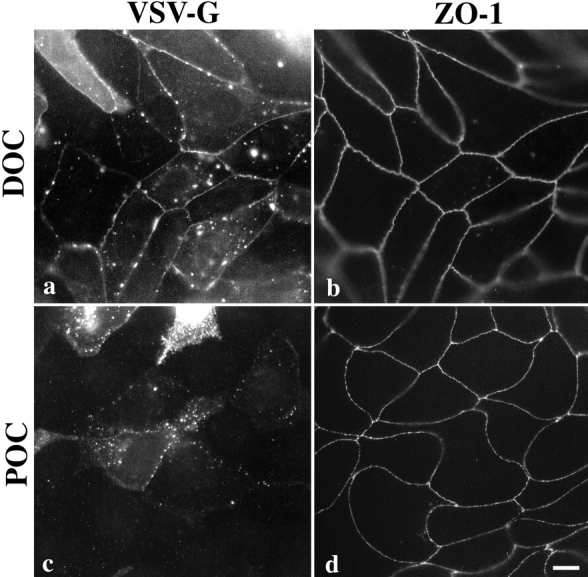
Immunolocalization of DOC and POC chimeras in MDCK cells. DOC (a), which contains the ZO-binding region, colocalizes primarily with ZO-1 (b) at tight junctions and also occasionally at discrete puncta on the lateral surface. Note that continuous circumferential VSV-G staining appears even in cells that neighbor nonexpressing cells. POC (c), which lacks the ZO-binding site, does not colocalize with ZO-1 (d). Instead it appears as puncta around the periphery of the cell in a focal plane basal to ZO-1, similar to cx32 (Fig. 2). Bar, 30 μm.
Endogenous Occludin Is Not Required to Colocalize Connexin-Occludin Chimeras with ZO-1 at NRK Sites of Cell Contact
If an interaction with ZO proteins is independently sufficient to localize occludin in the absence of endogenous occludin and claudin, we predict that connexin-occludin chimeras will be able to localize to ZO-1–containing cell–cell contacts, even in cells lacking these transmembrane tight junction proteins. We tested this prediction in NRK fibroblasts that express ZO-1 and ZO-2 at cadherin-based contacts (Itoh et al. 1993) but lack immunodetectable occludin, claudin-1 and-2 (data not shown) and lack freeze-fracture fibrils. In support of this prediction is our previously published observation that full-length occludin stably expressed in NRK cells colocalizes with ZO-1 at cell–cell contacts (Van Itallie and Anderson 1997). In contrast, occludin lacking the ZO-binding domain does not localize in NRK cells, yet the same construct localizes to tight junctions in MDCK cells (Van Itallie, C., personal communication).
When cx32 is transiently expressed in NRK cells its localization depends on whether adjacent cells also express the protein. In isolated cells that are not in contact with other transfected cells, the protein is detected diffusely in the cytoplasm or in perinuclear aggregates (data not shown). In contrast, when adjacent cells both express cx32, it localizes at cell contacts between them (Fig. 6 a, arrowhead), but not at the contacts with neighboring, nontransfected cells (Fig. 6 a, arrows). cx32 contacts in adjacent transfected cells occasionally appeared to overlap ZO-1 immunoreactivity (Fig. 6 b), but were quite different in shape and size. These results suggest that clustering of cx32 at cell–cell contacts in NRK cells requires expression of cx32 on both cells and that cx32 has no inherent tendency to associate with ZO-1 at cell contacts.
Figure 6.
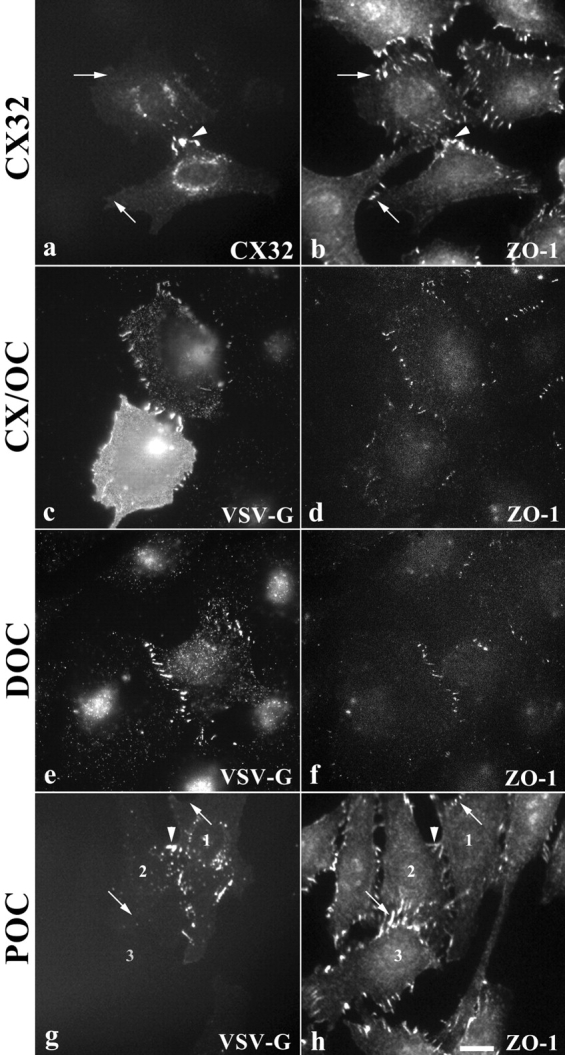
Immunolocalization of connexin-occludin chimeras in NRK fibroblasts. cx32 localizes at sites of shared contact between two transfected cells (a, arrowhead), but does not localize at sites of cell–cell contact with untransfected cells (a, arrows). ZO-1 immunolabeling occasionally overlaps cx32 localization, but is usually different in size and shape (b). Chimeras containing the ZO-binding site, CX/OC (c) and DOC (e), colocalize with ZO-1 (d and f, respectively) at fingerlike sites of cell contact around the periphery of occludin null NRKs. POC (g), which lacks the ZO-binding domain, only localizes at the plasma membrane if it is expressed in a cell adjacent to another transfected cell (g, arrowhead). ZO-1 immunolabeling in POC-transfected cells (h) is distinct from VSV-G immunolabeling. Note the lack of VSV-G immunoreactivity at other ZO-1–containing cell contacts in POC-expressing cells (g, arrows). Bar, 30 μm.
In contrast, CX/OC (Fig. 6 c) colocalized with ZO-1 (Fig. 6 d) at cell–cell contacts, implying that endogenous occludin or claudins are not necessary for targeting to ZO-1–containing cell contacts. This behavior is distinct from that seen with cx32, which only localized at contacts between two transfected cells (Fig. 6, a and b). Consistent with this is the observation that full-length occludin expressed in NRK cells will colocalize with ZO-1 at cell contacts even when the adjacent cell does not express occludin (Van Itallie, C., personal communication).
Since endogenous occludin is not necessary for localization of connexin-occludin chimeras in NRK cells, we next tested the requirement for the ZO-binding domain. DOC (Fig. 6 e) colocalized with ZO-1 (Fig. 6 f) in NRK cells. However, POC (Fig. 6 g), which lacks the ZO-binding domain, did not (Fig. 6 h). Instead, it localized only at contacts between adjacent transfected cells (Fig. 6 g, arrowhead) and not between adjacent, nontransfected cells (Fig. 6 g, arrows). This is a pattern identical to that seen with full-length cx32. Thus, the region of occludin that binds ZO MAGUKs is sufficient to target connexin-occludin chimeras to cell contacts in cells that do not contain endogenous occludin or claudins. Further, these results suggest that occludin need not bind occludin on an adjacent cell (transcellular interactions) to enter the contact.
Discussion
We present evidence that the ZO-binding domain of occludin's cytoplasmic tail is sufficient to target connexin-occludin chimeras to the tight junction and organize them into the linear fibrils that create the paracellular barrier. In fibroblasts, which lack endogenous occludin and claudins, the ZO-binding domain is also sufficient to localize chimeras to ZO-1–containing cell contacts. Extrapolating these results to occludin suggests interactions with ZO proteins are important for organizing occludin in tight junction fibrils. This conclusion is different from, but not in conflict with, that of previous studies that focused on the intramembrane and extracellular regions of occludin, and concluded that the cytoplasmic tail plays no role in localization. We suggest further study of cytoplasmic scaffolding is important since these connections may ultimately be most responsible for initial fibril assembly and subsequent barrier regulation.
We demonstrate using immunogold freeze-fracture labeling that connexin fused to the cytoplasmic tail of occludin intercalates directly into the occludin- and claudin-containing tight junction fibrils. This conclusion is further supported by the observation that tight junction fibrils in cells stably expressing CX/OC appear dotted, even after fixation with glutaraldehyde, with individual particles. This response to fixation is characteristic of connexin, but not of occludin (van Deurs and Luft 1979). Although gap junctions have been reported near or in continuity with tight junctions, they are always confined to the regions around the fibrils and are not found in continuity with them. In addition, although cx43 is reported to bind ZO-1 in cardiac myocytes (Toyofuku et al. 1998), cx32 is not known to form hemichannels with cx43. Moreover, MDCK cells do not contain cx43 (Jordan et al. 1999).
Further, our chimeric protein approach narrowed this targeting region to occludin's COOH-terminal 150–amino acids, designated the ZO-binding domain; DOC localizes at the tight junction in MDCK cells and at cell contacts in NRK fibroblasts. In contrast, chimeras that contain the membrane proximal region of the occludin cytoplasmic tail (POC) localize in MDCK cells exclusively to gap junctionlike plaques or to intracellular aggregates. In NRK fibroblasts POC localizes, as does full-length connexin 32, to plaques between neighboring transfected cells. Together, these data strongly suggest that the ability to bind ZO proteins is necessary and sufficient to localize connexin-occludin chimeras in tight junction fibrils and fibroblast cell contact sites.
Previous studies have demonstrated the ability of occludin lacking the ZO-binding domain to target to tight junctions in cultured MDCK cells (Balda et al. 1996) and Xenopus embryos (Chen et al. 1997). Balda et al. 1996 observed that occludin truncated after the fourth membrane-spanning region, and, thus, lacking the ZO-binding region, was able to localize to tight junctions. Similarly, Chen et al. 1997 reported tight junction localization in Xenopus embryos of an occludin construct missing almost the entire COOH-terminal cytoplasmic domain. These authors demonstrated the coimmunoprecipitation of endogenous and truncated transfected occludin, suggesting the possibility that tight junction targeting of truncated occludin resulted from an association with endogenous occludin through its transmembrane and/or extracellular regions (Chen et al. 1997). Interestingly, Furuse et al. 1998b recently demonstrated that claudin-1 and -2 can recruit occludin to fibrillike structures in L-cells; thus, interactions between transmembrane proteins are clearly important for occludin localization. Alternately, it is also possible that the transfected claudins recruited ZO proteins, which then functioned to localize occludin. Nonetheless, the apparent dispensability of the ZO-binding region in the localization of truncated occludin in these previous studies may be due to the ability of truncated occludin to self-oligomerize with full-length occludin (Chen et al. 1997) or co-oligomerize with claudins, either of which are already interacting with plaque proteins.
In contrast to our data, Matter and Balda 1998 recently demonstrated that the entire COOH-terminal tail of occludin targets Fc chimeras to the basolateral surface but is not sufficient to localize them at tight junctions. The chimeras reported by Matter and Balda 1998 and those used in this study differ in two respects: this study used the human occludin sequence, whereas Matter and Balda 1998 used chicken's, and the NH2-terminal region of the Fc protein used by Matter and Balda 1998 is a single span protein with no known ability to aggregate, whereas cx32 oligomerizes to form multisubunit connexons. As human and chicken occludin are only 35.3% identical across the entire COOH-terminal tail and 44.9% identical between ZO-binding domains, it is possible that species-specific sequence differences contributed to our contrary results. However, chimeras containing the entire COOH-terminal human occludin tail fused to the extracellular and membrane-spanning domains of glycophorin C, a single span transmembrane protein unable to oligomerize, localized basolaterally, not junctionally, in MDCK cells, and did not colocalize with ZO-1 in NRK cells (Mitic, L., E. Schneeberger, and J.M. Anderson, unpublished data). Similar results were obtained using the COOH-terminal 150–amino acids of human occludin fused to the amino half of glycophorin C. Thus, it is possible that retention of occludin chimeras at tight junctions and cell contacts requires an NH2 terminus capable of aggregation. cx32 may be able to substitute functionally for occludin in this respect.
Our results indicate that connexin can be aligned side-by-side with occludin into ordered, linear fibrils when it is fused to the ZO-binding region, thus, suggesting a scaffolding function for ZO-1, ZO-2, or ZO-3. The ZO proteins are members of the MAGUK protein family, many of whose members have already been shown to function as cytoplasmic scaffolds in organizing membrane proteins into specialized membrane domains (for review see Anderson 1996; Fanning and Anderson 1996). Genetic evidence from invertebrates supports this idea. For example, in Caenorhabditis elegans loss of LIN-2, a MAGUK, results in membrane mislocalization of the LET-23 receptor tyrosine kinase, a subsequent lack of receptor activation during development and a vulvaless phenotype (Simske et al. 1996). In Drosophila, the Discs-large MAGUK binds Shaker-type K+ channels at neuromuscular junctions (Tejedor et al. 1997). Mutations in Discs-large are associated with a loss of Shaker-type K+ channels from the neuromuscular junctions and their redistribution over the plasma membrane (Tejedor et al. 1997). Likewise, a vertebrate MAGUK called postsynaptic density protein-95 kD (PSD-95) has also been demonstrated in vertebrate synapses to bind and cluster both the N-methyl-d-aspartate receptor and the Shaker-type K+ channel (Kim et al. 1995; Kornau et al. 1995). Thus, the precedent for MAGUK involvement in organizing membrane proteins at specialized surface domains is well established.
MAGUK protein family members are characterized by a multidomain organization that includes one or three PSD-95/ Discs-Large/ZO-1(PDZ) domains (for review see Fanning and Anderson 1996; Fanning et al. 1996). PDZ domains are 80–100-amino acid protein binding cassettes that recognize a three residue peptide motif in the COOH termini of their binding partners (Songyang et al. 1997). MAGUK proteins also serve as linkers between integral membrane proteins and cytoskeletal networks. For example, p55, a MAGUK protein that contains one PDZ, anchors the transmembrane protein glycophorin C and also binds protein 4.1 (Marfatia et al. 1995). Similarly, the human homologue of the C. elegans protein Lin-2, a MAGUK protein, likely ties the membrane protein syndecan-2 to the cytoskeleton via the actin-binding protein 4.1. Recently, ZO-1 has been reported to bind directly to actin in fibroblasts via its proline-rich COOH terminus (Itoh et al. 1997). Likewise, ZO-1 itself appears to directly link occludin to actin (Fanning et al. 1998), suggesting that it may mediate cytoskeletally induced changes in occludin's sealing properties. Of note, members of the claudin family contain PDZ-binding motifs at their COOH termini.
Our results together with previously published data suggest the possibility of multiple steps for the assembly of occludin into fibrils. Free occludin in the lateral plasma membrane concentrates at the tight junction via contacts with a preformed, cytoplasmic ZO-containing scaffold. Concomitant self-aggregation, or oligomerization with other transmembrane proteins such as claudins, is necessary to assemble and/or maintain occludin in linear fibrils. Incorporation into a fibril could be regulated at either of these two steps: binding to ZOs or oligomerization. This idea is consistent with the recent observation of a second pool of occludin that is present on the lateral cell surface and not organized into fibrils (Sakakibara et al. 1997). The junctional pool of occludin is hyperphosphorylated relative to the nonfibrillar pool, suggesting phosphorylation might be one mechanism for regulating assembly (Sakakibara et al. 1997). Lending further support for this model, ZO-1 is localized at cell–cell contacts in the absence of occludin, such as in the NRK fibroblasts used in this study, but no examples of occludin localization in the absence of ZO-1 are known.
Several published experimental observations suggest cadherin is the initial organizer of the tight junction plaque. First, extracellular antibodies against cadherin inhibit formation of tight junctions (Gumbiner et al. 1988; Balda et al. 1991). Second, the tight junction and the adherens junction may be physically linked, particularly during formation of the tight junction, via ZO-1, which binds the cadherin-associated proteins α- (Itoh et al. 1997) and β-catenin (Rajasekaran et al. 1996). Moreover, cells containing a mutant form of αE-catenin that is unable to bind vinculin do not localize ZO-1 at tight junctions or form continuous fibrils (Watabe-Uchida et al. 1998). Lastly, ZO-1 assembles at cell–cell contacts before occludin and coincident with E-cadherin in studies of tight junction formation in the early mouse embryo (Sheth et al. 1997). These observations, together with those reported here, suggest that occludin is recruited into a preformed ZO-containing structure whose assembly is initiated at the adherens junction. We have demonstrated that the ZO-binding domain is sufficient for localization in fibrils. Such a model implies that regulation of the tight junction barrier may occur through reversible recruitment of occludin into fibrils organized by a ZO-containing scaffold. The occludin–ZO interactions and the overall organization of the cytoplasmic plaque will be important subjects of future inquiry into the assembly and regulation of tight junctions.
Acknowledgments
The authors thank Drs. Lynne Lapierre (Medical College of Georgia) and Christina Van Itallie (Yale University) for technical advice, Joanne McCormack (Massachusetts General Hospital) for expert freeze-fracture technical assistance, and Dr. Zenta Walther (Yale University) for critical reading of the manuscript.
This work was supported by the National Institutes of Health grants DK45134, CA66263, and DK38979 (all to J.M. Andersen), HL25822 (to E.E. Schneeberger), and DK34989 (to Yale Liver Center).
Footnotes
1.used in this paper: cx32, rat connexin 32; DOC, distal COOH-terminal residues 373–522 of occludin; HOC, human occludin cDNA; MAGUK, membrane-associated guanylate kinase; NRK, normal rat kidney; P-face, protoplasmic face; POC, proximal residues 266–372 of the occludin cytoplasmic tail; VSV-G, vesicular stomatitis virus glycoprotein; ZO-1, zonula occludens-1
References
- Anderson J.M. Cell signallingMAGUK magic. Curr. Biol. 1996;6:382–384. doi: 10.1016/s0960-9822(02)00501-8. [DOI] [PubMed] [Google Scholar]
- Ando-Akatsuka Y., Saitou M., Hirase T., Kishi M., Sakakibara A., Itoh M., Yonemura S., Furuse M., Tsukita S. Interspecies diversity of the occludin sequencecDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J. Cell Biol. 1996;133:43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M.S., Matter K. Tight junctions. J. Cell Sci. 1998;111:541–547. doi: 10.1242/jcs.111.5.541. [DOI] [PubMed] [Google Scholar]
- Balda M.S., Gonzalez-Mariscal L., Contreras R.G., Macias-Silva M., Torres-Marquez M.E., Garcia-Sainz J.A., Cereijido M. Assembly and sealing of tight junctionspossible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J. Membr. Biol. 1991;122:193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- Balda M.S., Whitney J.A., Flores C., Gonzalez S., Cereijido M., Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth S.D., Kniesel U., Wolburg H., Engelhardt B., Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J. Cell Sci. 1999;112:1879–1888. doi: 10.1242/jcs.112.12.1879. [DOI] [PubMed] [Google Scholar]
- Chen C.A., Okayama H. Calcium phosphate-mediated gene transfera highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- Chen Y., Merzdorf C., Paul D.L., Goodenough D.A. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J. Cell Biol. 1997;138:891–899. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.R., Woods D.F., Marfatia S.M., Anderson Z.M. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 localizes to the basolateral membrane of epithelial cells. J. Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragsten P.R., Blumenthal R., Handler J.S. Membrane asymmetry in epitheliais the tight junction a barrier to diffusion in the plasma membrane? Nature. 1981;294:718–722. doi: 10.1038/294718a0. [DOI] [PubMed] [Google Scholar]
- Fanning A.S., Anderson J.M. Protein-protein interactionsPDZ domain networks. Curr. Biol. 1996;6:1385–1388. doi: 10.1016/s0960-9822(96)00737-3. [DOI] [PubMed] [Google Scholar]
- Fanning A.S., Lapierre L.A., Brecher A.R., Van Itallie C.M., Anderson J.M. Protein interactions in the tight junctionthe role of MAGUK proteins in regulating tight junction organization and function. Curr. Top. Membr. 1996;43:211–235. [Google Scholar]
- Fanning, A.S., and J.M. Anderson. 1998. PDZ domains and the formation of protein networks at the plasma membrane. In Current Topics in Membranes: Membrane Protein-Cytoskeleton Complexes. N.J. Nelson, editor. Vol. 43. 211–235. [DOI] [PubMed]
- Fanning A.S., Jameson B.J., Jesaitis L.A., Anderson J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J. Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S. Occludina novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Itoh M., Hirase T., Nagafuchi A., Yonemura S., Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Fujimoto K., Sato N., Hirase T., Tsukita S. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J. Cell Sci. 1996;109:429–435. doi: 10.1242/jcs.109.2.429. [DOI] [PubMed] [Google Scholar]
- Furuse M., Fujita K., Hiiragi T., Fujimoto K., Tsukita S. Claudin-1 and -2novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin J. Cell Biol. 141 1998. 1539 1550a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Sasaki H., Fujimoto K., Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts J. Cell Biol. 143 1998. 391 401b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Stevenson B., Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins J., Gu L., Wittchen E.S., Hibbard J., Stevenson B.R. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase T., Staddon J.M., Saitou M., Ando-Akatsuka Y., Itoh M., Furuse M., Fujimoto K., Tsukita S., Rubin L.L. Occludin as a possible determinant of tight junction permeability in endothelial cells. J. Cell Sci. 1997;110:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Itoh M., Nagafuchi A., Yonemura S., Kitani-Yasuda T., Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cellscDNA cloning and immunoelectron microscopy. J. Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Nagafuchi A., Moroi S., Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to α-catenin and actin filaments. J. Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Morita K., Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J. Biol. Chem. 1999;274:5981–5986. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- Jesaitis L.A., Goodenough D.A. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J. Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K., Solan J.L., Dominguez M., Sia M., Hand A., Lampe P.D., Laird D.W. Trafficking, assembly, and function of a Connexin43-green fluorescent protein chimera in live mammalian cells. Mol. Biol Cell. 1999;10:2033–2050. doi: 10.1091/mbc.10.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Niethammer M., Rothschild A., Jan Y.N., Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kornau H.C., Schenker L.T., Kennedy M.B., Seeburg P.H. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Leube R.E. The topogenic fate of the polytopic transmembrane proteins, synaptophysin and connexin, is determined by their membrane-spanning domains. J. Cell Sci. 1995;108:883–894. doi: 10.1242/jcs.108.3.883. [DOI] [PubMed] [Google Scholar]
- Marfatia S.M., Leu R.A., Branton D., Chishti A.H. Identification of the protein 4.1 binding interface on glycophorin C and p55, a homologue of the Drosophila discs-large tumor suppressor protein. J. Biol. Chem. 1995;270:715–719. doi: 10.1074/jbc.270.2.715. [DOI] [PubMed] [Google Scholar]
- Matter K., Balda M.S. Biogenesis of tight junctionsthe C-terminal domain of occludin mediates basolateral targeting. J. Cell Sci. 1998;111:511–519. doi: 10.1242/jcs.111.4.511. [DOI] [PubMed] [Google Scholar]
- McCarthy K.M., Skare I.B., Stankewich M.C., Furuse M., Tsukita S., Rogers R.A., Lynch R.D., Schneeberger E.E. Occludin is a functional component of the tight junction. J. Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- Rajasekaran A.K., Hojo M., Huima T., Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J. Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M., Fujimoto K., Doi Y., Itoh M., Fujimoto T., Furuse M., Takano H., Noda T., Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A., Furuse M., Saitou M., Ando-Akatsuka Y., Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J. Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth B., Fesenko I., Collins J.E., Moran B., Wild A.E., Anderson J.M., Fleming T.P. Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha+ isoform. Development. 1997;124:2027–2037. doi: 10.1242/dev.124.10.2027. [DOI] [PubMed] [Google Scholar]
- Simske J.S., Kaech S.M., Harp S.A., Kim S.K. LET-23 receptor localization by the cell junction protein LIN-7 during C. elegans vulval induction. Cell. 1996;85:195–204. doi: 10.1016/s0092-8674(00)81096-x. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Fanning A.S., Fu C., Xu J., Marfatia S.M., Chishti A.H., Crompton A., Chan A.C., Anderson J.M., Cantley L.C. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Staehelin L.A. Further observations on the fine structure of freeze-cleaved tight junctions. J. Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- Stevenson B.R., Siliciano J.D., Mooseker M.S., Goodenough D.A. Identification of ZO-1a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor F.J., Bokhari A., Rogero O., Gorczyca M., Zhang J., Kim E., Sheng M., Budnik V. Essential role for dlg in synaptic clustering of Shaker K+ channels in vivo. J. Neurosci. 1997;17:152–159. doi: 10.1523/JNEUROSCI.17-01-00152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T., Yabuki M., Otsu K., Kuzuya T., Hori M., Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J. Biol. Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- Troyanovsky S.M., Eshkind L.G., Troyanovsky R.B., Leube R.E., Franke W.W. Contributions of cytoplasmic domains of desmosomal cadherins to desmosome assembly and intermediate filament anchorage. Cell. 1993;72:561–574. doi: 10.1016/0092-8674(93)90075-2. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Luft J.H. Effects of glutaraldehyde fixation on the structure of tight junctionsa quantitative freeze-fracture analysis. J. Ultrastruct. Res. 1979;68:160–172. doi: 10.1016/s0022-5320(79)90151-5. [DOI] [PubMed] [Google Scholar]
- Van Itallie C., Anderson J.M. Occludin confers adhesiveness when expressed in fibroblasts. J. Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- van Meer G., Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO (Eur. Mol. Biol. Organ.) J. 1986;5:1455–1464. doi: 10.1002/j.1460-2075.1986.tb04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M., Uchida N., Imamura Y., Nagafuchi A., Fujimoto K., Uemura T., Vermeulen S., van R.F., Adamson E.D., Takeichi M. α-Catenin–vinculin interaction functions to organize the apical junctional complex in epithelial cells. J. Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott E., Balda M.S., Fanning A.S., Jameson B., Van Itallie C.M., Anderson J.M. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc. Natl. Acad. Sci. 1993;90:7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V., Gumbiner B.M. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J. Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]