Abstract
Integrin-associated protein (CD47) is a multiply membrane spanning member of the immunoglobulin superfamily that regulates some adhesion-dependent cell functions through formation of a complex with αvβ3 integrin and trimeric G proteins. Cholesterol is critical for the association of the three protein components of the supramolecular complex and for its signaling. The multiply membrane spanning domain of IAP is required for complex formation because it binds cholesterol. The supramolecular complex forms preferentially in glycosphingolipid-enriched membrane domains. Binding of mAb 10G2 to the IAP Ig domain, previously shown to be required for association with αvβ3, is affected by both the multiply membrane spanning domain and cholesterol. These data demonstrate that cholesterol is an essential component of the αvβ3/IAP/G protein signaling complex, presumably acting through an effect on IAP conformation.
Keywords: cholesterol, integrin, cell adhesion, plasma membrane, vitronectin
The plasma membrane contains specialized glycosphingolipid and cholesterol enriched domains that may regulate cell surface receptor signal transduction via the segregation of membrane constituents (reviewed in Simons and Ikonen 1997; Hooper 1998; Okamoto et al. 1998). These membrane domains, which can be purified because of their low density and resistance to solubilization by nonionic detergents and are therefore called detergent-insoluble glycolipid domains (DIGs)1, are enriched in glycosylphosphatidylinositol (GPI)-linked and palmitoylated membrane proteins, as well as acylated intracellular signaling molecules. It has been suggested that these DIGs are especially efficient at initiating signal transduction. However, the alternative hypothesis, that they represent sites where signaling molecules can be sequestered away from signaling stimuli, has been advanced as well (Rodgers and Rose 1996).
The integrin-associated protein (IAP, CD47) is a widely expressed 50-kD protein which is comprised of an extracellular immunoglobulin variable domain, a domain containing five membrane spanning segments, and a cytoplasmic tail that can exist in four alternatively spliced forms (Lindberg et al. 1993; Reinhold et al. 1995). IAP was initially identified through copurification with the integrin αvβ3 from human placenta (Brown et al. 1990) and was later shown to be identical to CD47 (Lindberg et al. 1994). IAP has a role in multiple αvβ3-integrin mediated functions, such as binding to vitronectin (Vn)-coated beads (Lindberg et al. 1996b), PMN activation by Arg-Gly-Asp (RGD)-containing ligands (Gresham et al. 1992; Senior et al. 1992; Van Strijp et al. 1993; Zhou and Brown 1993), the increase of intracellular calcium in endothelial cells during adhesion to fibronectin (Schwartz et al. 1993), and integrin cross-talk (Van Strijp et al. 1993; Blystone et al. 1994). Absence of IAP leads to a host defense defect manifest as increased susceptibility to Escherichia coli infection because of deficient leukocyte migration to and activation at the site of infection (Lindberg et al. 1996a). αvβ3-mediated activation also is lacking in IAP-deficient PMN, demonstrating a requirement for IAP in this signaling event. Nonetheless, αvβ3-mediated adhesion and spreading of many cells is normal in the absence of IAP (Lindberg et al. 1993, Lindberg et al. 1996b), suggesting that IAP regulates only a subset of αvβ3-mediated functions. Furthermore, IAP and αvβ3 differ from some other supramolecular receptor signaling complexes because each can be expressed at the plasma membrane without complex assembly, suggesting that both complexed and independent molecules may exist simultaneously on the same plasma membrane, thus increasing the potential complexity of mechanisms of regulation of signaling by IAP and αvβ3.
IAP also is a receptor for thrombospondin (TSP), recognizing the FYVVM sequence present in all TSP isoforms (Gao et al. 1996b). Through IAP, TSPs can modulate the function of αvβ3 on endothelial and melanoma cells (Gao et al. 1996a), αIIbβ3 on platelets (Chung et al. 1997), and α2β1 on smooth muscle cells (Wang and Frazier 1998). Recently, heterotrimeric G proteins have been identified as a third component of the αvβ3/IAP signaling complex (Frazier et al. 1999), and TSP binding to IAP affects integrin function through a pertussis-toxin sensitive Gi-dependent mechanism (Gao et al. 1996a). However, the molecular pathway through which IAP ligation affects the function of αvβ3 or any other integrin has not been determined. Here, we show that the multimolecular complex among αvβ3, IAP, and G proteins requires cholesterol for physical assembly and signal transduction and occurs preferentially in DIGs. Cholesterol interacts with IAP and affects its antigenicity, presumably inducing a conformation favorable to complex formation. These data implicate cholesterol as a fourth component of this supramolecular signaling complex, required for assembly of the proteins into an effective initiator of signal transduction. Signaling by this multimolecular membrane complex is thus closely associated with DIGs localization.
Materials and Methods
Cells, Antibodies, and Materials
The C32 human melanoma cells (American Type Culture Collection) and the human ovarian carcinoma OV10 cells were maintained as described (Gao et al. 1996a; Lindberg et al. 1996b). The following mAbs were used in this study: 2D3, B6H12, 2B7, 1F7, and 10G2 (anti-IAP, CD47; Brown et al. 1990); MAR4 (anti-β1, CD29; PharMingen); 7G2 and 1A2 (anti-β3, CD61; Brown and Goodwin 1988); P1F6 (anti-αvβ5; Wayner et al. 1991); and anti-Gβ (Upstate Biotechnology Inc.). Vn was prepared as described (Blystone et al. 1994). The amino acid sequence of the IAP-binding TSP-derived peptide 4N1K peptide is KRFYVVMWKK; it was synthesized as previously described (Kosfeld and Frazier 1992, Kosfeld and Frazier 1993).
Cell Lysis and Equilibrium Centrifugation
mAbs were iodinated using Iodobeads (Pierce Chemical Co.). Cells were preincubated with saturating levels of 125I-labeled mAbs in RPMI-1640 with 10% FCS for 30 min on ice, followed by extensive washing to remove excess antibody. Preliminary experiments including 200-fold excess unlabeled antibody showed that >98% of the bound radioactivity represented specific binding. Cells were lysed in 20 mM Tris-HCl, pH 8.2, 140 mM NaCl, 2 mM EDTA, 25 μg/ml aprotinin, 25 μg/ml leupeptin, 1 mM PMSF, and 0.5% vol/vol Brij58 for 10 min on ice, homogenized using 10 strokes of a Dounce homogenizer, then lysed 20 min more on ice. The resulting lysate was adjusted to 40% wt/wt sucrose and applied onto a 60% wt/wt sucrose cushion. A sucrose step-gradient consisting of 25% wt/wt sucrose and 5% wt/wt sucrose were layered on top of the lysate. Gradients were centrifuged 16–20 h at 170,000 g at 4°C in a SW55 rotor (Beckman Instruments, Inc.). Fractions (0.5 ml) were taken from the top of the gradient using an auto densi-flow gradient harvester (Labconco). The amount of 125I present in each fraction was measured using a Packard Crystal II γ counter. Sucrose solutions were made in buffer containing 20 mM Tris-HCl, pH 8.2, 140 mM NaCl, and 2 mM EDTA. Sucrose density was determined by refractive index using a refractometer. The amount of protein in each fraction was determined using the BCA Protein Assay Kit (Pierce Chemical Co.).
Isolation of the Supramolecular Complex
Vn or peptides were coupled to 4.5-μm tosyl-activated magnetic beads and mAb were coupled to sheep anti–mouse 4.5-μm magnetic beads (Dynal) as described by the manufacturer. Cells were resuspended in HBSS with 0.1 mM MnCl2. Substrate-coated magnetic beads were added to the cells and the sample was rotated for 15 min at 37°C. Cells bound to the magnetic beads were collected by placing the sample in a magnet. Lysis buffer (20 mM Hepes, pH 7.5, 0.1 mM MnCl2, 250 mM sucrose, 25 μg/ml aprotinin, 25 μg/ml leupeptin, 1 mM PMSF, and 10 mM CHAPS) was added and the sample was incubated with agitation for 10 min at 37°C. Magnetic beads were resuspended and incubated 5 min in HBSS with 3 mM MgCl2 and 2.5 units DNase I. 2× SDS-PAGE sample buffer was added and the samples were heated for 10 min at 65°C. The samples were analyzed by SDS-PAGE and Western blotting.
Alteration of Plasma Membrane Cholesterol Content Using Methyl-β-Cyclodextrin
Cells were resuspended at 2.5 × 105 cells/ml in RPMI/0.1% fatty acid-free BSA (Sigma Chemical Co.) with or without 5–15 mM methyl-β-cyclodextrin (MβCD; Aldrich Chemical Co.) and incubated 15 min at 37°C. After washing, cells were added to the prepared tissue culture wells and allowed to spread 30 min to 1 h at 37°C. Cholesterol was reintroduced into cells using 1.33 mg/ml cholesterol-MβCD inclusion complexes in RPMI/0.1% fatty acid-free BSA, which results in a 0.1 mM solution of cholesterol (Klein et al. 1995). To quantitate the amount of cholesterol in membranes after these treatments, cellular lipids were extracted by the method of Bligh and Dyer 1959, and cholesterol content was assayed by the cholesterol oxidase method (Wako Chemicals USA). Approximately 30% of cellular cholesterol was removed in OV10 cells by this treatment.
Preparation of Cholesterol-Methyl-β-Cyclodextrin Inclusion Complexes
Cholesterol-MβCD inclusion complexes were prepared as described (Klein et al. 1995). In brief, a solution of 30 mg cholesterol (Sigma Chemical Co.) in 400 μl 2:1 vol/vol methanol/chloroform was added drop-wise to a stirred solution of 1 g of MβCD in 11 ml PBS prewarmed in an 80°C waterbath. The solution was stirred at 80°C until a clear solution resulted. The cholesterol-MβCD inclusion complexes were used immediately or freeze-dried. Inclusion complexes of the steroid analogues were made similarly using 30 mg 5-cholestene-3-one, 20 mg 5-cholestene, or 24.7 mg pregnenolone.
Cell Spreading Assay
IAP-enhanced spreading of C32 cells on suboptimal doses of Vn was performed as described (Gao et al. 1996a). In brief, 12-well plates (Costar Corp.) were coated for 2 h at room temperature with 0.125 μg/ml Vn in HBSS. Plates were blocked with 1% BSA/PBS for 2 h at room temperature and washed three times with PBS. After MβCD treatment, C32 cells were plated in HBSS with 1 mM CaCl2 and 1 mM MgCl2 with or without the addition of 20 μM 4N1K peptide. MβCD inclusion complexes made with cholesterol or the steroid analogues were added to some wells. Cells were allowed to spread for 30 min at 37°C.
FACS® Staining
Indirect immunofluorescence was analyzed on OV10 cells with or without pretreatment with MβCD as described (Rosales et al. 1992). The primary antibodies used were 10G2, a murine mAb that detects a subset of IAP on some cells (Hermann et al. 1999), and 1F7, a murine mAb that detects all IAP (Brown et al. 1990). FITC-labeled antimurine μ chain and antimurine γ chain (Sigma Chemical Co.) was used to detect cell-bound 10G2 and 1F7, respectively.
Results
Cholesterol Is Required For IAP/αvβ3/G Protein Complex
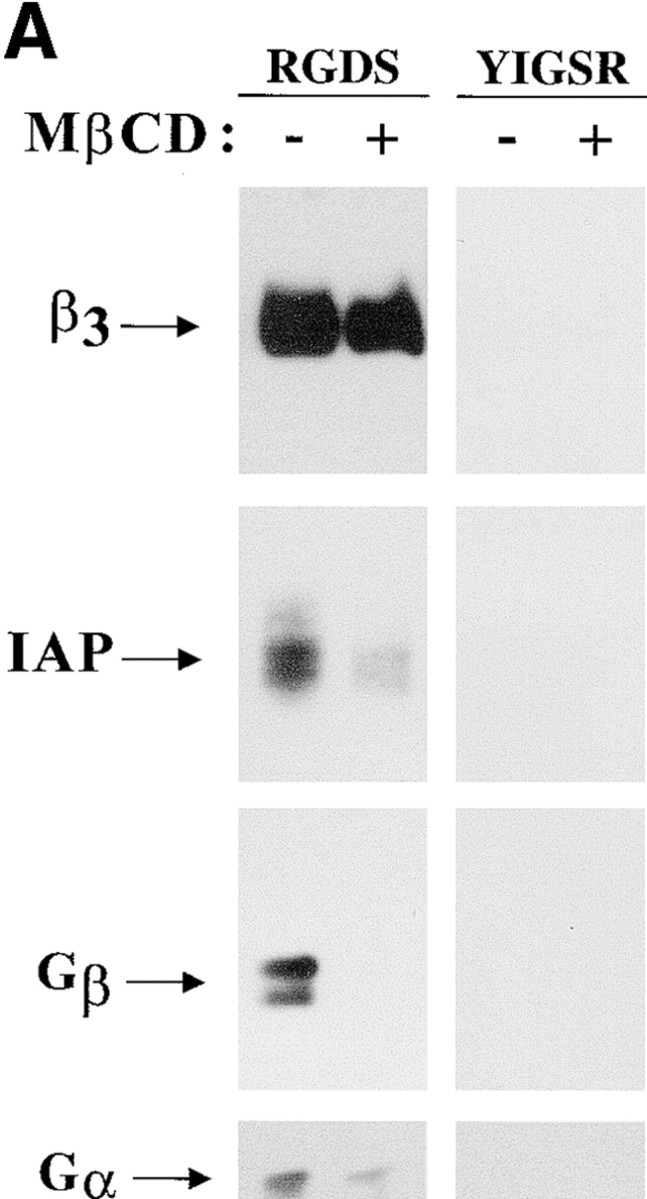
Previous studies using liposomes reconstituted with αvβ3 showed that lipid composition affected ligand binding by this integrin and that a cholesterol-containing environment markedly enhanced ligand binding (Conforti et al. 1990). Ligand binding by αvβ3 also is influenced by IAP (Gresham et al. 1992; Lindberg et al. 1996b). Because IAP likely contaminated the αvβ3 used to prepare the liposomes, we determined whether cholesterol affected αvβ3 association with IAP. Trimeric G proteins have been identified as a signal transduction component associated with IAP and αvβ3 (Frazier et al. 1999). αvβ3/IAP/G protein complexes were isolated from OV10 cells expressing both β3 integrin and IAP (Lindberg et al. 1996b), and treated in vitro with the cholesterol-chelating agent MβCD (Klein et al. 1995; Gimpl et al. 1997; Keller and Simons 1998). Treatment with MβCD markedly decreased association of both IAP and Gβ with the purified αvβ3 (Fig. 1 A). Anti-Gα Western blots showed a similar decrease in Gα association with αvβ3 (Fig. 1 A). Thus, removal of cholesterol compromised association of αvβ3 with IAP and trimeric G protein. No caveolin was identified in these isolated complexes, although OV10 cells do express caveolin (data not shown). No αvβ3, IAP, or G protein was precipitated with YIGSR-coated beads (Fig. 1 A). These control beads bind to a nonintegrin laminin receptor (Graf et al. 1987a) expressed on OV10 cells.
Figure 1.
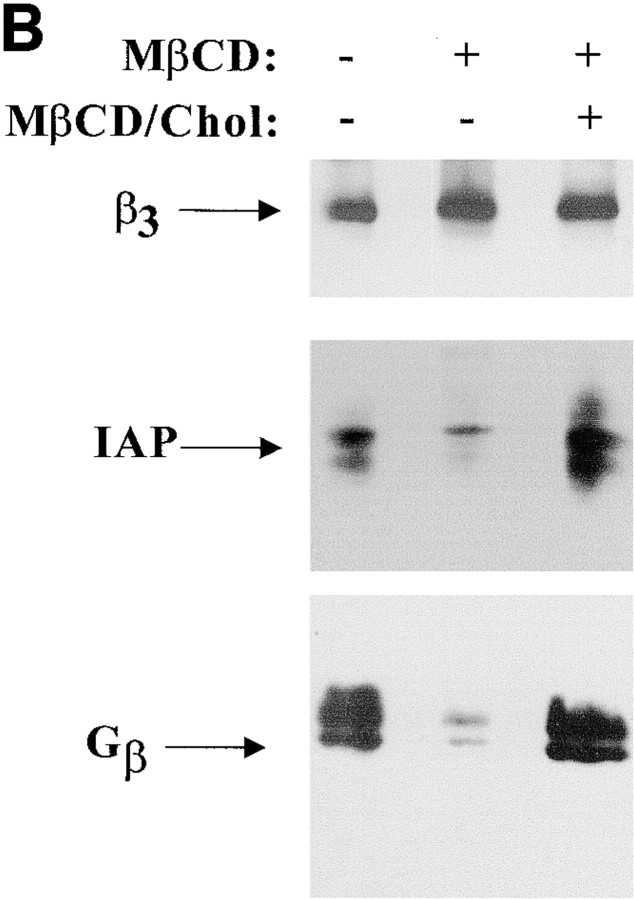
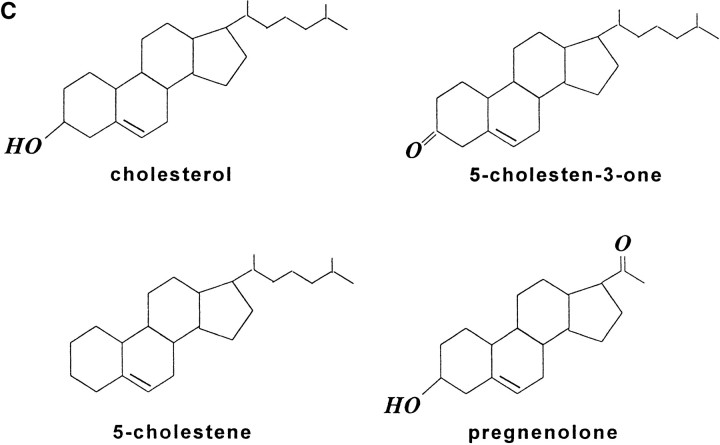
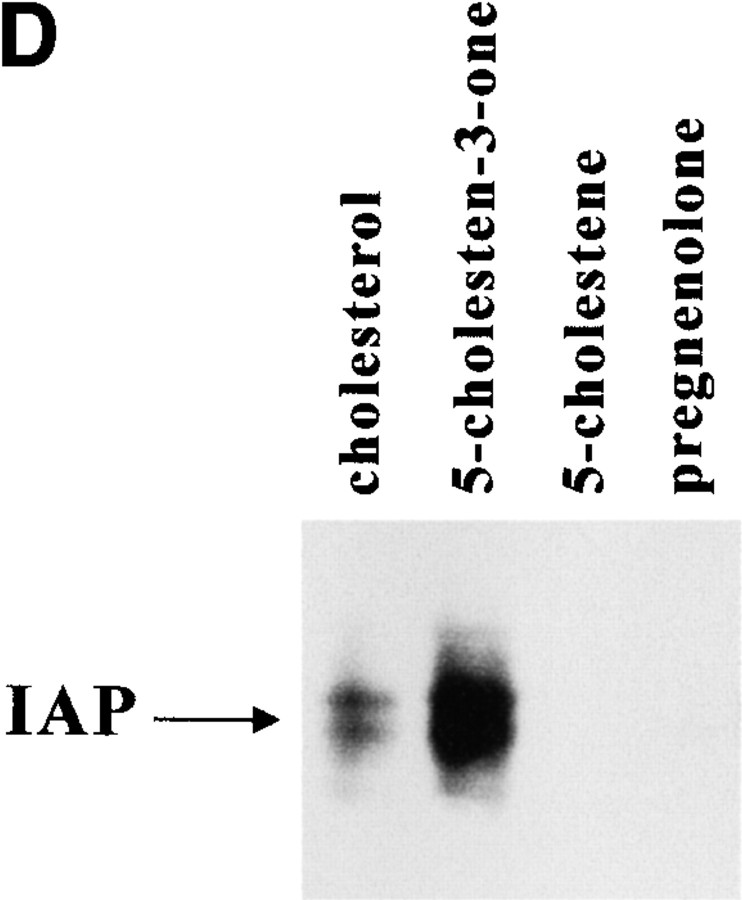
Cholesterol is in IAP/αvβ3/G protein complexes. A, Complexes of αvβ3, IAP, and G proteins from αvβ3- and IAP-expressing OV10 cells were isolated using beads coated with the αvβ3 ligand, RGDS, as described in Materials and Methods, then treated in vitro with buffer or 1 mM MβCD, and finally analyzed by SDS-PAGE and Western blotting using the β3 specific mAb 7G2, the IAP specific mAb B6H12, or rabbit antibodies specific for Gα or Gβ. Identical results were obtained with complexes obtained with Vn-coated beads or with anti-αvβ3–coated beads (data not shown). No protein reactive with any of the detecting antibodies was obtained using beads coated with the cell binding laminin peptide, YIGSR (Iwamoto et al. 1987) that does not bind integrins (Graf et al. 1987b). B, Coimmunoprecipitations were performed using beads coated with the β3 specific mAb 1A2 from buffer treated (lane 1), cholesterol-depleted (lane 2), or cholesterol-repleted (lane 3) OV10 cells expressing αvβ3 and IAP. Cholesterol depletion with MβCD and repletion with MβCD-cholesterol complexes were performed as described in Materials and Methods. No protein was immunoprecipitated using the irrelevant mAb 543 (data not shown). C, Structures of the cholesterol analogues used for preparation of the MβCD inclusion complexes. D, Coimmunoprecipitations were performed using beads coated with the β3 specific mAb 1A2 from cholesterol-depleted cells repleted with cholesterol or one of the analogues, 5-cholestene-3-one, 5-cholestene, or pregnenolone. Western blotting for IAP is shown. The amount of αvβ3 immunoprecipitated was similar for each sample, as determined by Western blotting (data not shown). Each experiment is representative of at least three repetitions.
Cholesterol also was required for complex assembly in intact cells, since little associated IAP or G protein was copurified with αvβ3 isolated from OV10 cells treated with MβCD (Fig. 1 B). Whereas MβCD removes cholesterol from cell membranes, MβCD–cholesterol inclusion complexes mediate the incorporation of cholesterol into membranes (Klein, et al. 1995). Treatment of OV10 cells with MβCD decreased cell cholesterol content 30–40%; subsequent incubation with MβCD-cholesterol inclusion complexes restored cellular cholesterol to basal levels (data not shown). In cells treated first with MβCD to disassemble the integrin/IAP/G protein signaling complexes and then incubated with cholesterol-charged MβCD to restore membrane cholesterol, IAP/αvβ3/G protein complexes were isolated to the same extent as in untreated cells (Fig. 1 B).
To examine the structural requirements for cholesterol in complex formation, inclusion complexes were made with several cholesterol analogues. Steroids with minimal structural differences to cholesterol were chosen, all having the hydrophobic, planar, core ring structure (Fig. 1 C). 5-cholestene lacks the 3β-hydroxyl group. Pregnenolone contains a methyl ketone group instead of the aliphatic tail. A carbonyl replaces the 3β-hydroxyl group in 5-cholestene-3-one. These compounds yield an equilibrium between MβCD-complexed steroid and steroid incorporated into the plasma membrane, similar to what is observed with cholesterol itself (Klein et al. 1995). Neither 5-cholestene nor pregnenolone was able to restore association of IAP/αvβ3/G protein complexes in MβCD treated cells (Fig. 1 D). However, 5-cholestene-3-one was even better than cholesterol (∼2.3-fold better by densitometry) in restoring the IAP/αvβ3 association. Thus, there is structural specificity in the lipid requirement for complex formation, with both the aliphatic tail and an oxygen in the 3 position of the cholestene ring apparently required.
Cholesterol Is Required for IAP-αvβ3 Signaling
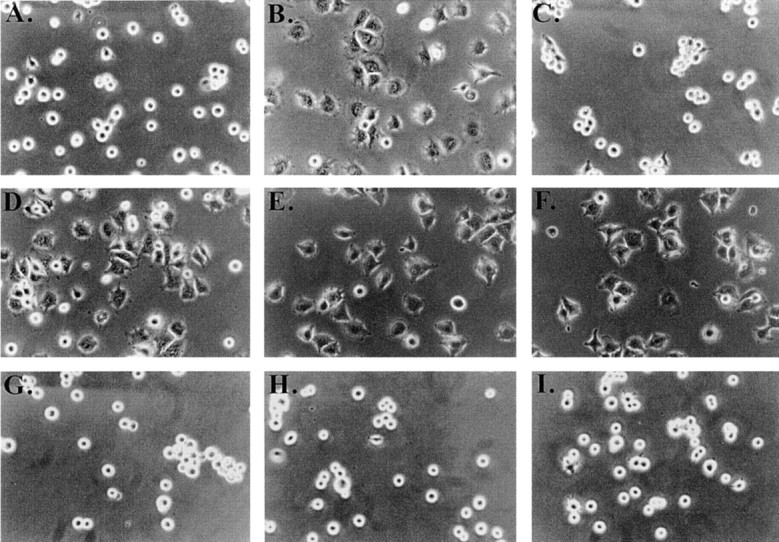
To determine the role for the cholesterol-dependent supramolecular complex in IAP/αvβ3 signaling, we examined the role of the complex in TSP modulation of αvβ3 function in C32 cells. This is an excellent model to test signaling by the complex since the COOH-terminal domain of TSP (TSP-1) has been shown to modulate αvβ3 integrin-mediated adhesion and spreading of these cells through interaction with IAP, and this effect requires activation of a heterotrimeric G protein (Gao et al. 1996a; Frazier et al. 1999). Moreover, cholesterol depletion resulted in no loss of viability and no obvious morphologic change in these cells (data not shown). Only when C32 cells were treated with the IAP-binding agonist peptide from TSP-1 (4N1K; Kosfeld and Frazier 1993; Gao et al. 1996b) did they spread on surfaces coated with low concentrations of Vn (Fig. 2A and Fig. B; Gao et al. 1996b), an effect abolished by cholesterol chelation with MβCD (Fig. 2 C). Cholesterol repletion using MβCD inclusion complexes restored IAP-dependent spreading (Fig. 2 D). In contrast, MβCD had no effect on C32 spreading on high density Vn (Fig. 2E and Fig. F), which is known to be IAP-independent (Gao et al. 1996a). None of the cholesterol analogues were able to restore 4N1K-induced spreading in MβCD treated cells (Fig. 2, G–I). The failure of 5-cholestene-3-one, which restores complex formation, to restore TSP induction of C32 cells spreading, resulted from a general inhibition of cell spreading by this cholesterol analogue, since C32 spreading on high concentration Vn was inhibited in cells repleted with this cholesterol analogue (data not shown). In contrast, neither 5-cholestene nor pregnenolone affected C32 spreading on high Vn substrates. Thus, cholesterol is required for cooperation between IAP and αvβ3 in C32 cells, but not for cell spreading in general.
Figure 2.
Effect of cholesterol depletion on spreading and tyrosine phosphorylation of C32 melanoma cells. (A–D, G–I) C32 cells were allowed to spread on plates coated with a suboptimal amount of Vn (0.125 μg/ml) for 30 min at 37°C in the absence (A) or presence (B–D, G–I) of 20 μM TSP peptide 4N1K in solution. C, Cells were pretreated with 10 mM MβCD for 15 min to remove plasma membrane cholesterol. D, Cholesterol–MβCD inclusion complexes were added to cholesterol-depleted cells to replenish plasma membrane cholesterol. E and F, Cells spreading on plates coated with 1 μg/ml Vn in the absence (E) or presence (F) of 10 mM MβCD. No 4N1K peptide was added. Spreading was complete within 30 min at 37°C, when pictures were taken. G–I, Cholesterol-depleted cells were treated with 4N1K peptide and MβCD-inclusion complexes made with the cholesterol analogues 5-cholestene-3-one (G), 5-cholestene (H), and pregnenolone (I). 5-cholestene-3-one inhibited C32 spreading on high (1 μg/ml) Vn, demonstrating a nonspecific inhibition of cell spreading, but the other two analogues had no such effect (data not shown). J, C32 cells, with or without treatment with 10 mM MβCD, were incubated on low density Vn (0.125 μg/ml) in the absence or presence of 20 μM 4N1K or the scrambled control peptide 4NGG (Gao et al. 1996b). Alternatively, cells were allowed to spread on high concentrations of Vn (1 μg/ml). After cell lysis and SDS-PAGE, Western blotting was performed using antiphosphotyrosine mAb 4G10.
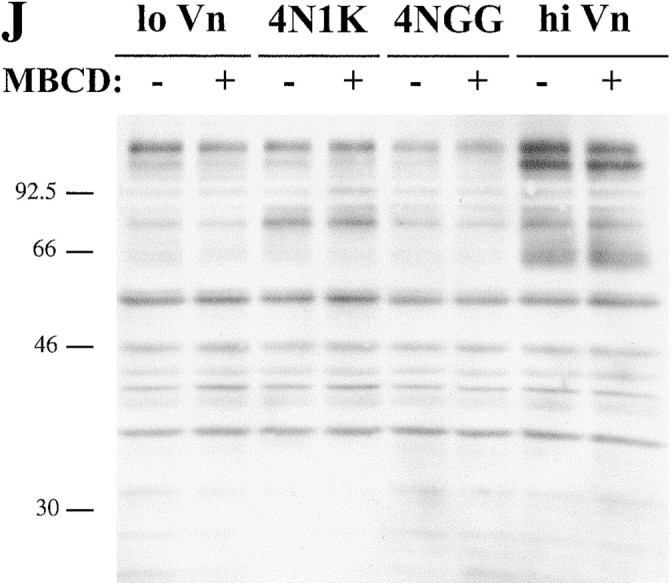
While cell spreading in response to 4N1K likely requires IAP-dependent signal transduction, the biochemical mediators of this signaling are not known (Gao et al. 1996a). Therefore, we measured 4N1K-mediated inhibition of adenylate cyclase in prostaglandin E1 (PGE1)-treated resting platelets, an event known to require heterotrimeric G protein signaling (Frazier et al. 1999). 4N1K caused an 80% decrease in cAMP levels in platelets (81.4 ± 5.2 fmol/2 × 107 cells to 16.0 ± 3.2). In MβCD-treated platelets, the cAMP was unchanged by 4N1K (33.4 ± 3.3 fmol without and 31 ± 3.2 with 4N1K). Repletion of cholesterol in MβCD-treated platelets allowed 4N1K to again induce a drop in cAMP to 18 ± 2.4 fmol, not different from the cAMP in 4N1K-treated platelets without cholesterol perturbation. The reason that MβCD caused a decrease in the basal level of cAMP in resting platelets is unknown, but may reflect a failure of PGE1 signaling, since it binds to a seven transmembrane Gs-coupled receptor that likely resides in DIGs (Kerins et al. 1991; Woodward et al. 1997). Removal of cholesterol did not abolish all cell signaling since tyrosine phosphorylation in response to both 4N1K treatment and adhesion to high concentrations of Vn was normal in MβCD treated cells (Fig. 2 J). Thus, while cholesterol depletion by MβCD does not affect integrin-dependent tyrosine phosphorylation, it does block 4N1K-initiated spreading and signaling, which are dependent on heterotrimeric G proteins.
IAP/αvβ3/G Protein Complex Requires the IAP Multiply Membrane Spanning Domain and Extracellular Domain
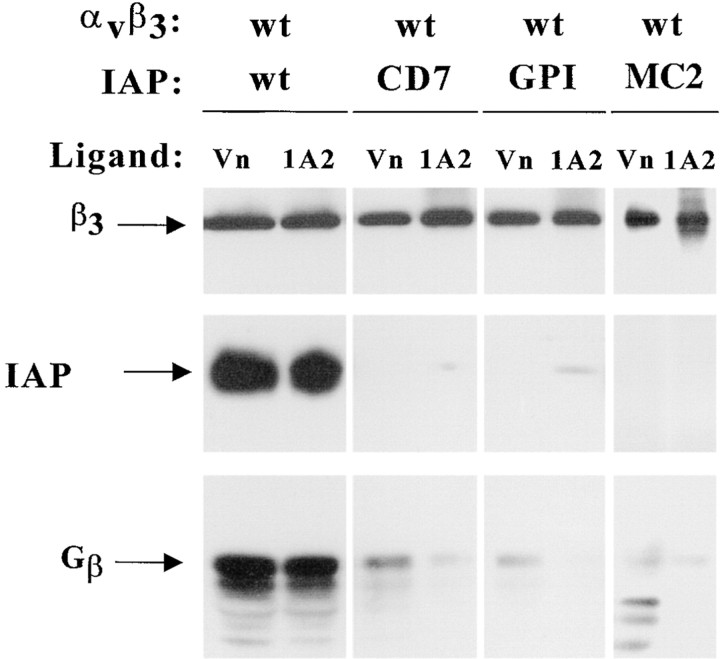
To determine how cholesterol affected assembly of the supramolecular signaling complex, we determined the role of the IAP multiply membrane spanning (MMS) domain and extracellular domain in complex formation. αvβ3 integrins were isolated from transfected OV10 cells expressing normal IAP, IAP in which the MMS domain had been replaced either by a single pass CD7 transmembrane domain (IAP/CD7) or by a glycan phosphoinositol anchor (IAP/GPI), or IAP in which the extracellular domain of IAP was replaced with a FLAG epitope (MC2). The IAP Ig domain was expressed equivalently on OV10 cells transfected with each of the Ig domain-expressing constructs (Lindberg et al. 1996b) and the MMS domain was equivalently expressed in wild-type and IAP/MC2 transfected cells, as assessed by antibody against the IAP cytoplasmic tail (data not shown). While the complex was easily isolated using either the αvβ3 ligand Vn or anti-β3 mAb (1A2) from cells expressing normal IAP, minimal IAP or G protein was copurified with integrin from cells expressing either IAP/CD7, IAP/GPI, or IAP/MC2 (Fig. 3). Furthermore, the IAP MMS domain influenced cholesterol association with αvβ3, since there was 1.7 ± 0.85-fold (P < 0.03, n = 4) more cholesterol associated with anti-β3 1A2 immunoprecipitates from OV10 cells expressing IAP than from cells deficient in IAP. In addition, there was 2.1 ± 0.46-fold (P < 0.03, n = 3) more cholesterol associated with anti-IAP immunoprecipitates from OV10 cells expressing wild-type IAP than from cells expressing either IAP/GPI or IAP/CD7. Thus, both the MMS and the Ig domains of IAP are required for complex assembly, and the MMS domain enhances cholesterol association with the αvβ3 integrin.
Figure 3.
Requirement of the IAP MMS domain for supramolecular complex formation. αvβ3 was isolated from OV10 cells expressing wild-type IAP, IAP/CD7, IAP/GPI, or IAP/MC2 using beads coated with the αvβ3 ligand Vn or the anti-β3 mAb 1A2. Samples were analyzed by SDS-PAGE and Western blotting using the β3 specific mAb 7G2, the IAP specific mAb B6H12, or rabbit antibodies specific for Gβ. mAb 131, specific for the cytoplasmic tail of IAP, was used to probe for IAP/MC2. Similar results were obtained using beads coated with the β3 ligand, RGDS (data not shown).
IAP/αvβ3/G Protein Complex Is Preferentially Formed in DIGs
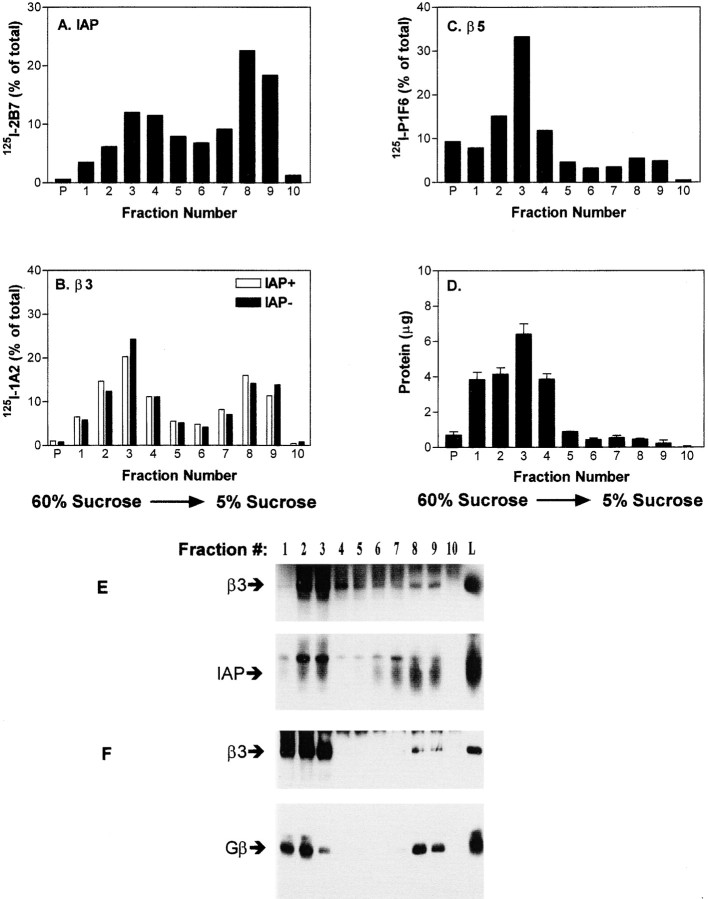
Because cholesterol and heterotrimeric G proteins are concentrated in DIGs in many cell types (Brown and Rose 1992; Sargiacomo et al. 1993), it was possible that the IAP-dependent signaling complex was preferentially formed in these domains. Sucrose density ultracentrifugation demonstrated that both αvβ3 and IAP were enriched in DIGs, which are found at the interface of the 25 and 5% sucrose layers (Fig. 4, fractions 7–9). Approximately half of IAP (Fig. 4 A) was isolated in this low density membrane fraction, together with about one-fourth of the αvβ3 (Fig. 4 B). In contrast, the closely related αvβ5 integrin localized predominantly to the bulk membranes (Fig. 4 C, fractions 2–4), together with >95% of total cellular protein (Fig. 4 D). To determine whether complex formation occurred preferentially among the IAP, αvβ3, and G proteins in DIGs, αvβ3 was purified from individual fractions of the sucrose gradient and coassociation of αvβ3, IAP, and heterotrimeric G proteins determined (Fig. 4 E). Together with 15% of the bead-bound αvβ3 (as determined by densitometry), ∼40% of the associated IAP and Gβ were coprecipitated in the DIGs. This represents a minimum estimate of DIGs-associated complex, since the purification procedure was presumably not 100% efficient. Thus, αvβ3 in DIGs was at least four times more likely to be involved in complex formation than αvβ3 in the bulk membrane fractions of the cell, demonstrating a preferential association of the complex with DIGs. To determine whether complex formation was required for localization of IAP or αvβ3 to DIGs, fractionation studies were performed on cells lacking IAP or αvβ3. In OV10, αvβ3 localized to DIGs similarly whether or not IAP was present (Fig. 4 B). In the Jurkat T lymphoid line, which expresses little if any αvβ3 (Reinhold et al. 1997) and as a consequence have no detectable αvβ3/IAP complex, >60% of the IAP was in low density fractions (data not shown). Thus, IAP and αvβ3 localize to DIGs independent of complex formation. It is already known that trimeric G proteins localize to DIGs because of acylation and/or interaction with caveolin (Sargiacomo et al. 1993; Couet et al. 1997; Mumby 1997). Thus, each of the three protein components of the complex is targeted to DIGs independent of complex formation, suggesting that DIGs are the membrane sites where these signaling complexes form.
Figure 4.
DIGs localization of components of the signaling complex. Specific radioactivity in sucrose density fractions obtained from OV10 cells labeled with 125I mAbs specific for IAP (mAb 2B7; A), αvβ3 (mAb 1A2; B), or αvβ5 (mAb P1F6; C) are shown. Data is presented as a percentage of the total input counts in each fraction. In B, distribution of radioactivity in cells with and without IAP are compared. D, Distribution of total protein, measured by the BCA assay. The sucrose concentration is highest in fraction 1 and least in fraction 10. P denotes radioactivity or protein in the pellet. DIGs migrate to the interface of the 25 and 5% sucrose solutions (fraction 7–9), while the vast majority of membrane protein is contained within the 40% sucrose fractions. Data is representative of three or more experiments. E and F, αvβ3 was immunoprecipitated with mAb 1A2 from each fraction of a sucrose density gradient prepared with unlabeled OV10 cells expressing wild-type IAP. The samples were analyzed by SDS-PAGE and Western blotting using anti-β3 mAb 7G2 and anti-IAP mAb B6H12 (E) or anti-β3 and rabbit antibodies specific for Gβ (F).
DIGs Localization Is Not Sufficient for Complex Formation
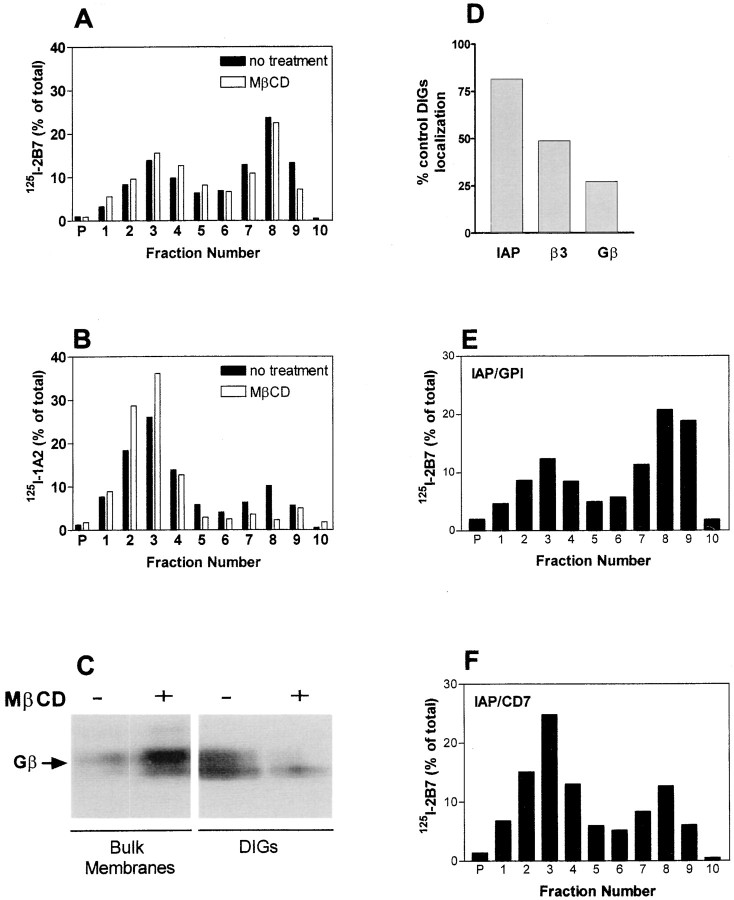
To determine the effect of cholesterol removal on localization of the protein components of the complex to DIGs, OV10 were treated with MβCD before sucrose density gradient centrifugation. The localization of IAP was minimally affected by MβCD treatment (Fig. 5 A). However, both αvβ3 (Fig. 5 B) and Gβ (Fig. 5 C) localization were substantially diminished (Fig. 5 D). To determine if DIGs localization of each of the components was sufficient for complex formation, the membrane localization of IAP/CD7 (Fig. 5 E) and IAP/GPI (Fig. 5 F), both of which failed to form complexes, was determined. While less IAP/CD7 was in DIGs, IAP/GPI localized to the DIGs to a similar extent to wild-type IAP (see Fig. 4 A and 5A). This is consistent with the known propensity of GPI-linked proteins to be enriched in DIGs (Brown and Rose 1992; Sargiacomo et al. 1993; Friedrichson and Kurzchalia 1998; Varma and Mayor 1998). Since complexes did not form in cells expressing IAP/GPI (Fig. 3) despite the ability of the IAP Ig domain to mediate interaction with αvβ3 (Lindberg et al. 1996b), this result demonstrates that localization of the IAP Ig domain to DIGs is not sufficient for complex formation and that the MMS domain of IAP must serve another function in complex formation. Furthermore, this experiment demonstrates that the protocol for isolating the αvβ3/IAP/G protein complex does not simply nonspecifically copurify all proteins found in DIGs. However, DIGs localization of each component of the complex may be necessary for complex formation. Since cholesterol is an essential component of the complex, DIGs localization may provide a mechanism for focusing the proteins of the complex at a site where an adequate concentration of cholesterol exists for complex assembly.
Figure 5.
Effect of cholesterol depletion on DIGs localization of signaling components. A–D, OV10 cells expressing IAP treated with or without 10 mM MβCD before Brij lysis and sucrose density centrifugation. Distribution of 125I-labeled anti-IAP mAb 2B7 (A) or anti-β3 mAb 1A2 (B) was determined as described in Fig. 4. C, Bulk membranes (fractions 2–4 combined from a sucrose gradient) and DIGs containing fractions (fractions 7–9 combined) were analyzed by SDS-PAGE and Western blotting using rabbit antibodies specific for Gβ after sucrose density fractionation of unlabeled cells. D, The percent of IAP, β3, and Gβ remaining in the DIGs-containing fractions 7–9 after MβCD treatment. Percentage of total counts was used to determine the amount of IAP or β3 present, and densitometry of Western blots was used to determine the amount of Gβ present. E and F, Distribution of IAP Ig domain-expressing molecules after sucrose density centrifugation of Brij-lysed OV10 cells expressing IAP/GPI (E) or IAP/CD7 (F) was determined as described in Fig. 4.
Cholesterol Affects IAP Expression of the 10G2 Epitope
A previous study suggested that the interaction between IAP and αvβ3 could occur through the IAP Ig domain (Lindberg et al. 1996b). Thus, it was possible that cholesterol interaction with the IAP MMS domain could affect the IAP conformation to facilitate complex formation. To determine whether any cholesterol-dependent conformation of IAP could be discovered, FACS® analysis was performed with 10G2, a mAb that recognizes only a subset of IAP on many cells (Hermann et al. 1999). 10G2 recognizes the IAP Ig domain, as demonstrated by ELISA and Western blotting with purified Ig domain (data not shown). On OV10 cells, 10G2 recognized wild-type IAP approximately fourfold better than IAP/CD7 and did not bind IAP/GPI at all (Fig. 6, top). This difference in detection was not due to differences in expression, as detected by the conventional anti-IAP mAb B6H12, which detects equivalent expression of the three constructs (Lindberg et al. 1996b; data not shown). When OV10 expressing wild-type IAP were treated with MβCD, 10G2 binding increased another sixfold (Fig. 6, middle). Repletion of cellular cholesterol with MβCD/cholesterol complexes returned 10G2 binding to the level of untreated cells. MβCD did not affect 10G2 binding to IAP/CD7 or IAP/GPI (data not shown). Furthermore, MβCD did not affect the binding of the anti-IAP mAbs 2D3 or 2B7 (Fig. 6, bottom). These data demonstrate that the availability of the 10G2 epitope on the IAP Ig domain on OV10 cells is markedly influenced by the MMS domain and is significantly modulated by cholesterol. Its ability to modulate 10G2 binding to the Ig domain suggests that cholesterol binding to the IAP MMS domain affects IAP conformation.
Figure 6.
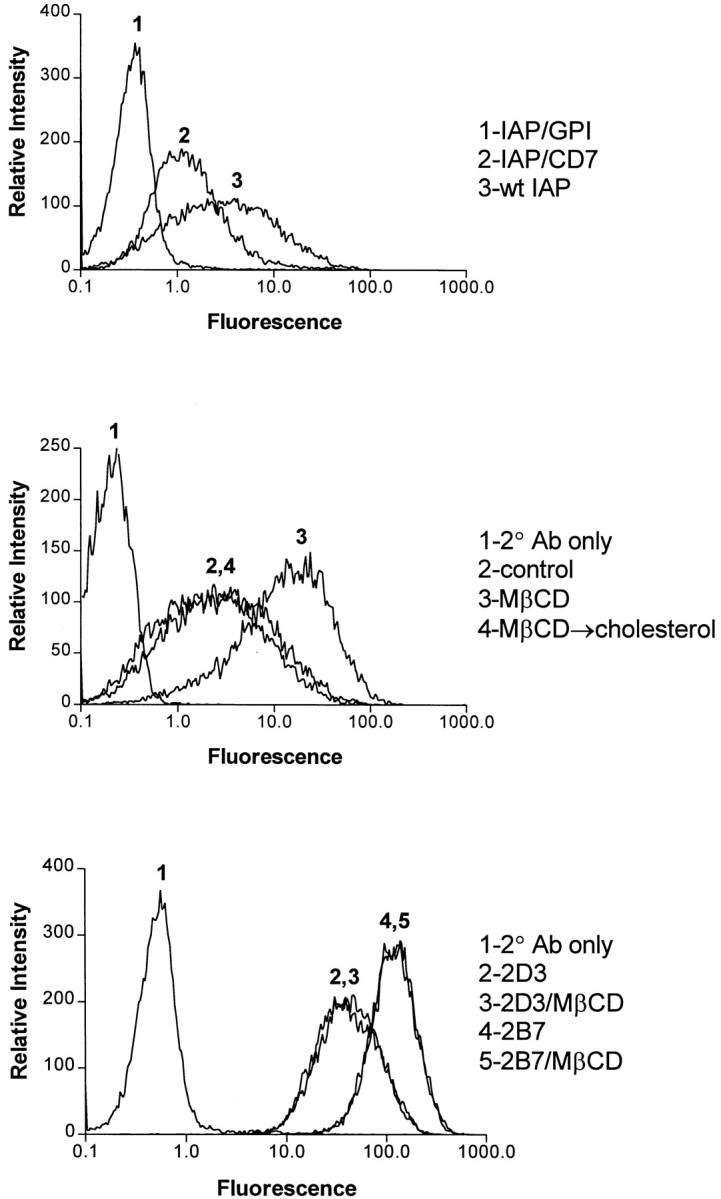
Binding of mAb 10G2 to IAP. Top, OV10 cells expressing IAP, IAP/GPI, or IAP/CD7 were incubated with the mAb 10G2. FACS® analysis with anti-IAP mAb B6H12 showed that both IAP/GPI and IAP/CD7 were expressed at a level at least equivalent to that of wild-type IAP (mean channel fluorescence wild-type IAP, 3.8 ± 0.1; IAP/GPI, 6.1 ± 0.3; IAP/CD7, 11.7 ± 1.4; Lindberg et al. 1996b; data not shown). Middle, 10G2 binding was determined on buffer-treated OV10, cholesterol-depleted OV10, and cholesterol-repleted OV10 expressing wild-type IAP. Bottom, Binding of conventional anti-IAP mAb 2D3 and 2B7 (Brown et al. 1990) was determined on buffer-treated and MβCD-treated OV10 expressing wild-type IAP.
Discussion
IAP is a plasma membrane protein first isolated by copurification with αv integrins. Subsequent studies have demonstrated a functional association of IAP with β3 integrins in leukocytes and endothelial cells (Senior et al. 1992; Schwartz et al. 1993; Van Strijp et al. 1993; Zhou and Brown 1993) and coimmunoprecipitation of IAP with αvβ3 from several cells and tissues (Brown et al. 1990). Moreover, IAP is required for Vn bead binding by both αvβ3 and αvβ5 integrins in OV10 cells (Lindberg et al. 1996b). However, IAP appears to be unnecessary for adhesion of these same cells to Vn-coated surfaces and IAP-deficient mice develop normally, in contrast to αv-deficient mice, demonstrating that IAP is not absolutely required for αv integrin function. This has led to the hypothesis that IAP and αvβ3 form a signaling complex in at least some cells that can influence specific cell functions. Recently, association of αvβ3 and IAP with trimeric G proteins has been demonstrated (Frazier et al. 1999), suggesting a mechanism by which the αvβ3/IAP complex may signal. However, the molecular mechanisms involved in association of αvβ3 with IAP or of this membrane complex with G proteins have not been resolved.
An unusual feature of IAP is its MMS domain, which could allow for enhanced interactions with and regulation by membrane lipids. To determine whether this was the case, we examined whether cholesterol was required for association of IAP with αvβ3 integrin and with trimeric G proteins. Although the removal of cholesterol with MβCD has been shown to disrupt receptor signaling in a variety of cells (Fernandez-Ballester et al. 1994; Gimpl et al. 1997; Xavier et al. 1998; Zhang et al. 1998; Sheets et al. 1999a), a specific role for cholesterol in the formation of supramolecular receptor complexes or in regulation of integrin function has not been demonstrated previously. We found that cholesterol is required both for maintenance of complexes in cells and for maintenance of isolated complexes in vitro. Thus, cholesterol is the fourth molecular and first described nonprotein component of this signaling complex. Likely, the cholesterol interacts with the IAP MMS domain, since replacement with a single transmembrane domain in IAP/CD7 or with a GPI-anchor in IAP/GPI abolished complex formation as efficiently as cholesterol removal. Moreover, removal of cholesterol or replacement of the MMS domain with CD7 also abolished functional responses to IAP ligation in Jurkat T cells and murine fibroblasts (unpublished data). Thus, cholesterol is required not only for physical association of IAP with integrins and G proteins, but for integrin-independent functions of IAP as well.
Recently, the understanding has emerged that there are distinct domains within the lipid bilayer in which specific lipids are concentrated (Brown and Rose 1992; Sargiacomo et al. 1993). Because they can be purified and studied due to their low density and relative resistance to solubilization by some detergents, domains enriched in glycosphingolipids and cholesterol are the best characterized (Brown and Rose 1992; Sargiacomo et al. 1993). These domains are called DIGs, GEMs (for glycosphingolipid-enriched membranes), or rafts, to reflect these properties. In addition to lipids, these domains are enriched in specific integral membrane proteins, of which caveolin is the best known (Kurzchalia et al. 1992). However, it is now clear that caveolin is not required for organization of the domains and DIGs can exist even in cells lacking caveolin (Fra et al. 1994). In addition to caveolin, DIGs contain disproportionately high concentrations of GPI-linked membrane proteins, as well as a variety of cytoplasmic proteins that associate with the plasma membrane through lipid modifications, including NH2-terminal myristoylation and/or palmitoylation (Simons and Ikonen 1997). In this latter category are a variety of signal transduction molecules, such as heterotrimeric G proteins and some src family kinases, which have been shown to localize to these domains (Xavier et al. 1998). However, the significance of domain localization is somewhat controversial. While specialization of these domains as sites for initiation of signal transduction has been proposed (Field et al. 1995; Xavier et al. 1998; Zhang et al. 1998), so has the opposite, that these are sites where signal transduction proteins can be sequestered in inactive form (Rodgers and Rose 1996).
IAP and αvβ3 both preferentially localize to DIGs and their association is greatly enhanced in these domains. This may imply that the functional signaling complex localizes to these membrane domains and that DIGs are essential for signal propagation across the membrane, perhaps because of proximity to other cytoplasmic molecules required for the signaling cascade. Alternatively, the localization to DIGs may simply reflect the tight association with cholesterol that apparently characterizes the complex. However, it is clear that localization to DIGs is not sufficient for stable physical association, since IAP/GPI, which localizes to DIGs equally as well as wild-type IAP does not participate in complex formation. Moreover, removal of cholesterol fails to disrupt localization of IAP to DIGs, even though the functional association among integrin, IAP, and G proteins is disrupted, and IAP no longer functions in integrin-mediated spreading. It is possible that a particularly tight or specific association of IAP with cholesterol would require more extensive depletion of cholesterol than is achieved with 10 mM MβCD to affect localization.
It is interesting that localization of IAP to DIGs and formation of stable complexes with αvβ3 and G proteins appears not to be required for IAP enhancement of αv integrin bead binding, since both IAP/CD7 and IAP/GPI can mediate this effect of IAP (Lindberg et al. 1996b). This suggests that IAP's regulation of αvβ3 ligand binding does not depend on its signaling capacity, but relies instead on direct interaction of its Ig domain with αv integrins, potentially altering the Vn-binding ability of these integrins. It is possible that this effect of IAP does not require stable association with the integrin and that once Vn binding is activated, the αvβ3 integrin can bind ligand tightly without IAP (Orlando and Cheresh 1991). Nonetheless, these experiments suggest that the IAP Ig domain is an important component of the interaction of IAP with αvβ3. Consistent with this, no complex formation can be demonstrated in cells transfected with a chimeric molecule in which the IAP Ig domain has been replaced with an irrelevant domain. Based on these observations, we propose a model in which the conformation of IAP is influenced by the MMS domain and further influenced by the tight binding of cholesterol to the MMS domain. This is consistent with the pattern of binding of the 10G2 mAb to OV10-expressed IAP, for which the MMS domain is required and mAb binding enhanced by cholesterol removal. In this model, the cholesterol-replete conformation of IAP can interact with αvβ3, and for this, the Ig domain is required. This entity, in turn, associates with the trimeric G protein to form the complete signaling complex. Since there is specificity in the ability of cholesterol analogues to mediate IAP-αvβ3 complex formation, it will be interesting to determine whether the failure of some analogues to mediate complex formation reflects failure to interact with IAP or failure to induce the change in IAP conformation and function.
In summary, we have demonstrated that cholesterol has a critical role in assembly of the αvβ3/IAP/G protein signaling complex. This is a novel role for a membrane lipid and suggests a new and direct mechanism for regulation of signal transduction by supramolecular complexes in the plasma membrane. It has been shown that cell activation signals can regulate the association of the high affinity Fc∈ receptor with DIGs (Sheets et al. 1999b). It is possible that IAP association with these cholesterol rich domains also is regulated and that this, in turn, affects formation and maintenance of the signaling complex containing αvβ3 and heterotrimeric G proteins.
Acknowledgments
The authors gratefully acknowledge helpful discussions with Linda Pike and Maureen Linder (Washington University School of Medicine).
This work was supported by National Institutes of Health grants GM38330 and AI24674 (to E.J. Brown), GM54390 (to W.A. Frazier), and GM57573 (to F.P. Lindberg). J.M. Green was a Markey Foundation Fellow during the pursuit of these studies.
Footnotes
1.used in this paper: DIG, detergent-insoluble glycolipid domain; GPI, glycosylphosphatidylinositol; IAP, integrin-associated protein (CD47); MβCD, methyl-β-cyclodextrin; MMS, multiply membrane spanning; TSP, thrombospondin; Vn, vitronectin
References
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blystone S.D., Graham I.L., Lindberg F.P., Brown E.J. Integrin alpha-v beta-3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor alpha-5 beta-1. J. Cell Biol. 1994;127:1129–1137. doi: 10.1083/jcb.127.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.A., Rose J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown E.J., Goodwin J.L. Fibronectin receptors of phagocytescharacterization of the Arg-Gly-Asp binding proteins of human monocytes and polymorphonuclear leukocytes. J. Exp. Med. 1988;167:777–793. doi: 10.1084/jem.167.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.J., Hooper L., Ho T., Gresham H.D. Integrin-associated proteina 50-kD plasma membrane antigen physically and functionally associated with integrins. J. Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Gao A.G., Frazier W.A. Thrombospondin acts via integrin-associated protein to activate the platelet integrin αIIbβ3 . J. Biol. Chem. 1997;272:14740–14746. doi: 10.1074/jbc.272.23.14740. [DOI] [PubMed] [Google Scholar]
- Conforti G., Zanetti A., Pasquali-Ronchetti I., Quaglino D., Jr., Neyroz P., Dejana E. Modulation of vitronectin receptor binding by membrane lipid composition. J. Biol. Chem. 1990;265:4011–4019. [PubMed] [Google Scholar]
- Couet J., Li S., Okamoto T., Ikezu T., Lisanti M.P. Identification of peptide and protein ligands for the caveolin–scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ballester G., Castresana J., Fernandez A.M., Arrondo J.L., Ferragut J.A., Gonzalez-Ros J.M. Role of cholesterol as a structural and functional effector of the nicotinic acetylcholine receptor. Biochem. Soc. Trans. 1994;22:776–780. doi: 10.1042/bst0220776. [DOI] [PubMed] [Google Scholar]
- Field K.A., Holowka D., Baird B. Fc epsilon RI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc. Natl. Acad. Sci. USA. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra A.M., Williamson E., Simons K., Parton R.G. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- Frazier W.A., Gao A.-G., Dimitry J., Chung J., Brown E.J., Lindberg F.P., Linder M.E. The thrombospondin receptor integrin-associated protein (CD47) functionally couples to heterotrimeric Gi. J. Biol. Chem. 1999;274:8554–8560. doi: 10.1074/jbc.274.13.8554. [DOI] [PubMed] [Google Scholar]
- Friedrichson T., Kurzchalia T.V. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- Gao A.-G., Lindberg F.P., Dimitry J.M., Brown E.J., Frazier W.A. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein J. Cell Biol. 135 1996. 533 544a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A.-G., Lindberg F.P., Finn M.B., Blystone S.D., Brown E.J., Frazier W.A. Integrin associated protein is a receptor for the carboxy terminal domain of thrombospondin J. Biol. Chem. 271 1996. 21 24b [DOI] [PubMed] [Google Scholar]
- Gimpl G., Burger K., Fahrenholz F. Cholesterol as modulator of receptor function. Biochemistry. 1997;36:10959–10974. doi: 10.1021/bi963138w. [DOI] [PubMed] [Google Scholar]
- Graf J., Iwamoto Y., Sasaki M., Martin G.R., Klienman H.K., Robey F.A., Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding Cell. 48 1987. 989 996a [DOI] [PubMed] [Google Scholar]
- Graf J., Ogle R.C., Robey F.A., Sasaki M., Martin G.R., Yamada Y., Kleinman H.K. A pentapeptide from the laminin B1 chain mediates cell adhesion and binds the 67,000 laminin receptor Biochemistry. 26 1987. 6896 6900b [DOI] [PubMed] [Google Scholar]
- Gresham H.D., Adams S.P., Brown E.J. Ligand binding specificity of the leukocyte response integrin expressed by human neutrophils. J. Biol. Chem. 1992;267:13895–13902. [PubMed] [Google Scholar]
- Hermann P., Armant M., Brown E., Rubio M., Ishihara H., Ulrich D., Caspary R.G., Lindberg F.P., Armitage R., Maliszewski C. The vitronectin receptor and its associated cd47 molecule mediates proinflammatory cytokine synthesis in human monocytes by interaction with soluble CD23. J. Cell Biol. 1999;144:767–775. doi: 10.1083/jcb.144.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N.M. Membrane biologydo glycolipid microdomains really exist? Curr. Biol. 1998;8:R114–R116. doi: 10.1016/s0960-9822(98)70984-4. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y., Robey F.A., Graf J., Sasaki M., Kleinman H.K., Yamada Y., Martin G.R. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Keller P., Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerins D.M., Murray R., FitzGerald G.A. Prostacyclin and prostaglandin E1molecular mechanisms and therapeutic utility. Prog. Hemostasis Thromb. 1991;10:307–337. [PubMed] [Google Scholar]
- Klein U., Gimpl G., Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 1995;34:13784–13793. doi: 10.1021/bi00042a009. [DOI] [PubMed] [Google Scholar]
- Kosfeld M.D., Frazier W.A. Identification of active peptide sequences in the carboxyl-terminal cell binding domain of human thrombospondin-1. J. Biol. Chem. 1992;267:16230–16236. [PubMed] [Google Scholar]
- Kosfeld M.D., Frazier W.A. Identification of a new cell adhesion motif in two homologous peptides from the COOH-terminal cell binding domain of human thrombospondin. J. Biol. Chem. 1993;268:8808–8814. [PubMed] [Google Scholar]
- Kurzchalia T.V., Dupree P., Parton R.G., Kellner R., Virta H., Lehnert M., Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi network-derived transport vesicles. J. Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F.P., Gresham H.D., Schwarz E., Brown E.J. Molecular cloning of integrin-associated proteinan immunoglobulin family member with multiple membrane spanning domains implicated in alpha-v, beta-3-dependent ligand binding. J. Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F.P., Lublin D.M., Telen M.J., Veile R.A., Miller Y.E., Donis-Keller H., Brown E.J. Rh-related antigen CD47 is the signal-transducer integrin associated protein. J. Biol. Chem. 1994;269:1567–1570. [PubMed] [Google Scholar]
- Lindberg F.P., Bullard D.C., Caver T.E., Gresham H.D., Beaudet A.L., Brown E.J. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice Science. 274 1996. 795 798a [DOI] [PubMed] [Google Scholar]
- Lindberg F.P., Gresham H.D., Reinhold M.I., Brown E.J. Integrin-associated protein immunoglobulin domain is necessary for efficient vitronectin bead binding J. Cell Biol. 134 1996. 1313 1322b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S.M. Reversible palmitoylation of signaling proteins. Curr. Opin. Cell Biol. 1997;9:148–154. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Schlegel A., Scherer P.E., Lisanti M.P. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Orlando R.A., Cheresh D.A. Arginine-glycine-aspartic acid binding leading to molecular stabilization between integrin alpha v beta 3 and its ligand. J. Biol. Chem. 1991;266:19543–19550. [PubMed] [Google Scholar]
- Reinhold M.I., Lindberg F.P., Plas D., Reynolds S., Peters M.G., Brown E.J. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47) J. Cell Sci. 1995;108:3419–3425. doi: 10.1242/jcs.108.11.3419. [DOI] [PubMed] [Google Scholar]
- Reinhold M.I., Lindberg F.P., Kersh G.J., Allen P.M., Brown E.J. Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway. J. Exp. Med. 1997;185:1–11. doi: 10.1084/jem.185.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W., Rose J.K. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J. Cell Biol. 1996;135:1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales C., Gresham H.D., Brown E.J. Expression of the 50kD integrin associated protein on myeloid cells and erythrocytes. J. Immunol. 1992;149:2759–2764. [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M.P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J. Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.A., Brown E.J., Fazeli B. A 50 kDa integrin-associated protein is required for integrin-regulated calcium entry in endothelial cells. J. Biol. Chem. 1993;268:19931–19934. [PubMed] [Google Scholar]
- Senior R.M., Gresham H.D., Griffin G.L., Brown E.J., Chung A.E. Entactin stimulates neutrophil adhesion and chemotaxis through interactions between its Arg-Gly-Asp (RGD) domain and the leukocyte response integrin (LRI) J. Clin. Invest. 1992;90:2251–2257. doi: 10.1172/JCI116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets E.D., Holowka D., Baird B. Critical role for cholesterol in lyn-mediated tyrosine phosphorylation of Fc∈RI and their association with detergent-resistant membranes J. Cell Biol. 145 1999. 877 887a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets E.D., Holowka D., Baird B. Membrane organization in immunoglobulin E receptor signaling Curr. Opin. Chem. Biol. 3 1999. 95 99b [DOI] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Van Strijp J.A.G., Russell D.G., Tuomanen E., Brown E.J., Wright S.D. Ligand specificity of purified complement receptor type 3 (CD11b/CD18, Mac-1, alphaM beta2)indirect effects of an Arg-Gly-Asp sequence. J. Immunol. 1993;151:3324–3336. [PubMed] [Google Scholar]
- Varma R., Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Wang X.Q., Frazier W.A. The thrombospondin receptor CD47 (IAP) modulates and associates with alpha-2-beta-1 integrin in vascular smooth muscle cells. Mol. Biol. Cell. 1998;9:865–874. doi: 10.1091/mbc.9.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E.A., Orlando R.A., Cheresh D.A. Integrins αvβ3 and αvβ5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J. Cell Biol. 1991;113:919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward D.F., Regan J.W., Lake S., Ocklind A. The molecular biology and ocular distribution of prostanoid receptors. Surv. Ophthalmol. 1997;41:S15–S21. doi: 10.1016/s0039-6257(97)80003-3. [DOI] [PubMed] [Google Scholar]
- Xavier R., Brennan T., Li Q., McCormack C., Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Zhang W., Trible R.P., Samelson L.E. LAT palmitoylationits essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- Zhou M.-J., Brown E.J. Leukocyte response integrin and integrin associated protein act as a signal transduction unit in generation of a phagocyte respiratory burst. J. Exp. Med. 1993;178:1165–1174. doi: 10.1084/jem.178.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]