Abstract
For nearly as long as lithium has been in clinical use for the treatment of bipolar disorder, depression, and other conditions, investigators have attempted to characterize its effects on behaviors in rodents. Lithium consistently decreases exploratory activity, rearing, aggression, and amphetamine-induced hyperlocomotion; and it increases the sensitivity to pilocarpine-induced seizures, decreases immobility time in the forced swim test, and attenuates reserpine-induced hypolocomotion. Lithium also predictably induces conditioned taste aversion and alterations in circadian rhythms. The modulation of stereotypy, sensitization, and reward behavior are less consistent actions of the drug. These behavioral models may be relevant to human symptoms and to clinical endophenotypes. It is likely that the actions of lithium in a subset of these animal models are related to the therapeutic efficacy, as well the side effects, of the drug. We conclude with a brief discussion of various molecular mechanisms by which these lithium-sensitive behaviors may be mediated, and comment on the ways in which rat and mouse models can be used more effectively in the future to address persistent questions about the therapeutically relevant molecular actions of lithium.
Keywords: mood stabilizer, antidepressant, animal model, endophenotype, bipolar disorder, manic-depressive illness, mania, depression, rat, mouse
I. Introduction
The development of novel therapeutics for bipolar disorder, as well as other mood disorders, has been hindered by limited knowledge both of the underlying neurobiology of the disorders, and of how the most useful medications actually exert their beneficial effects (Gould et al., 2004; Quiroz et al., 2004). Without a firm understanding of these issues, new treatments for mood disorders are not likely to be discovered by any method other than testing medications previously approved for other conditions (such as antipsychotics and anticonvulsants), or by pure serendipity. Thus, ongoing studies to elucidate both the complex etiologies of these disorders and the relevant direct and downstream actions of mood-stabilizers hold the promise of important future discoveries.
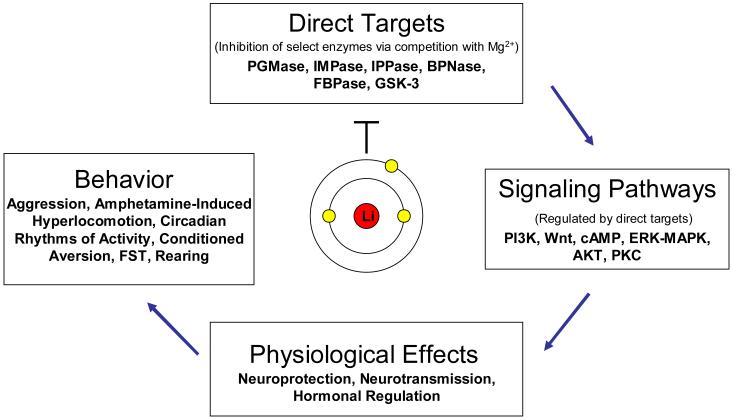
Over the past five decades, preclinical studies have investigated the actions of lithium on a number of levels, including biochemical (identifying either direct targets or secondary signaling pathways), physiological, and behavioral effects of the drug (Figure 1). However, we have yet to determine with certainty how lithium exerts its therapeutic effects. Much of the work on the behavioral actions of lithium was accomplished early in the history of the drug (Smith, 1980). The advanced techniques that have since developed have given us greater insight into why certain behaviors are elicited or modified upon administration of lithium, and biochemical studies suggest that the targets relevant to the behavioral actions of this cation could soon be decisively identified. The behavioral actions of lithium have received far less attention recently than the biochemical actions; however, we are likely currently in a situation where, using novel genetic and pharmacological approaches, we can more firmly relate the behavioral and biochemical actions of lithium to one another, and perhaps even to the direct action of the drug. By integrating the knowledge gathered from modern techniques with behaviors that have long been familiar but incompletely understood, we are armed with a new approach with which to tackle behavioral complexity.
Figure 1. Lithium: loci of preclinical research.
The mechanism by which lithium exerts its therapeutic effects is investigated from a number of perspectives. The cation directly inhibits a select group of enzymes, included in which are inositol monophosphatase (IMPase), inositol polyphosphate 1-phosphatase (IPPase), phosphoglucomutase (PGMase), biphosphate nucleotidase (BPNase), fructose 1,6-biphosphatase (FBPase), and glycogen synthase kinase-3 (GSK-3). The study of these direct targets can provide insight to the ability of lithium to modulate intracellular signaling cascades, the downstream consequences of which are its effects on physiology and behavior. Ideally, the study of behavior will in turn provide insight on which of the immediate targets of lithium is relevant to its therapeutic effects. Abbreviations: cAMP: cyclic adenosine monophosphate; ERK-MAPK: extracellular signal-regulated kinase-mitogen-activated protein kinase; FST: forced swim test; PI3K: phosphatidylinositol 3-kinase; PKC: protein kinase C.
The Continued Utility of Animal Models
As our knowledge and tools have developed, we have increasingly recognized the diagnosis heterogeneity inherent within our current psychiatric classification system, the American Psychiatric Association Diagnostic and Statistical Manual (Charney et al., 2002). At the present time, the search for a complete animal model of a particular disorder may be as equally naïve as a classification system not based upon underlying neurobiology, genetics, or response to medications; no single genetic mutation or behavior it might elicit will reveal all the intricacies behind a human syndrome. However, if animal models are to make a contribution to the long process of unraveling such diseases, their relevance must be assessed in light of observations and data obtained from human patients (Matthews et al., 2005). The endophenotype approach to deconstruct complex diseases has been increasingly utilized in psychiatric research (Gottesman and Shields, 1973; Gottesman and Gould, 2003). This approach identifies a quantifiable measure—neuropsychological or electrophysiological are two examples—which is ideally strongly influenced by genes known to be associated with a given illness. It is an intermediate phenotype, found in (perhaps only a subset of) patients with a disorder, which can begin to unfold the convoluted pathway from genes to behavior in psychiatric illness. Animal models are being utilized both to demonstrate the validity of endophenotypes by their reproduction in relevant models, and to understand the genetic and biological underpinnings of endophenotypes (Seong et al., 2002; Gould and Gottesman, 2006; Cryan and Slattery, 2007).
The utility of animal models is not, however, limited to their ability to replicate a particular physiological process or endophenotype, and the premature dismissal of traditional behavioral models should be avoided. Indeed, animal models are often studied for their ability to predict treatment response, and in this case the phenomenology of the model is secondary to its empirical validity. For example, a model in which rodent behavior is responsive to antidepressants should not, without appropriate validation, be considered a model of depression. Much of the criticism stems from the fact that a distinction is seldom made between the various goals of different behavioral models, as McKinney describes (McKinney, 1984). It is unlikely that an animal model of any psychiatric disease will ever simultaneously mirror the etiology, pathophysiology, symptoms, and treatment response characteristic of a psychiatric illness. Instead, these models permit the controlled study of a particular aspect of illness and treatment; as such, an evaluation of their validity—and thus, utility—demands consideration of which aspect(s) a model purports to address. Models intended to study particular symptoms, which would have face validity, should be distinguished from models of endophenotypes, which might exhibit no phenotypic similarity whatever to the diseases they probe. Although the endophenotype approach offers a promising route to understanding pathophysiology, more traditional models remain invaluable. The fundamental limitations of all models should be acknowledged, an effort which requires that the three-pronged face-construct-predictive validity prototype not be taken too literally.
Here we review the literature reporting the effects of lithium on rodent behavior. As the drug is primarily used in the treatment of mania and bipolar disorder, our discussion of the models relevant to these disorders will be the most extensive; however, studies have also explored the effect of lithium on preclinical models of conditions such as aggression, depression, circadian rhythms, schizophrenia, and tardive dyskinesia, and we will also review those data. For the interested reader we include a table describing the existing literature for the effects of lithium on each behavior. The differences in species, strain, and specific procedures across laboratories complicate generalization; however, Table 1 provides our overall interpretation of the consistency of the effects of lithium on each behavior.
Table 1. Summary of the behavioral effects of lithium.
The effects of lithium on rodent behavior vary depending on the paradigm used, and results are frequently inconsistent across laboratories. Here we indicate the relative number and consistency of reports for each behavior discussed in the review, with * denoting relatively low, ** intermediate, and *** relatively high consistency/number for each behavior. We also indicate the consistency of effect of other drugs used to treat bipolar disorder, both depression and mania, namely antidepressants, mood stabilizers, and antipsychotics on each particular model. We illustrate the most consistent action of lithium as attenuating (↓), potentiating (↑), or not affecting (↔) the particular behavior.
| Test | Effect | Number of Studies | Consistency of Effect | Effect of Other Antidepressants, Mood Stabilizers, and Antipsychotics |
|---|---|---|---|---|
| Aggression | ↓ | ** | *** | * |
| Alcohol Consumption | ↔ | * | * | * |
| Amphetamine-induced Hyperlocomotion | ↓ | *** | *** | ** |
| Behavioral Supersensitivity | ↓ | * | ** | * |
| Circadian Rhythm Length | ↑ | ** | *** | * |
| Conditioned Place Preference | ↓ | * | ** | ** |
| Conditioned Taste Aversion | ↑ | *** | *** | * |
| Exploratory Behavior | ↓ | ** | ** | * |
| Forced Swim Test immobility | ↓ | ** | *** | *** |
| Intracranial Self-Stimulation | ↓ | ** | * | * |
| Learned Helplessness | ↔ | * | * | *** |
| Learning and Memory | ↓ | ** | * | * |
| Morphine Administration | ↔ | * | * | * |
| Ouabain-induced changes | ↓ | * | * | * |
| Pilocarpine Seizures | ↑ | *** | *** | * |
| Prepulse Inhibition | ↔ | * | * | *** |
| Rearing | ↓ | *** | *** | * |
| Reserpine-induced Hypoactivity | ↓ | ** | *** | *** |
| Sensitization to Psychostimulants | ↔ | ** | * | ** |
| Stereotyped Behavior | ↔ | ** | * | ** |
| Stress-induced Hypoactivity | ↓ | * | *** | *** |
| Tail Suspension Test immobility | ↓ | * | * | *** |
Abbreviations: AD: antidepressants; MS: mood stabilizers
Because our focus in this review is the present status of animal behavioral models on which the effect of lithium has been studied (Table 1), our discussion of the biochemical antecedents of particular models will be limited, and primarily in our concluding remarks. For a more extensive discussion of the biochemical and physiological actions of lithium, the interested reader is directed to a number of reviews of such data (Jope, 1999; Phiel and Klein, 2001; Shaldubina et al., 2001; Chuang, 2004; Gould et al., 2004; Williams et al., 2004). This review begins with a discussion of baseline behaviors, and the effect of lithium upon them. We subsequently address behavioral models induced by amphetamine, followed by models of aggressive, depression-like, and reward-related behaviors. These are followed by a discussion of circadian rhythms, lithium augmentation of pilocarpine-induced seizures, and finally by a discussion of adverse effects of lithium such taste aversion. Each section begins with a short introduction to the model, followed by any available relevant clinical findings addressing the effect of lithium on the disease state, which the model is intended to mimic. Thereafter, we discuss the relevant tests more specifically, and describe the data relating the effects of lithium on rodents in those tests. If applicable, we also mention the effects of other mood stabilizers on the model.
II. Baseline Behaviors
Background
For the purposes of this review we define “baseline behaviors” as those behaviors not elicited by an influence beyond what the animal would experience during daily living. Therefore, locomotion and rearing that are not altered or induced by stress, drugs, or other such probes, are considered in this section. Animal behavior in a novel environment is, however, included in this section, since it is distinguished from “spontaneous” locomotion and rearing only by the length of time the animal has been in the test environment.
When investigating the effects of lithium in a behavioral paradigm, a dose and dosing schedule should be chosen which yield serum lithium levels equivalent to therapeutic levels in humans, ranging from 0.5 to 1.2 mEq/kg. Care must be taken to undertake studies with serum and brain lithium levels not much higher than this range to both prevent toxic side effects that are equally observed in rodents at higher dosages (see Box 1).
Box 1: Lithium dosage in behavioral studies.
When investigating the effect of lithium on a behavioral paradigm, a dose and dosing schedule are generally chosen which yield serum lithium levels equivalent to therapeutic levels in humans, ranging from 0.5 to 1.2 mEq/kg. Because the pharmacokinetics of lithium in humans is considerably different from what is found in rodents, a considerably higher lithium-by-weight dose may be applied in behavioral models. However, lithium metabolism in rats and mice is not identical, and particular caution must be taken when attempting to apply to rats a dosing paradigm used with mice, as the former metabolize lithium more slowly, and thus often require a lower dose than would mice (Wood et al., 1986). High levels of lithium can cause non-specific toxic effects, and can influence baseline behavior (thus confounding test results), making appropriate dosing all the more critical (Smith, 1978). Generalizing about such doses is difficult, however, because the time between the administration of lithium and the behavioral test can dramatically affect the level of lithium both inside the brain and in the serum. Studies have shown that acute i.p. administration of lithium leads rapidly to high serum levels, which subsequently decrease, and low brain levels, which subsequently increase (Morrison et al., 1971; Ghoshdastidar et al., 1989). These normalize between five and ten hours after administration. The serum and brain levels of chronically treated animals are approximately equivalent. For acute intraperitoneal (i.p.) or subcutaneous (s.c.) injections administered within approximately one hour before the test is begun, therapeutic serum levels are achieved by doses between 1.5 and 3 mEq/kg. Chronic administration of 0.2% (rats and mice) to 0.4% (mice) LiCl by weight in rodent chow has yielded predictable serum levels within the therapeutic range, while rats treated with 0.15% to 0.2% Li2CO3 chow have yielded the same (Lerer et al., 1980; Hamburger-Bar et al., 1986; O’Brien et al., 2004; Kitaichi et al., 2006; Youngs et al., 2006). For chronic administration in the drinking water, 20-30 mEq/L has been seen to be effective in rats (Prasad and Sheard, 1982; Hines, 1986b). Doses within the therapeutic range generally have no effect on baseline locomotor activity, so behavioral effects cannot generally be attributed to general motor changes.
A common test of baseline behavior is locomotor activity in an open field. A mouse or rat is placed in an arena, and measurements are made of its locomotor activity either by means of an automated system or by manual scoring, in which a record is kept of the number of times the animal crosses a given region. In addition to general locomotion, behaviors measured in an open field include rearing and hole-poking, which are considered to be measures of exploratory activity. Rearing can be measured either by means of an automated photocell system, or by manual scoring. In the hole-poke test, an animal is placed in an arena with holes lining the floor and/or walls. Exploratory activity is measured by the number of times the animal pokes its head (or its nose, depending on sensitivity settings) through the holes.
In the event that exploratory behavior alone is investigated, the test is generally of short duration in a novel environment. If the activity of interest is spontaneous locomotion and the effect of novelty must be entirely avoided, rodents are pre-exposed to the open field prior to test day, to habituate them to the environment. In the absence of such habituation, which is not generally considered not as critical for mice as for rats, investigators often characterize only the earliest fraction as exploratory behavior (generally the first 10-30 minutes, depending on the length of the test), while baseline locomotion is determined from the remaining time (Mukherjee et al., 1977).
Effect of Lithium on Baseline Behaviors in Rodents
The effect of lithium on spontaneous behavior is limited to certain behaviors and certain doses (see Table 2). Changes that occur at therapeutic doses are not caused by general motor impairment (Smith and Smith, 1973). Indeed, it has been shown consistently that therapeutic doses of lithium, which effectively alter drug-induced locomotion, do not change baseline locomotion in tests with a sufficient time course (see Stimulant-induced Behaviors, below) (Johnson, 1972a; Johnson, 1972b; Gould et al., 2001). Nevertheless, acute toxicity, a novel environment, and—in the case of injections—general discomfort can confound analyses of baseline locomotion. For these reasons, as mentioned above, baseline locomotion is typically measured only after such acute effects can be expected to have abated.
Table 2.
Studies of the effect of lithium on spontaneous locomotion and exploratory behaviors.
| Species, Strain | Lithium | Experimental design | Results | Reference |
|---|---|---|---|---|
| Rat, RCA | Li s.c. | Rearing | Decreased | (Johnson and Wormington, 1972) |
| Rat, RCA | Li i.p. | OFT, Rearing | Decreased rearing; LOCO: No change | (Johnson, 1972a) |
| Rat, Roman | Li i.p. | OFT, Rearing | Decreased rearing; LOCO: no change | (Johnson, 1972b) |
| Rat, W | Li in chow | OFT, activity wheel | Decreased LOCO; Wheel: no change, but decreased compared to weightmatched controls | (Smith and Smith, 1973) |
| Rat, W | Li i.g. | OFT, rearing | Decreased rearing and LOCO | (Smith, 1975) |
| Rat, H | Li in water | OFT, rearing | Decreased rearing and LOCO | (Langham et al., 1975) |
| Rat, CD | Li s.c. | Rearing | Decreased | (Gray et al., 1976) |
| Rat, W | Li i.p. | OFT, rearing | Decreased biting, LOCO, and rearing | (Johnson, 1976) |
| Rat, F | Li i.p. | OFT | Decreased LOCO | (Mukherjee et al., 1977) |
| Rat, W | Li i.p. | Rearing | Decreased | (Smith, 1978) |
| Rat, W | Li i.p. | OFT, hole-board | Decreased hole-poking and entries into center | (Johnson, 1981) |
| Rat, W | Li i.p. | OFT, rearing | Dose-dependent effects on LOCO | (Cappeliez and White, 1981) |
| Rat | Li chow | OFT, rearing | Decreased rearing; LOCO: no change | (Kofman et al., 1995) |
| Rat, SD | Li chow | OFT, rearing | Decreased rearing; LOCO: no change | (Kofman and Bersudsky, 2000) |
| Mouse, C57BL/6 | Li chow | Hole pokes, rearing, OFT | Decreased hole pokes only | (O’Brien et al., 2004) |
| Rat, LE | Li i.p. | OFT, rearing | Decreased rearing and LOCO | (Tenk et al., 2005) |
| Rat, SD | Li or VPA i.p. | OFT | Decreased early, then normalized | (Tomasiewicz et al., 2006) |
Abbreviations: LE: Long-Evans; Li: lithium; LOCO: locomotion; i.g.: intragastrically; i.p.: intraperitoneally; OFT: open field test; s.c.: subcutaneously; SD: Sprague-Dawley; VPA: valproic acid; W: Wistar.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
Unlike baseline locomotion, exploratory locomotion, measured immediately after being placed in a novel environment, is dose-dependently decreased by lithium (Table 2). Some have found similarities between these effects of lithium and those induced by comparable doses of other alkali metal chlorides or toxic substances, raising the question of whether they are caused by general toxicity (Smith, 1978; Johnson, 1981). However, the ability of therapeutic lithium levels to affect exploratory behavior without affecting baseline locomotion is an argument against this hypothesis, and the drug’s effects at therapeutic doses are generally regarded as specific.
An attenuating effect of lithium on rearing, also considered an exploratory behavior, is also consistently reported, even when locomotor activity is reported as unchanged (Table 2). One group (Gray et al., 1976) reported that this effect is seen only with rats which have been stressed, but in the majority of studies lithium decreased exploratory rearing even in untreated animals.
III. Stimulant-induced Behaviors
Background
One of the most common models by which the mood-stabilizing action of lithium is studied requires the induction of hyperactivity by a stimulant. Most studies have focused primarily upon one stimulant, amphetamine, the central action of which is the release and reuptake inhibition of norepinephrine and dopamine. Amphetamine, which has been widely studied, is frequently used to induce a variety of behavioral patterns, and thus to model many behavioral states other than mania.
In rodents, both the behavioral and the biochemical effects of amphetamine appear to be dose-dependent, in that the stimulant induces two ostensibly distinct patterns of behavior. In rodents, hyperlocomotion and rearing are elicited by a low dose of amphetamine, whereas higher doses induce stereotypy, a behavioral pattern in which various motor activities are characterized by their repetitiveness and intensity, typically to the exclusion of locomotion. Considerable overlap of the two behaviors is seen at intermediate doses. After a discussion of the effect of lithium on hyperlocomotion and stereotypy individually, we will discuss models, which make use of these behaviors within the context of behavioral supersensitivity and sensitization.
Lithium and Stimulants in Humans
Stimulants alone have been shown to precipitate mania or hypomania in bipolar patients (Murphy et al., 1971; Mamelak, 1978; Anand et al., 2000), which is one reason why the effect of lithium on amphetamine models of mania might be of interest. In a double-blind study of depressed patients, the euphoriant and/or activating effects of amphetamine were also either attenuated or blocked by lithium (Van Kammen and Murphy, 1975). Other double-blind studies have shown the same effect of lithium on schizophrenic patients treated with amphetamine (Van Kammen et al., 1985), as well as on patients with a variety of other psychiatric illnesses (Huey et al., 1981).
Preclinical behavioral models that make use of amphetamine as a pharmacological probe include putative models of mania, schizophrenia, tardive dyskinesia, and drug addiction. While the ability of lithium to attenuate mania in a clinical setting has been definitively established, its effect on other disease states is less clear. The effect of lithium on tardive dyskinesia, a movement disorder most often caused by prolonged exposure to neuroleptics, has been evaluated in open studies. These have yielded mixed results, and lithium is not generally regarded as a therapy for this disorder (Dalen, 1973; Reda et al., 1975; Mukherjee et al., 1986). A recent comprehensive meta-analysis of the literature describing the effect of lithium on schizophrenia suggested that lithium administered alone was effective neither when compared to antipsychotics, nor when compared to placebo (Leucht et al., 2004). While lithium is effective in the treatment of bipolar disorder but not schizophrenia, antipsychotics are effective treatments for both diseases. One would predict that animal models used for predictive studies of schizophrenia treatments would respond to antipsychotics more consistently than to lithium, while predictive models of mania, like the disease state, would respond to both.
The Effect of Lithium on Amphetamine-induced Hyperlocomotion
The effect of low doses of amphetamine (generally less than 3 mg/kg) on rodent behavior can be evaluated with various tests and apparatuses. Among these are activity wheels, with which activity is determined by the number of wheel turns (Flemenbaum, 1975; Flemenbaum, 1977). Others have used the hole-board described in the section on baseline and exploratory behavior, and calculated the effect of amphetamine to increase exploration, as measured by the number of times the animal pokes it head through a hole (e.g. (Davies et al., 1974)). The effect of amphetamine to increase locomotion within an arena is more extensively studied than these other measures, and for this reason our discussion of hyperactivity will focus mainly on hyperlocomotion. Amphetamine-induced hyperlocomotion is typically measured by means of a computerized apparatus, which monitors distance traversed over time. Photocell activity cages, in which locomotion is measured by interruptions of photocell beams, are a common example. Such automatic methods have the advantage of eliminating subjectivity over observer-based scoring common prior to technological advances. Indeed, some data have been acquired using observation alone to evaluate hyperlocomotion, often making use of rating scales which allow the rater to evaluate locomotor activity and stereotypy simultaneously (Borison et al., 1978; Fessler et al., 1982).
In research utilizing the acute administration of lithium to modify hyperlocomotion induced by either amphetamine or a mixture of amphetamine and chlordiazepoxide, the overwhelming consensus is that lithium has an attenuating effect. As discussed in the introduction, the toxicity of high doses of lithium is certainly capable of lowering even baseline activity (Berggren et al., 1978; Ebstein et al., 1980), so groups of animals treated with lithium or saline in the absence of amphetamine can verify the specificity of the effects. Many studies, however, have shown that at a moderate dose, lithium is capable of decreasing the hyperactivity without affecting spontaneous locomotion (Cox et al., 1971; Davies et al., 1974; Namima et al., 1999). Specifically, in 1971 Cox and colleagues first reported lithium attenuation of stimulant-induced hyperlocomotion in rats (Cox et al., 1971). This effect of lithium was soon found to exist in mice as well, and results in both species have been widely replicated (Berggren et al., 1978; Borison et al., 1978) (Table 3). While exceptions in the literature exist, they can often be attributed to an inappropriate dose of either amphetamine or lithium. As mentioned above, a decrease in hyperlocomotion is often concomitant with a rise in stereotypy at higher doses of amphetamine; this fact is likely the reason why Ebstein and colleagues found no effect of lithium on amphetamine-induced hyperlocomotion in rats, as they used doses of amphetamine ranging from 5 to 15mg/kg, the lowest of which is itself a dose high enough to elicit considerably stereotypy (Borison et al., 1978; Ebstein et al., 1980; Fessler et al., 1982). At this elevated dose, stereotypy was likely to be a confounding factor in the measure of hyperlocomotion. As we discuss below, lithium has been shown by some to be ineffective at attenuating an increase in amphetamine-induced stereotypy.
Table 3.
Studies of the impact of lithium on both hyperlocomotion and other measures of hyperactivity (hole pokes, activity wheel turns) induced by stimulants.
| Species, strain | Lithium | Experimental design | Effect on hyperactivity | Reference |
|---|---|---|---|---|
| Rat, Hooded | Li i.p. | CDP and d-AMP i.p.. Y-shaped maze | Decreased by acute, not chronic Li | (Cox et al., 1971) |
| Rat, Porton | Li i.p. | d-AMP and CDP i.p.. Hole-pokes and OFT | Decreased hole pokes; no effect on HyL | (U’Prichard and Steinberg, 1972) |
| Mouse, Rat, Porton | Li i.p. | d-AMP and CDP i.p.. Hole-pokes, Y-shaped maze, and OFT | Decreases hole-pokes, maybe HyL | (Davies et al., 1974) |
| Rat, SD | Li i.p. | d-AMP, i.p.. Activity wheel | Decreased HyA | (Flemenbaum, 1975) |
| Rat, W; Mouse, BALB/c | Li i.p. | d-amp s.c. OFT | Decreased HyL | (Wielosz, 1976) |
| Rat, SD | Li s.c. | d-AMP, l-AMP, m-AMP, APO, or COC, all i.p.; Activity wheel | Decreased HyA | (Flemenbaum, 1977) |
| Mouse, NMRI | Li i.p. | d-AMP, i.p.. HyL in OFT | Decreased HyL | (Berggren et al., 1978) |
| Mouse, Swiss | Li i.p. | d-AMP i.p. OFT | Li attenuates d-AMP-induced HyL | (Borison et al., 1978) |
| Rat, SAB | Li chow | d-AMP, i.p. Activity wheel | No attenuation of HyA | (Ebstein et al., 1980) |
| Rat, SD | Li chow | PCP or d-AMP i.p. OFT and SB | PCP-induced HyL was not affected; decreased AMP-induced HyL is concomitant with increased SB | (Fessler et al., 1982) |
| Rat, SD | Li chow | d-AMP or PCP i.p. OFT | No effect | (Fessler et al., 1982) |
| Rat, SAB | Li chow | d-AMP i.p. OFT | Decreased HyL induced by low dose | (Lerer et al., 1984) |
| Mouse, C3H, C57BL, A, BALB/c, AKR, CBA/LAC | Li chow | 2 mg/kg d-AMP i.p. OFT | Decreased HyL in C3H and A, not in C57 and BALB/c. AKR not d-AMP-responsive | (Hamburger-Bar et al., 1986) |
| Mouse, ddY | Li or CBZ i.p. | m-AMP + CDP, then OFT | Both decreased HyL | (Okada et al., 1990) |
| Mouse, NIH Rat, SD |
VPA i.p., SC, or PO | d-AMP + CDP. Head dips and Y-maze | Decreased head dips and arm entries | (Cao and Peng, 1993) |
| Rat, W | Li s.c. | m-AMP i.p. then OFT | Decreased | (Takigawa et al., 1994) |
| Mouse, ddY | Li s.c. | m-AMP i.p. then OFT | Decreased | (Namima et al., 1999) |
| Mouse, C57BL/6nCrlBR and C3H/HenCrlBR | Li i.p. | d-AMP i.p. then OFT | Decreased HyL in C57BL/6nCrlBr mice. C3H/HenCrlBR were unresponsive to d-AMP | (Gould et al., 2001) |
| Mouse, CD-1 | VPA, CBZ, or LMG, p.o. | d-AMP + CDZ i.p. then OFT | VPA, CBZ decreased HyL. No effect of LMG |
(Arban et al., 2005) |
| Mouse, 12 strains | Li i.p. | d-AMP i.p. then OFT | Decreased HyL in C57BL/6J, C57BL/6Tac, Black Swiss, CBA/J; no change in CD-1, DBA, 129, FVB, SWR, or NIH Swiss | (Gould et al., 2006) |
| Mouse, C57BL/6J | Li i.p. | d-AMP i.p., then OFT | Decreased | (Gould et al., 2007) |
Abbreviations: APO: apomorphine; CBZ: carbamazepine; CDP: chlordiazepoxide; COC: cocaine; d-AMP: dexamphetamine; HyA: hyperactivity (other than locomotion); HyL: hyperlocomotion; l-AMP: levoamphetamine; Li: lithium; LOCO: locomotion; LMG: lamotrigine; i.p.: intraperitoneally; OFT: open field test; PCP: phencyclidine; p.o.: orally; SAB: Sabra; s.c.: subcutaneously; SD: Sprague-Dawley; VPA: valproic acid; W: Wistar.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
Other inconsistencies in the literature might be attributed to the use of a strain inappropriate to the model. Indeed, a few studies have reported strain differences in lithium attenuation of amphetamine hyperlocomotion. In 1986 Hamburger-Bar and colleagues reported differences in amphetamine-induced hyperlocomotion in six mouse strains, following 3 weeks of feeding with lithium chow (4 g/kg lithium chloride) (Hamburger-Bar et al., 1986). They found that in C3H and A strains, d-amphetamine stimulated activity, which lithium was able to block; in AKR and Balb/c mice, lithium showed no effect. Two more strains (C57BL and CBA/LAC) were unresponsive to d-amphetamine. In 2001 Gould, Keith, and Bhat compared C57BL/6nCrlBr and C3H/HenCrlBR mice (Gould et al., 2001). They found that lithium, when administered 50 minutes prior to 3 mg/kg d-amphetamine and 60 minutes prior to a 10-minute test, prevented amphetamine-induced hyperlocomotion in C57BL/6nCrlBr mice. C3H/HenCrlBR mice, by contrast, were unresponsive to d-amphetamine. Recently, Gould and colleagues undertook an extensive strain-differences study, testing both inbred and outbred strains (12 total) (Gould et al., 2006). Acute lithium attenuated hyperlocomotion in four strains (C57/BL6J, C57BL/6Tac, Black Swiss, and CBA/J), increased hyperlocomotion in C3H/HeJ mice, and had no significant effect on six strains (CD-1, DBA/2J, 129S6/SvEv, FVB/NJ, SWR/J and NIH Swiss). A/J mice were found to be unresponsive to d-amphetamine. These acute findings were confirmed with chronic lithium administration in C57BL/6J, FVB/NJ, and C3H/HeJ mice (Gould et al., 2006). These three studies illustrate that in rodent strains, as in human patients, differential responsiveness to lithium is common. Genetic studies predicated on these results might begin to investigate these differences; in the meantime, it must be noted that the strain of mouse (and most likely rat) must be taken into consideration when evaluating the effect of a drug, in this case lithium, on a particular behavior.
Given the fact that a single dose of lithium does not diminish overt behavioral symptoms in mood disorder patients, the fact that amphetamine-induced hyperlocomotion, as well as other behaviors discussed later in the text, can be so rapidly modified is a limitation of the model. However, as we discussed in the Introduction, the utility of these models must be considered in light of what facets of a disease and treatment they purport to address, as all such models have significant limitations. Amphetamine-induced hyperlocomotion, for example, is a behavior, not a mood; as such, the ability of lithium to modify that behavior will not necessarily mirror the effect of the drug to alter mania or depression. These behavioral measures may, for example, be measures of the function of receptors or G-proteins located on the cell membrane, which may lead to further changes within the cell. In a clinical population, those interior changes may be manifested as mood stabilization. If these behavioral models provide a measure of receptor/G-protein function, an acute response would be unsurprising, as the concentration of lithium on the membrane, the site of these receptors and G-proteins, would be higher than that inside the cell following acute treatment.
Overall, the results describing the effect of chronic lithium on the hyperlocomotion model are somewhat less consistent than acute studies, which again, may be due in part to dosage and strain issues (Table 3). For example, Cox and colleagues utilized the same sex and strain of rat (female, hooded) for both acute and chronic studies, the latter of which did not reveal a distinct attenuation of hyperlocomotion, so the strain alone is not responsible (Cox et al., 1971). Inasmuch as amphetamine-induced hyperlocomotion is a model relevant to mania, the effect of other mood-stabilizers on the model is of interest. A wide range of antipsychotics are capable of attenuating both hyperlocomotion and stereotypy (Arnt, 1995), and for this reason the model is often used in the screening of new antipsychotic drugs (Skuza et al., 1997; Lautar et al., 2005; Tort et al., 2005). As antipsychotics are frequently used to treat bipolar disorder, their efficacy in this model is supportive of its use in the study of mania. Valproic acid, carbamazepine, and lamotrigine, all mood stabilizers different in structure both from each other and from lithium, have also been studied in the hyperlocomotion model. Like lithium, they have been shown to attenuate the hyperlocomotion induced by a mixture of d-amphetamine and chlordiazepoxide (Cao and Peng, 1993; Arban et al., 2005). In the cited cases, all three anticonvulsants were able to decrease hyperlocomotion without affecting baseline activity. However, Arban et al (2005) also found that neither valproate nor carbamazepine was able to attenuate the hyperlocomotion induced by 1.25mg/kg d-amphetamine alone, though lamotrigine had a partially attenuating effect, suggesting that a mixture of d-amphetamine and chlordiazepoxide may be critical.
Effect of Lithium on Amphetamine-induced Stereotypy
Stereotyped behavior, elicited by high doses (generally 5 mg/kg and higher) of amphetamine, is commonly used to investigate disorders including, but not limited to, psychosis, autism, obsessive-compulsive disorder, and schizophrenia (Kelley, 2005). Stereotypy consists of motor activity that is patterned, lacking in both variation and purpose; as such, accurate measurement demands that the character of the behavior, rather than the behavior itself, be considered. Such behaviors can include sniffing, gnawing, and rearing among many others. Most procedures typically do not monitor behavior over the entire time course of drug action, but rather apply an observations time-sampling method. However, a large number of protocols are in use, including some automated systems.
The variations in scoring methods are but one of many differences between studies evaluating the effect of lithium upon stereotypy. As with all behavioral studies, species and strain differences are significant, as are the dose, duration, and route of lithium administered. Neither the length of time between lithium and stimulant administration nor the type and dose of stimulant are consistent across studies (see Table 4). d-Amphetamine is the most widely used dopamine agonist, but cocaine, apomorphine, and methamphetamine are also common. The heterogeneity of testing materials and methods may be a reason why no consensus exists on the effects of lithium on stereotypy. Within the studies of rats, lithium has been found to decrease amphetamine-induced stereotypy (Flemenbaum, 1977), to have no effect on it (Wielosz, 1976; Fessler et al., 1982), or to potentiate it (Miyauchi et al., 1981), but both the dose and the isomer of amphetamine varied between the studies. There was no consistent association with strain, as all three possible outcomes were found even among Sprague-Dawley rats. Studies with mice have also been inconclusive, with strain, dosage, and isomer differences again obfuscating any firm conclusions. As in rats, lithium administration was found to reduce (Frances et al., 1981a), to potentiate (Ozawa and Miyauchi, 1977; Miyauchi et al., 1981), or to have no effect on (Gould et al., 2007) stereotypy in mice.
Table 4.
Studies of the effect of lithium on stimulant-induced stereotyped behavior.
| Species, strain | Lithium | Experimental design | Effect on stereotyped behavior | Reference |
|---|---|---|---|---|
| Rat, W | Li s.c. | d-AMP for SB | No effect | (Wielosz, 1976) |
| Rat Rat, SD |
Li s.c. | d-AMP, l-AMP, m-AMP, APO, COC i.p. for SB | Decreased | (Flemenbaum, 1977) |
| Mouse, ddI | Li i.p. | m-AMP, i.p. for SB | Decreased | (Ozawa and Miyauchi, 1977) |
| Mouse, Swiss | Li i.p. | d-AMP i.p., for SB | No effect | (Borison et al., 1978) |
| Mouse, Swiss | Li water | APO s.c., for SB | Decreased | (Frances et al., 1981a) |
| Mouse, ddl, ddY | Li i.p. | m-AMP i.p. for SB | Increased | (Miyauchi et al., 1981) |
| Rat, SD | Li chow | PCP or d-AMP i.p., for SB | Increased PCP-induced SB; no effect on AMP-induced SB | (Fessler et al., 1982) |
| Rat, SD | Li chow | AMP i.p., for SB | Increased | (Rubin and Wooten, 1984) |
| Rat, W | CBZ i.p. | APO i.p., for SB | Increased | (Barros and Leite, 1986) |
| Mouse, C57BL/6J | Li i.p. | d-AMP i.p., for SB | No effect | (Gould et al., 2007) |
Abbreviations: APO: apomorphine; CBZ: carbamazepine; COC: cocaine; d-AMP: dexamphetamine; i.g.: intragastrically; i.p.: intraperitoneally; l-AMP: levoamphetamine; Li: lithium; OFT: open field test; PCP: phencyclidine; SB: stereotyped behavior; s.c.: subcutaneously; SD: Sprague-Dawley; W: Wistar.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
The lack of consensus regarding the effects of lithium in this model may be telling in regard to the diseases which the model intends to study. Antipsychotics, developed initially for the treatment of schizophrenia, reproducibly attenuate stereotyped behavior (Randrup and Munkvad, 1975; Kokkinidis and Anisman, 1981) as well as hyperlocomotion (Arnt, 1995). Although antipsychotics are also used in the treatment of bipolar disorder, lithium is not generally considered an effective treatment for psychosis without a mood component, or for schizophrenia. Therefore, the clear effects of drugs that attenuate amphetamine stereotypy may have predictive validity in the development of antipsychotic agents over mood stabilizers.
The Effect of Lithium on Neuroleptic-induced Behavioral Supersensitivity
After chronic treatment with neuroleptics, the response to stimulants is markedly enhanced. Thus, a single administration of apomorphine (dopamine D1 and D2 receptor agonist) or amphetamine elicits a more intense hyperlocomotor or stereotyped response following neuroleptic administration than that seen without such pre-treatment (Tarsy and Baldessarini, 1973; Smith and Davis, 1976). Biochemical studies reveal that chronic exposure to antipsychotics renders particular dopamine receptors supersensitive (Burt et al., 1977; Gianutsos and Moore, 1977). These effects are of interest because neuroleptic-induced behavioral supersensitivity has been proposed as a model for tardive dyskinesia, which chronic neuroleptic treatment induces in some patients. Lithium has been shown to block the changes in dopamine metabolism caused by exposure to neuroleptics in rodents, and some evidence suggests that chronic lithium lowers extracellular dopamine levels in rodents (Sternberg et al., 1983; Ferrie et al., 2005). As mentioned previously, lithium has had modest positive effects in both open and double-blind studies of patients with tardive dyskinesia; however, these results have not been pursued further, and lithium is not generally used to treat the disorder (Gerlach et al., 1975; Reda et al., 1975).
Rodent studies of the effect of chronic lithium on supersensitivity induced by chronic administration of neuroleptics vary (Table 5). Following chronic exposure to haloperidol, rats treated with a single injection of apomorphine showed intense hyperlocomotion and stereotypy, which chronic lithium chow or i.p. administration was able to block (Pert et al., 1978; Verimer et al., 1980). Electrophysiological and biochemical studies have suggested that lithium protects against the effect of haloperidol to increase dopamine receptor sensitivity (Gallager et al., 1978; Pert et al., 1978). However, subsequent studies found that lithium had no effect on dopamine turnover or receptor supersensitivity, even though it continued to affect behavior in this model. Whether this behavioral alteration is mediated by a change in dopamine receptor function or through another biochemical target is thus unclear (Reches et al., 1982; McIntyre et al., 1983; Reches et al., 1984).
Table 5.
Studies of the effect of lithium on neuroleptic-induced behavioral supersensitivity.
| Species, strain | Lithium | Experimental design | Results | Reference |
|---|---|---|---|---|
| Rat, SD | Li chow | Daily HAL i.p., then OFT | HAL-treated animals showed HyA, which lithium decreased | (Pert et al., 1978) |
| Rat, SD | Li i.p. | HAL i.p., then OFT | HAL-treated animals showed sedation, which lithium decreased | (Verimer et al., 1980) |
Abbreviations: HAL: haloperidol; HyA: hyperactivity; i.p.: intraperitoneally; Li: lithium; OFT: open field test; SD: Sprague-Dawley.
Limitations to the animal model include the fact that receptor supersensitivity and heightened behavioral sensitivity can be elicited in rodents within weeks, whereas tardive dyskinesia is typically seen only after years of neuroleptic use. Nevertheless, the movement disorder seen in patients is thought to be mediated at least in part by dopamine receptor supersensitivity, though limited understanding of the disorder leaves the validity of the model in question.
The Effect of Lithium on Behavioral Sensitization
Behavioral sensitization, in which repeated administration of stimulants such as amphetamines, methylphenidate, and cocaine leads to the progressive elevation of psychomotor stimulating effects, is used to model of a variety of illnesses, particularly drug craving and drug-induced psychosis (Robinson et al., 1985; Robinson and Berridge, 1993). It is a phenomenon that is not unique to rodents; sensitization to the effects of stimulants is seen in human users of amphetamines. The sensitization that appears after chronic use in humans can result in the development of psychosis, which can disappear with abstinence, but which can fully return following the use of even a small quantity of amphetamine (Sato et al., 1983; Sato, 1986). Owing to the behavioral parallels between drug-induced psychosis and schizophrenia, behavioral sensitization has also been proposed as a model of the latter (Kokkinidis and Anisman, 1981). Lithium is not utilized clinically for the treatment of either schizophrenia or drug addiction. As would be expected from a putative model of schizophrenia, behavioral sensitization can be prevented by the administration of typical and atypical antipsychotics (Meng et al., 1998).
Behavioral sensitization, like supersensitivity, is not entirely distinct from the stimulant-induced behaviors discussed above. In this model, the behavioral response (for example, hyperlocomotion or focused stereotypy) to a stimulant is progressively amplified with multiple exposures to stimulants. The measurement of sensitization is the measurement of the difference between the initial and subsequent behavioral effects. This is a difference in intensity, not in the type of behavior. A single low dose of amphetamine will often cause hyperlocomotion; as amphetamine is repeatedly administered, the same (or even a lower) dose will thus lead to an increasingly greater degree of hyperlocomotion. The effects of sensitization are long lasting, and for this reason rodents should not be reused for other behavioral studies involving stimulants once a single dose has been administered, unless sensitization effects are taken into account.
Reports of the effect of lithium on sensitization vary (Table 6). In rats, the inability of chronic lithium administration to inhibit sensitization to amphetamine or apomorphine has been reported (Rubin and Wooten, 1984; Cappeliez and Moore, 1990). In the Rubin et al. (1984) study, the measure of sensitization was amphetamine-induced stereotyped behavior. The study by Cappeliez and Moore (1990), however, tested hyperlocomotion induced by a 1.5 mg/kg dose of d-amphetamine administered twice daily, but found no effect of lithium. By contrast, lithium prevented cocaine-induced sensitization as measured by hyperlocomotion, when carbamazepine had no effect on the increase; however, the authors of this study reportedly had difficulty replicating the findings (Post et al., 1984). Yang and colleagues tested the effect of lithium on sensitization to methylphenidate in rats, and found it to be capable of suppressing the expression of sensitization early on, but unable to prevent its expression after a later re-challenge (Yang et al., 2001). They also showed that lithium has no effect on sensitization to methylphenidate once it has developed. In another study this group demonstrated that, like lithium, valproate has no effect on sensitization to methylphenidate that has already been induced (Yang et al., 2000). Administration of valproate both during methylphenidate exposure and during the washout period preceding re-challenge does, however, suppressed the subsequent expression of sensitization. The positive results, small in number, are thus limited to the prevention, rather than the abolition, of sensitization.
Table 6.
Studies of the effect of lithium on stimulant-induced behavioral sensitization, as measured through hyperlocomotion, stereotyped behavior, or hypoalgesia.
| Species, strain | Lithium | Experimental design | Effect on sensitization | Reference |
|---|---|---|---|---|
| Rat, SD | Li chow | AMP or APO s.c.. SENS measured by SB | Possibly increased | (Rubin and Wooten, 1984) |
| Rat | Li chow | COC i.p. SENS measured by HyL | Decreased | (Post et al., 1984) |
| Rat, SD | Li chow | d-AMP i.p. SENS measured by HyL | No effect | (Cappeliez and Moore, 1990) |
| Rat, SD | Li water | COC i.p. SENS measured by shock-induced hypoalgesia | Decreased | (Antelman et al., 1998) |
| Mouse, ddy | Li i.p. | m-AMP s.c. SENS measured by HyL | Decreased | (Namima et al., 1999) |
| Rat, SD | VPA i.p. | MPD s.c. SENS measured by HyL | No effect of acute VPA; chronic decreased | (Yang et al., 2000) |
| Rat, SD | Li i.p. | MPD s.c. SENS measured by HyL and SB | No effect | (Yang et al., 2001) |
| Mouse, Kunming | VPA i.p. | m-AMP or COC i.p., measured by HyL | No effect of acute VPA; chronic decreased | (Li et al., 2005) |
| Rat, SD | Clozapine, HAL, or SCH 23390 s.c. | d-AMP-induced SENS, measured by HyL | Decreased | (Tenn et al., 2005) |
| Mouse, C57BL/6J | Li i.p. | d-AMP-induced SENS, measured by HyL | No effect | (Gould et al., 2007) |
Abbreviations: APO: apomorphine; COC: cocaine; d-AMP: dexamphetamine; HyL: hyperlocomotion; i.p.: intraperitoneally; Li: lithium; m-AMP: methamphetamine; MPD: methylphenidate; SB: stereotyped behavior; s.c.: subcutaneously; SD: Sprague-Dawley; SENS: sensitization; VPA: valproic acid.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
With mice, the literature with lithium and sensitization is less extensive than with rats. Gould and colleagues have found that acute lithium administration, while continuing to attenuate hyperactivity, does not prevent sensitization. Over five days, amphetamine-induced hyperactivity in lithium-treated mice was consistently lower than in the untreated controls, but higher than it had been the first day (Gould et al., 2007). Importantly, these data were derived from a dosing paradigm that showed positive results in the attenuation of amphetamine-induced hyperactivity, without causing toxic effects. Namima et al (Namima et al., 1999), using a considerably higher dose of lithium, also administered five times but at intervals of three days, reported lithium’s prolonged attenuation of the hyperactivity induced by amphetamine. Chronic administration of valproate has been found to attenuate amphetamine-induced sensitization in mice (Li et al., 2005). All of these studies used different mouse strains (C57BL/6J, ddY, and Kunming, respectively), which, along with different dosing paradigms, further complicates comparison.
In experiments with rats using chronic cocaine administration followed by a single amphetamine challenge, certain groups have also used a hot plate apparatus to measure the treated rat’s sensitivity to pain, which is affected by cocaine. In this test, the animal’s latency to jump or lick its hind paw is measured (Antelman et al., 1998; Kucinski et al., 1999). Following continued administration of cocaine, this sensitization can lead to an oscillation in the response to pain. This behavioral oscillation is matched by an oscillation in amphetamine-induced dopamine efflux in the nucleus accumbens following chronic exposure to cocaine: a single administration of cocaine increases this efflux, whereas a sixth exposure can decrease it (Antelman and Caggiula, 1996). The finding that sensitization, at least with these behavioral and biochemical measures, is not unidirectional, coupled with its context-dependency, has led some to propose that it might be used to model bipolar disorder, insofar as the same manipulation (e.g. the administration of cocaine) can lead to different and even opposite responses, and is sensitive to lithium (Post et al., 1986; Antelman and Caggiula, 1996; Kucinski et al., 1999). With further replication of these results, this test might be another way to investigate bipolar disorder symptoms. Interestingly, however, lithium is not particularly effective in rapid-cycling bipolar disorder (Maj et al., 1998). It is also a relatively ineffective prophylactic agent for bipolar patients with mood-incongruent psychosis (van Kammen et al., 1985; Maj et al., 2002), and the possibility that behavior induced by sustained administration of cocaine is more akin to psychosis (or to drug abuse) cannot be ruled out.
IV. Lithium and Aggressive Behavior
Background
Unlike mania, schizophrenia, psychosis, or any of the other psychiatric disorders which the above stimulant-induced behaviors attempt to imitate, aggressive activity is arguably more straightforward to model in an animal. Nevertheless, the study of aggression in rodents is beset with many of the same difficulties as other models, not least of which is the fact that aggression itself is a heterogeneous phenomenon, one which can be manifested both in isolation and as one of a constellation of symptoms in over twenty psychiatric disorders, including bipolar disorder, ADHD, autism, major depressive disorder, ADHD, and schizophrenia (see (Fava, 1997) for a discussion and complete list). Also similar to the disorders investigated through the stimulant-induced behaviors, aggression is defined only symptomatically (generally as a destructive behavior intended to inflict harm on oneself, objects, or others). Though much is known about certain forms of aggression and related neural systems, no single biochemical origin or pathway can be looked to when attempting to discover the origin of aggressive behavior. Owing to the vast array of psychiatric disorders within which aggression is manifested, attempts are now being made to treat not merely the aggressive behavior, but the underlying disorder/disease process of which the behavior is a symptom (Connor and Steingard, 1996; Leibenluft et al., 2003).
In humans, two types of aggression have been distinguished: reactive (or impulsive) aggression, often the consequence of frustration and anger; and instrumental aggression, generally premeditated and carried out as a means of attaining a particular goal. Instrumental aggression, unlike its explosive counterpart, is often unassociated with emotion or later feelings of guilt (Cornell et al., 1996). Different disorders are associated with the different subtypes; for example, instrumental aggression and psychopathy have been associated with one another (Cornell et al., 1996), whereas increased risk for childhood bipolar disorder or explosive disorder is associated with impulsive aggression alone (Coccaro, 1998; Leibenluft et al., 2003). The two kinds of aggression respond differently to pharmacological treatment, and appear to have different neural substrates (see (Blair, 2005) for a concise review). As such, studies and models of aggression are well served to identify the type of aggression they seek to investigate; however, the incomplete understanding of the behavioral models complicates this prospect.
Lithium and Human Aggression
The effect of lithium on aggression in humans appears to be limited by the form of aggression under study, and by the subjects on whom the drug is tested. Malone and colleagues showed that lithium has a marked ability to attenuate aggression in impulsively aggressive children, but not in those with instrumental aggression (Malone et al., 1998). The effect of lithium on aggression symptomatic of particular psychiatric disorders is unclear. For example, in adolescent patients with conduct disorder, one double-blind, placebo-controlled trial showed a significant decrease in both incidence and severity of impulsive aggression (Campbell et al., 1995); however, another blinded, controlled study yielded no effect on this patient population (Rifkin et al., 1997). Double-blind studies of lithium and aggression in adults who suffer from psychiatric illness are lacking (see (Goedhard et al., 2006)).
Lithium has also been shown to attenuate some aggression that is not decisively attributed to an underlying psychiatric illness. One double-blind study, carried out on male prisoners without affective disorders, measured the effect of lithium on the number of violent/aggressive behaviors that each subject exhibited during and after treatment (Sheard, 1971; Sheard et al., 1976). In this study, lithium was shown to reduce significantly the number of infractions, despite unchanged (self-reported) irritability.
Effect of Lithium on Rodent Models of Aggression
The suppressive effect of lithium on rodent models of aggression is seen in tests of social conflict, in which two or more animals of the same species are paired. Often, only one of these animals is being tested; the other is frequently a reliably “dominant” or “submissive” animal, against which to compare the test animal. In these intraspecies tests, aggression can be spontaneous, or it can be induced, for example by drugs, isolation, or electrical shock (see Table 7). Approach/avoidance behaviors and dominant/submissive postures are scored in addition to more directly violent behaviors, such as biting attacks. Latency to attack is often measured as well. The tests typically last between 5 and 30 minutes, and scoring involves rating scales than may or may not take intensity into consideration (Crawley, 2000).
Table 7.
Studies reporting the effect of lithium on various rodent models of aggression.
| Species, strain | Lithium | Experimental design | Effect on aggression | Reference |
|---|---|---|---|---|
| Rat. SD | Li i.p. | Shock-induced | Decreased | (Sheard, 1970) |
| Rat, SD | Li i.p. | Shock-induced | Decreased | (Eichelman et al., 1973) |
| Mouse, ddI | Li i.p. | Induced by nialimide plus L-DOPA, or by clonidine | Increased | (Ozawa et al., 1975) |
| Rat, SD | MAOI and dibenzazepines, i.p. | Shock-induced | Increased | (Eichelman and Barchas, 1975) |
| Rat, WR | Li i.p. | Shock-induced alone, as well as potentiated by d-AMP or scopolamine, i.p. | Decreased | (Mukherjee and Pradhan, 1976) |
| Mouse, TO | Li i.p. | Resident intruder, maternal aggression, and locust killing | Decreased resident intruder. Other tests inconclusive | (Brain and Al-Maliki, 1979) |
| Rat, W | IMI, amitriptyline, mianserin, iprindole, i.p. | Shock-induced | Increased | (Mogilnicka and Przewlocka, 1981) |
| Rat, SD | Li water | Shock-induced | Decreased | (Prasad and Sheard, 1982) |
| Mouse, AB | Li, VPA, or CBZ in water | Isolation-induced | Li, CBZ decreased; VPA, CBZ: no change | (Oehler et al., 1985) |
Abbreviations: CBZ: carbamazepine; d-AMP: dexamphetamine; i.p.: intraperitoneally; Li: lithium; MAOI: monoamine oxidase inhibitor; SD: Sprague-Dawley; VPA: valproic acid; W: Wistar; WR: Walter Reed.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
Despite the methodological variability in scoring, lithium has frequently been shown to attenuate aggression in social conflict tests with both rats and mice, using varying methods of administration (Table 7). Studies are more extensive in rats than in mice, and almost invariably reveal that lithium attenuates aggression in social conflict tests. Intraspecies aggression is frequently elicited by means of light electric shocks to the rats’ paws. Lithium consistently attenuates this kind of aggression, and the attenuation persists even in the presence of a pharmacologic agent that enhances the aggressive behavior (Sheard, 1970; Eichelman et al., 1973). For example, the effects of both desipramine and d-amphetamine to potentiate shock-induced aggression are decreased by lithium (Mukherjee and Pradhan, 1976; Prasad and Sheard, 1982). Similar effects are seen in social conflict tests between mice. Lithium attenuates both isolation-induced fighting outside the home cage, and resident-intruder fighting within the home cage (Brain and Al-Maliki, 1979; Oehler et al., 1985).
The argument has been made that the anti-aggressive effect of lithium is a toxic effect, related to the emetic properties of high doses of the drug (McGlone et al., 1980). In this study, Sprague-Dawley rats were treated for five consecutive days with i.p. injections of 5 mEq/kg of lithium. Given that the investigators were trying to investigate whether the effect of lithium was specific, the choice of such a high dose of lithium is surprising, since the high serum levels that result are known to produce many non-specific effects (serum levels reported are between 6.9 and 7.6 mEq/l). Since the majority of studies illustrating the anti-aggressive properties of lithium use a much lower dose of lithium, the anti-aggressive actions are quite possibly different in the two cases, with the low dose eliciting a specific effect, and the high dose eliciting a non-specific effect that can be attributed to general malaise associated with lithium toxicity.
V. Depression-like Behaviors
Background
As with models of all affective disorders, rodent models of major depression are limited by the fact that a behavior, rather than a mood, is the output measure. Nevertheless, many models thought to be related to depression exist. With classical models of depression, their utility is often founded on their predictive validity, as these models, which we describe in each section below, are responsive to a variety of antidepressants. However, in many cases their construct validity has often not been fully ascertained, and for this reason the models we describe below are more appropriately referred to as screening tests for antidepressants, rather than behavioral models of depression per se. More recently, attention has been directed at developing models for specific features of the disease, whether particular symptoms or endophenotypes. For a review of the current status of depression models, the reader is referred to (Cryan and Holmes, 2005; Cryan and Slattery, 2007).
Effect of Lithium on Human Depression
While not as widely recognized as its effect on manic episodes, many studies have shown lithium to have a consistent antidepressant effect in clinical populations. It has been shown to augment antidepressant effectiveness in treatment-refractory patients (Heninger et al., 1983; Austin et al., 1991). A recent review revealed that approximately 45% of patients monitored in placebo-controlled studies responded to lithium as an adjunct medication for depression (Bauer et al., 2003), a rate higher than for any other medication. Further, some studies have suggested that lithium monotherapy is an effective prophylactic agent in unipolar depressed patients (see (Souza and Goodwin, 1991) for meta-analysis and review). Given the strong clinical data, there is considerable interest and importance in fully understanding the action of lithium in rodent models of depression.
Effect of Lithium on the Forced Swim Test
The forced swim test (FST) was introduced by Porsolt and colleagues as an animal model for depressive behavior in 1977 (Porsolt et al., 1977b). It is among the most common tests used to predict antidepressant efficacy in both mice and rats (Porsolt et al., 1977a; Porsolt et al., 1977b). It is a simple test, lasting only six minutes and replicable across laboratories. A mouse or rat is placed in a cylinder of water, and an initial period of intense activity is followed by a period of immobility, in which the animal generally makes only enough movements to keep its head above water. A wide range of antidepressants reduce immobility time in the model. The administration of stimulants also can decrease immobility, giving a false positive result for the test; likewise, gastrointestinal discomfort caused by an acute injection of lithium might affect mobility. For this reason, a test of spontaneous locomotor activity is often used to complement the FST, to determine whether the decreased immobility can be attributed to a non-specific activating effect of either the drug or the injection itself.
An effect of lithium on the FST was first reported in 1987, when chronic i.p. administration of lithium chloride to rats was shown to decrease immobility time (Eroglu and Hizal, 1987) (see Table 8). The effect was reported in mice seven years later (Hascoet et al., 1994). In this experiment, a range of doses of lithium were used, to determine whether lithium had an effect on its own, and also to determine whether a subactive dose of lithium, in conjunction with a subactive dose of an antidepressant, would reduce immobility time. As in the test with rats, lithium was administered i.p., but only once, rather than chronically. Two, 4, and 8 mEq/kg lithium were shown to have a small but significant effect on immobility time, and when the subactive 1 mEq/kg was administered 15 minutes before a subactive dose of the 5HT(1A) agonist gepirone, immobility time was also reduced. This adjunctive effect of lithium, which it shares (though not identically) with clonidine and quinine, has been shown for a number of different kinds of antidepressants, including SSRIs, MAOIs, tricyclic, and atypical antidepressants (Malinge et al., 1988; Bourin et al., 1991; Hascoet et al., 1994; Nixon et al., 1994; Guo et al., 1995; Bourin et al., 1996; Redrobe et al., 1998). This additive mechanism is likely to be mediated at least in part by the serotonergic system, as antidepressants specific for dopaminergic and noradrenergic transmission are not affected by lithium in this model (Nixon et al., 1994). The aforementioned data showing lithium to be effective alone in the FST were collected after the chronic or acute i.p. administration of lithium. Others have shown more recently that long-term administration of lithium in mouse feed also decreases immobility time (O’Brien et al., 2004; Shaldubina et al., 2006; Bersudsky et al., 2007; Cryns et al., 2007; Gould et al., 2007). The effect of lithium is dependent upon the level of lithium in the blood, and not upon lithium-induced weight loss (Bersudsky et al., 2007).
Table 8.
Studies of the effect of lithium on animal models of depression.
| Species, Strain | Lithium | Experimental design | Effect on depression-like behavior | Reference |
|---|---|---|---|---|
| Mouse, Swiss | Li i.p. | RES i.p. OFT | Decreased hypoactivity | (Borison et al., 1978) |
| Rat, Sabra | Li chow | RES i.p. OFT | Decreased hypoactivity | (Lerer et al., 1980) |
| Mouse, Swiss | Li water | Isolation, then OFT | Decreased hypoactivity | (Frances et al., 1981b) |
| Rat, Holtzmann | Li water | Shock, then OFT | Decreased hypoactivity | (Hines, 1986a) |
| Rat, Wistar | Li i.p. | FST | Decreased immobility | (Eroglu and Hizal, 1987) |
| Rat | Li i.p. | LH | No effect | (Stewart et al., 1991a) |
| Rat, Wistar | Li or IMI, i.p. | LH | Li increased deficit; IMI decreased | (Geoffroy et al., 1991) |
| Rat, Wistar | Li water | LH | Decreased deficit | (Faria and Teixeira, 1993) |
| Mouse, Swiss | Li i.p. | FST with SSRIs | Potentiated SSRI-induced decrease in immobility | (Nixon et al., 1994) |
| Mouse, Swiss | Li i.p., with AD | FST | Decreased immobility, and potentiated AD-induced decrease | (Hascoet et al., 1994) |
| Rat, Wistar | Li i.p. | Immobilization stress-induced hypokinesia | Li decreased hypokinesia | (Kofman et al., 1995) |
| Rat, Wistar | Li water | LH | Chronic Li decreased deficit; acute: no effect | (Teixeira et al., 1995) |
| Mouse, Swiss | Li i.p. | FST | Li + AD decreased immobility time | (Bourin et al., 1996) |
| Mouse, Swiss | Li i.p. | TST | Li potentiated AD-induced decrease in immobility | (Redrobe and Bourin, 1997) |
| Mouse, Swiss | Li i.p. | FST | Li + subactive venlafaxine decreased immobility time | (Redrobe et al., 1998) |
| Rat, SD | Li i.p. | LH | Sub-chronic: dose-dependent decrease in LH; chronic: Li induced spontaneous escape deficits | (Gambarana et al., 1999) |
| Mouse, C57BL/6 | Li chow | FST | Li decreased immobility | (O’Brien et al., 2004) |
| Mouse, C57BL/6 and 129 hybrid | Li chow | FST | Li decreased immobility | (Shaldubina et al., 2006) |
| Rat, SD | Li or VPA, i.p. | FST | High dose Li decreased immobility; low dose increased. VPA: no effect | (Tomasiewicz et al., 2006) |
| Mouse, ICR | Li chow | FST | Li decreased immobility | (Bersudsky et al., 2007) |
| Mouse, C57BL/6 and 129 hybrid | Li chow | FST | Li decreased immobility | (Cryns et al., 2007) |
| Mouse, C57BL/6J | Li chow | FST | Decreased immobility | (Gould et al., 2007) |
Abbreviations: AD: antidepressant(s); FST: forced swim test; IMI: imipramine; i.p.: intraperitoneally; LH: learned helplessness; Li: lithium; OFT: open field test; RES: reserpine; SD: Sprague-Dawley; SSRI: selective serotonin reuptake inhibitor(s); TST: tail suspension test; VPA: valproic acid.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
Effect of Lithium on the Tail Suspension Test
The tail suspension test (TST), established in 1985 by Steru and colleagues (Steru et al., 1985), has much in common with the FST. Mice are suspended by their tails for six minutes. During this time, as in the FST, we see both activity and immobility. The TST serves as a predictor of antidepressant activity, as a variety of drugs are capable of decreasing the time spent immobile. As with the FST, stimulants, too, are capable of decreasing immobility; so, to distinguish stimulant and antidepressant effects, the test is often administered in conjunction with a test for general locomotor activity. It is sensitive to the same range of antidepressants as is the FST, and serves as another simple test of antidepressant efficacy (Steru et al., 1985).
Very little has been published on the effect of lithium on immobility time in the TST (Table 8). A low dose of lithium (1 mEq/kg, the subactive dose used as an adjunct to subactive doses of antidepressants in the FST experiments), however, has been shown to potentiate the effect of various antidepressants to reduce immobility (Redrobe and Bourin, 1997). This study also showed that lithium shares this potentiating effect, as it did in the FST, with clonidine and quinine, although the specific antidepressants whose effect lithium potentiated did not always overlap.
The Effect of Lithium on Learned Helplessness
In the learned helplessness (LH) model, the mouse or rat is placed in a box from which it cannot escape, and is intermittently and repeatedly shocked. This can happen once or several times before the actual test day. When the animal is subsequently tested, it is placed in a shuttle box, which has two compartments. Shocks are administered in only one of these compartments at a time, and the test determines whether or not the animal escapes from the shock by moving to the other compartment. Scoring involves counting the number escape failures, and timing the latency to escape. Animals that had not received the inescapable shock generally learn quickly that the shock on test day can be evaded. Animals that did receive the inescapable shock, however, escape less frequently, a deficit theoretically mediated by the earlier inability to control or escape the shocks.
Reports have suggested that lithium administration either decreased (Geoffroy et al., 1991) or did not affect (Stewart et al., 1991a) the escape behavior of rats in the LH model using long-term lithium administration i.p. or in chow, respectively (Table 8). The doses used in these tests were outside the upper therapeutic serum level of 1.2 mEq/l (the low end of the range was 1.3 mEq/l); however, the effect is likely not attributable to toxicity, since lithium-treated animals that had not undergone inescapable shock did not differ from the controls, illustrating an effect which specifically altered the development of helplessness, rather learning or general motor activity. In mice, however, within the therapeutic serum range, chronic but not acute lithium administered via the drinking water was shown to decrease escape deficits 24 hours after exposure to inescapable shock (Faria and Teixeira, 1993; Teixeira et al., 1995). In these studies lithium did not have an effect on escape failures in mice that had not received the inescapable shock training; that is to say, lithium neither increased nor decreased the ability of the animal to learn to escape but, at least in these studies, prevented the development of helplessness specifically (Geoffroy et al., 1991).
The Effect of Lithium on Stress-, Isolation- and Reserpine-Induced Hypoactivity
Hypoactivity, generally measured in terms of locomotion, wheel turns, and/or rearing, can be induced by stress such as isolation or immobilization. It can also be induced by the administration of certain drugs; among these, reserpine, which depletes brain monoamines, is the most commonly used. In this case, a loading dose of the drug is often given some time before another dose on the actual test day. In these cases the induction of a decrease in activity is considered a model of depression and is consistently reversed by lithium. Lithium administered i.p. for eight days to mice, and in chow for 21 days for rats, was effective at reducing reserpine-induced hypolocomotion (Borison et al., 1978; Lerer et al., 1980) (Table 8). Chronic lithium has also been shown to decrease hypoactivity induced by isolation in mice (Frances et al., 1981b), by immobilization in rats (Kofman et al., 1995), and by shock exposure in rats (Hines, 1986a) (Table 8).
VI. Reward Behavior
Background
A characteristic of mania is an elevated hedonic tendency. Due to lithium’s action as anti-manic agent, its effect on behavioral models of such tendencies has been investigated. A common behavioral model for testing the addictive or hedonic effects of a drug is conditioned place preference, which will not be discussed in this section, as it will be addressed in the section below on the aversive effects of lithium. In other studies, however, the effect of lithium on drug consumption and addiction has been studied with tests that do not require the institution of a conditioned aversion. Such tests have measured the effect of lithium on intracranial self-stimulation (ICSS), as well as on alcohol and morphine addiction behaviors. For ICSS, some studies have used electrodes implanted in the lateral hypothalamus, but the medial forebrain bundle is the site most frequently used. Measurements can be made of the rate of self-stimulation, as well as the threshold current at which self-stimulation begins.
Effect of Lithium on Reward Behavior in Humans
Early double-blind, controlled studies appeared to indicate that lithium decreased the consumption of alcohol in depressed alcoholics (Kline et al., 1974; Merry et al., 1976). This effect did not appear to be associated with a decrease in depressive symptoms, but non-depressed alcoholics were not affected similarly. Subsequent studies, however, were ultimately unable to replicate the results with any consistency (see (Lejoyeux and Ades, 1993) for review). Thus, in general, lithium is not considered an appropriate treatment for alcoholism. The impact of lithium on the effects of morphine in humans has not been extensively studied, but one double-blind study indicated that lithium is not able to attenuate the euphoric effects of morphine; in fact, lithium appeared to potentiate morphine-induced euphoria (Jasinski et al., 1977).
Effect of Lithium on Intracranial Self-Stimulation in Rats
The studies of lithium administration and ICSS have all, to the best of our knowledge, used i.p. administration of lithium to rats (Table 9). An early negative study (Ramsey et al., 1972) was challenged by Edelson et al., who found that not only did lithium show an early effect to decrease the rate of self-stimulation, but that the same effect could be shown in the earlier study, if the early results were reexamined (Edelson et al., 1976). The difficulty with this positive result is that it was temporary; lithium only decreased the rate of self-stimulation for the first three of five days. A recent study of the effect of lithium on the self-stimulation threshold also reported that the effects of lithium to increase the threshold were most pronounced after a single injection of 100 mg/kg LiCl (Tomasiewicz et al., 2006), and another positive study only reported the results of acute administration of 1 mEq/kg lithium (Cassens and Mills, 1973). The negative results reported used a longer dosing paradigm, with eight or nine days of lithium injections prior to measuring the change in ICSS (Ramsey et al., 1972; Takigawa et al., 1994). The fact that the acute result is not sustained might suggest that the decreased self-stimulation results from the irritation of the lithium injection. An alternative method of administration (for example in rodent chow) could address this possibility, but we are not aware of any such studies.
Table 9.
Studies of the effect of lithium on reward behaviors in rodents.
| Species, strain | Lithium | Experimental Design | Effect on reward behavior | Reference |
|---|---|---|---|---|
| Rat, SD | Li i.p. | ICSS | No effect | (Ramsey et al., 1972) |
| Rat, LE | Li or d-AMP, i.p. | ICSS | Li decreased; AMP increased | (Cassens and Mills, 1973) |
| Rat, SD | Li i.p. | ICSS | Decreased, then normalized | (Edelson et al., 1976) |
| Rat, SD | Li i.p. | Alcohol consumption | Lithium decreased alcohol consumption, but increased severity of withdrawal symptoms | (Ho and Tsai, 1976) |
| Rat, Holtzmann | Li water | Alcohol consumption | Li produced earlier onset of adjunctive consumption of alcohol and water | (Hines, 1986b) |
| Rat, Holtzmann | Li water | Alcohol consumption paired with inescapable footshock | Alcohol consumption not affected by foot shock, but increased in animals treated with lithium and foot shock | (Hines, 1989) |
| Rat, W | Li i.p. | ICSS | No effect | (Takigawa et al., 1994) |
| Rat, SD | Li i.p. | ICSS | Decreased | (Tomasiewicz et al., 2006) |
Abbreviations: d-AMP: dexamphetamine; ICSS: intracerebral self-stimulation; i.p.: intraperitoneally; LE: Long-Evans; Li: lithium; SD: Sprague-Dawley; W: Wistar.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
Effect of Lithium on Alcohol and Morphine Consumption, Addiction, and Withdrawal
Lithium administration has yielded inconsistent effects on alcohol- and morphine-induced behaviors in rodents (Table 9). While some have reported that lithium can decrease voluntary consumption of alcohol (Ho and Tsai, 1976), morphine-facilitated ICSS (Liebman and Segal, 1976), and morphine ingestion in addicted rats (Tomkiewicz and Steinberg, 1974), it has also been shown to reduce or potentiate opioid-induced analgesia (Saarnivaara and Mannisto, 1976; Johnston and Westbrook, 2004; Hilal Karakucuk et al., 2006), and to reduce or potentiate morphine-induced hyperactivity (Carroll and Sharp, 1971; Sanghvi and Gershon, 1973; Jensen, 1974). One group showed that lithium administration can decrease withdrawal symptoms induced by naloxone in morphine-addicted rats (You et al., 2001). Some biochemical studies have indicated that lithium can increase the biosynthesis and release of endogenous opioids, reduce the number of opioid binding sites, and reduce the binding of opioid receptor antagonists (Wajda et al., 1981; Staunton et al., 1982; Stengaard-Pedersen and Schou, 1982; Stengaard-Pedersen and Schou, 1985; Sivam et al., 1986; Sivam et al., 1987). Lithium can also decrease adenylyl cyclase and cyclic AMP, the levels of which are increased by naloxone administration or rapid morphine withdrawal (Sharma et al., 1975; Sharma et al., 1977).
The effect of lithium on alcohol consumption and withdrawal in rodents has not been extensively studied. One group found that lithium decreased voluntary alcohol consumption, but also dramatically increased the severity of withdrawal symptoms (Ho and Tsai, 1976). Another study supported the ability of lithium to attenuate alcohol consumption (Alexander and Alexander, 1978). The mechanisms of these effects are not clear, but the involvement of the cholinergic system has been proposed (Ho and Tsai, 1976).
VII. Effect of Lithium on Learning and Memory
Background
Multiple preclinical tests purport to evaluate learning and memory in rodents, although not all monitor the same aspects of cognitive functioning (Crawley, 2000). For example, the Morris Water Maze is used to test two aspects of spatial memory. Spatial reference memory is measured by the animal’s ability to learn the location of a hidden platform in a large pool of water. Once learning has occurred, the platform can be moved, and the ability of the animal to learn the new location is a measurement of spatial working memory. Other conditioning tasks, such as active and passive avoidance, measure the ability of the animal to learn and remember an association between an aversive experience, such as mild foot-shocks, and environmental cues. In the active avoidance task, the animal must learn to exit the chamber in which foot-shocks are delivered; in the passive avoidance test, the animal must refrain from entering the chamber in which the shocks are administered. A test of latent inhibition first exposes the animal to a stimulus without a reinforcer. The animal then learns to ignore the stimulus. When that stimulus is later paired with a reinforcer, the ability to learn to associate stimulus with reinforcer is delayed, a phenomenon called latent inhibition. This test is thus a measure of the animal’s ability to learn to ignore irrelevant stimuli.
Effect of Lithium on Human Cognition
Reports on the effect of lithium on human cognition are inconsistent. Although subjective reports of cognitive “slowing” are often found in cohorts of lithium-treated bipolar disorder patients, few controlled studies have objectively investigated these complaints. In one double-blind crossover study researchers found impaired psychomotor performance, but no impairment in memory, in a psychiatric population following two weeks of lithium administration (Squire et al., 1980). In a series of double-blind, controlled studies with normal subjects given lithium for 14 days, Judd and colleagues found lithium-related performance deficits in visual-motor function and processing speed (Judd et al., 1977a; Judd et al., 1977b; Judd et al., 1977c). Another double-blind, controlled study with normal subjects showed a lithium-associated impairment in memory after two weeks (Kropf and Muller-Oerlinghausen, 1979). Reviews of these effects and attempts at meta-analysis have included studies with flawed methodological design, and of necessity have compared studies that use different outcome measures for the cognitive changes (Ananth et al., 1987; Pachet and Wisniewski, 2003). As such, they find the results to be equivocal, and the effect of lithium on learning and memory in humans remains uncertain.
Effect of Lithium on Learning and Memory in Rodents
As in humans, the effect of lithium on cognitive functioning in rodents is ambiguous (Table 10). One group found an improvement in spatial reference memory, as measured by the Morris Water Maze; however, other reports of spatial working memory have yielded inconclusive results (Vasconcellos et al., 2003; Tsaltas et al., 2006). Another group found that six days of i.p. lithium impaired both latent inhibition and the acquisition of the conditioned response in rats which had not been pre-exposed to the stimulus (Cappeliez and Moore, 1988). It has also been reported that while lithium appears to decrease the passive avoidance response to shock, its effect in an active avoidance paradigm is unchanged, leading to the suggestion that lithium decreases not cognition, but sensitivity to low-intensity stimuli (Hines and Poling, 1984; Hines, 1986a). Others, however, have found that lithium improves performance in passive avoidance tasks (Tsaltas et al., 2006). While on the one hand the perceived effect of lithium to cause cognitive impairment can be a serious threat to patient adherence, on the other hand, the neuroprotective effect of lithium might be expected to improve cognitive deficits associated with illness (Manji et al., 1999; Chuang, 2004). As such, the cognitive effects of lithium warrant further investigation in both preclinical and clinical studies.
Table 10.
Studies of the effect of lithium on learning and memory in rodents.
| Species, strain | Lithium | Experimental design | Results | Reference |
|---|---|---|---|---|
| Rat, Holtzman | Li water | AA and PA | Decreased acquisition of PA response. AA escape performance unaffected, but fewer avoid shock entirely. Conclude that learning isn’t changed, but sensitivity to low-intensity stimulus is | (Hines and Poling, 1984) |
| Rat, Wistar | Li i.p. | Latent inhibition | Li impaired filtering of irrelevant stimuli | (Cappeliez and Moore, 1988) |
| Rat, SD | Li i.p. | T maze following stimulation of α-1 adrenergic receptors | Pretreatment with Li reverses the impairment in delayed alternation performance | (Arnsten et al., 1999) |
| Rat | Li chow | Chronic variable stress, then MWM | Li attenuated spatial reference memory deficit in stressed mice. No change in working memory | (Vasconcellos et al., 2003) |
| Rat, LE | Li chow | Object recognition; holeboard spatial discrimination | 4 weeks Li impaired spatial reference memory, but not working memory, in the hole-board. No effect on object recognition memory | (Al Banchaabouchi et al., 2004) |
| Rat, Wistar and SD | Li i.p. | T maze, PA | In 30- and 45-s delayed alternation Li groups were more accurate than controls. In others, no difference. Li animals had higher step-through latencies impassive avoidance, relative to their baseline performance | (Tsaltas et al., 2006) |
Abbreviations: AA: active avoidance; i.p.: intraperitoneally; LE: Long-Evans; Li: lithium; MWM: Morris Water Maze; PA: passive avoidance; SD: Sprague-Dawley; W: Wistar.
Column “Lithium” is administration route of lithium.
VIII. Effects of Lithium on Other Rodent Behaviors
Ouabain-induced Behaviors
Although its precise relationship to the disorder is unknown, a finding in patients with bipolar disorder is dysregulated ion balance, as seen by increased intracellular sodium and calcium, and decreased sodium pump activity in peripheral cells (Looney and el-Mallakh, 1997). In order to investigate this relationship vis-à-vis rodent behavior, some investigators have administered ouabain, a sodium pump inhibitor, to rats. A short period of hyperlocomotion was observed following ouabain administration (El-Mallakh et al., 2003), and this effect could be prevented by two weeks of pretreatment with lithium in the chow (see Table 11). In another study, the same group observed the induction of hypoactivity by ouabain administration, an effect which could also be normalized by lithium, which suggests that under distinct paradigms lithium may reverse the adverse effects of sodium pump inhibition (Li et al., 1997).
Table 11.
Effect of lithium on other rodent behaviors.
| Species, strain | Lithium | Experimental Design | Results | Reference |
|---|---|---|---|---|
| Rat, SD | Li i.p. | ICV ouabain, then OFT | Li reversed hypoactivity | (Li et al., 1997) |
| Rat, SD | Li chow | CF | CIT and MKC-242 inhibited expression of CF. Li for 1 week enhanced the inhibitory effect | (Muraki et al., 1999) |
| Mouse, DBA and C3H | Li chow | PPI | Increased in DBA mice; decreased in C3H mice | (O’Neill et al., 2003) |
| Rat, SD | Li chow | Chronic restraint stress, then OFT | No change | (Wood et al., 2004) |
| Mouse, 129 and C57BL/6 | Li, VPA, or CBZ i.p. | PPI, and KET- or d-AMP-induced deficits | No effect on PPI alone. Li prevented PPI-disruptive effects of d-AMP, but not of KET. CBZ did opposite. VPA prevented none | (Ong et al., 2005) |
| Mice, ddY | Li p.o., or VPA, CBZ i.p. | PPI, alone and with APO or dizocilpine | No effect of any on PPI alone. All prevented APO-induced PPI disruption. Li exacerbated dizocilpine-induced PPI disruption | (Umeda et al., 2006) |
| Rat, SD | Li chow | CF with or without clorgyline | Li + clorgyline reduced freezing relative to lithium or clorgyline alone | (Kitaichi et al., 2006) |
| Rat, SD pups | Li chow | OFT, EPM | No effect on total LOCO, but decreased time in center of open field and in open arms of EPM | (Youngs et al., 2006) |
Abbreviations: APO: apomorphine CBZ: carbamazepine; CF: conditioned freezing; EPM: elevated plus maze; ICV: intracerebroventricularly; i.p.: intraperitoneally; KET: ketamine; Li: lithium; OFT: open field test; p.o.: orally; PPI: prepulse inhibition; SD: Sprague-Dawley; VPA: valproic acid.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
Prepulse Inhibition
Sensorimotor gating refers to the process by which an organism filters extraneous information from both the internal and the external environment. For example, the startle response to a new stimulus typically diminishes with each subsequent exposure, and the prepulse inhibition test (PPI) is a measure of this diminution. Deficits in PPI have been observed in a number of psychiatric illnesses, particularly schizophrenia (Braff et al., 2001). In rodent models, PPI can be disrupted experimentally, for example by d-amphetamine or ketamine administration. Drugs that are effective treatments for schizophrenia, such as antipsychotics, can reverse this deficit. While little has been reported concerning the effect of lithium on PPI in rodents, one group recently showed that lithium could inhibit the PPI deficit induced by d-amphetamine, but not that induced by ketamine in C57BL/6J mice (see Table 11) (Ong et al., 2005). A recent study showed that while acute lithium did not affect PPI alone in ddY mice, it could prevent the PPI deficit induced by apomorphine (Umeda et al., 2006). Another group, however, did find a strain-dependent effect of lithium on PPI in mice, with six weeks of lithium administration improving inhibition in DBA/2 mice, but worsening it in C3H mice (O’Neill et al., 2003). These results must thus be explored further before conclusions as to the effect of lithium on PPI can be drawn.
Fear and Anxiety-like Behaviors
Rodent behavior can be dramatically affected by exposure to stress, and such effects are visible in a number of paradigms, including the forced swim test discussed above. The reported effects of lithium on many tests of fear and anxiety are not consistent; for example, in the conditioned freezing model of fear, in which the duration of the freezing response of electric shock is measured, lithium has been shown to enhance, to inhibit, or to have no impact upon freezing (Table 11) (Muraki et al., 1999; Kitaichi et al., 2006; Youngs et al., 2006).
Lithium-Pilocarpine Seizures
Lithium can be used to induce a state of seizure in rats when combined with pilocarpine, a muscarinic receptor agonist (see Table 12). High doses (400 mg/kg) of pilocarpine alone are capable of inducing this state, but Honchar and colleagues were the first to show that the administration of lithium 24 hours prior to the administration of pilocarpine dramatically alters the dose-response curve, reliably resulting in continuous seizures and subsequent brain damage (Honchar et al., 1983). The length of time between lithium and pilocarpine administration, as well as the doses of both drugs, can impact the characteristics of the seizures, though as few as two hours between the two has yielded seizures in 100% of rats tested, and even 0.5 mEq/kg of lithium can induce the state when administered the full 24 hours before pilocarpine (Morrisett et al., 1987b).
Table 12.
Studies of the effect of lithium and pilocarpine to induce seizures in rats.
| Species, strain | Lithium | Experimental design | Results | Reference |
|---|---|---|---|---|
| Rat, SD | Li s.c. | Li added to PIL, physostigmine, or arecoline, s.c. | SE induced by Li-PIL and by Li-arecoline | (Honchar et al., 1983) |
| Rat | Li s.c. | Li-PIL | SE induced | (Clifford et al., 1987) |
| Rat, SD | Li i.p. | Li-PIL, then anticonvulsants | SE induced. Atropine, phenobarbital, diazepam, CBZ prevented SE. VPA increased latency to SE. Paraldehyde prevented SE, and was the only drug capable of terminating SE once induced | (Morrisett et al., 1987a) |
| Rat, SD | Li i.p. or chow | Li added to PIL, arecoline, or physostigmine, s.c. | SE induced. Li potentiated proconvulsant effects of arecoline and physostigmine | (Morrisett et al., 1987b) |
| Rat, SD | Li s.c. | Li-PIL. Groups tested later for Li-induced CTA | Less CTA in rats that had been SE two months before. Both acquisition and memory of CTA impaired | (Venugopal and Persinger, 1988) |
| Rat, SD | Li i.p. | Li-PIL, in rats of different ages | SE first seen in 7-10 day old rats, but only consistently induced at 11-14 days | (Hirsch et al., 1992) |
| Rat, W | Li i.p. | Li-PIL i.p. Nifedipine, atropine, or calcium channel agonist given pre-PIL to see if they affected SE | SE induced, prevented by atropine but increased by calcium channel agonist. Nifedipine increased mortality | (Wielosz et al., 1995) |
| Rat, W | Li i.p. | Li-PIL. Effect of various adenosinergic agents on SE studied | SE reliably induced, prevented by adenosine and adenosinergic agents | (George and Kulkarni, 1997b) |
| Rat, W | Li i.p. | 30 mg/kg PIL s.c.21 hrs after LiCl. Selective DA receptor antagonists also administered, 30 min. prior to PIL | SE reliably induced, prevented (not reversed) by D2 agonist and D1 antagonist | (George and Kulkarni, 1997a) |
| Rat, W | Li i.p. | Li-PIL | SE induced, blocked by atropine | (Marinho et al., 1997) |
| Rat, SD | Li i.p. | Li-PIL | SE induced, with spatial learning deficits and histological damage | (Wu et al., 2001) |
| Rat, W | Li i.p. | Li-PIL | SE induced. Pathologic EEG activity | (Suchomelova et al., 2002) |
| Rat, SD | Li i.p. | Li-PIL, then MWM | SE induced; SE rats that received topiramate performed better in Morris water maze than SE rats that received saline | (Cha et al., 2002) |
| Rat, SD | Li i.p. | Li-PIL | SE induced | (Nehlig et al., 2002) |
Abbreviations: CBZ: carbamazepine; CTA: conditioned taste aversion; DA: dopamine; EEG: electroencephalograph; i.p.: intraperitoneally; Li: lithium; MWM: Morris Water Maze; PIL: pilocarpine; s.c.: subcutaneously; SD: Sprague-Dawley; SE: status epilepticus; VPA: valproic acid; W: Wistar.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
Lithium-pilocarpine seizure has been proposed as a model of both status epilepticus (SE) and of the behavioral effect of lithium-induced inositol depletion. The relationship of this model to the human phenomenon of SE was shown by Treiman and colleagues, who demonstrated that the EEG pattern produced in rats by the lithium-pilocarpine model, with serum lithium concentrations within the therapeutic range, consistently resembled that seen in humans in a state of SE (Treiman et al., 1990). The model is further validated by the fact that common anticonvulsants have effects that resemble their respective clinical profiles in SE. While drugs such as diazepam and carbamazepine were able to prevent the onset of SE when administered after lithium but prior to pilocarpine, paraldehyde was the only anticonvulsant that was able to stop SE— with respect to behavior, biochemical changes, EEG, and mortality—once the condition had been induced (Morrisett et al., 1987a). This is similar to the clinical data, which show SE to be a treatment-resistant seizure type, influenced by paraldehyde alone out of the anticonvulsants.
While the lithium-pilocarpine seizure model lacks face validity as a model of mania and/or depression, the model can be reversed by increased levels of myo-inositol suggesting that it might have utility in understanding the behavioral relevance of inositol depletion (Kofman and Belmaker, 1993; Kofman et al., 1993). Studies have shown that lithium administration decreases brain inositol, some suggesting that the model might help to investigate the behavioral effects of inositol depletion by lithium, which themselves may be relevant to the mood-stabilizing effects of the drug. For further discussion of this model, see the recent review by Belmaker and Bersudsky (Belmaker and Bersudsky).
IX. Circadian Rhythms
Background
The term “circadian rhythm” describes an approximately 24-hour cycle of physiological and behavioral activity. The duration of these rhythms, which include the sleep-wake cycle as well as the regulation of hormones and temperature, is regulated centrally in the suprachiasmatic nucleus of the hypothalamus. Circadian rhythms are studied by monitoring them either continuously or at a range of time points over the course of the cycle. These sometimes take place in constant light or darkness, such that the rhythms become “free-running,” no longer conforming to the cycle that light-dark changes can impose.
The Effect of Lithium on Circadian Rhythms in Humans
Circadian rhythm abnormalities have been studied in mood disorders for decades. Convergent evidence suggests that abnormal circadian rhythms play a role in mood disorders (Wehr and Wirz-Justice, 1982; Klemfuss, 1992; Healy and Waterhouse, 1995; Bunney and Bunney, 2000). For example, alterations in the sleep/wake cycle and in the period of physiological processes are well documented in patients with bipolar disorder. Non-pharmacological treatments such as sleep deprivation, adjustment of sleep cycles, and light therapy are often helpful in the treatment of mood disorders. Further, sleep deprivation is a common precipitant of the manic phases of bipolar disorder, and a highly efficacious—albeit short-term—treatment for depression. Further, an extensive body of data describes lithium’s impact on circadian rhythms in human and rodent models. For example, human free running circadian rhythm length (in constant darkness) is reportedly increased by lithium (Johnsson et al., 1979).
The Effect of Lithium on Circadian Behaviors in Rodents
The effect of lithium on rodent circadian biochemistry has been studied extensively, and the drug has been shown to act directly on cultured neurons from the suprachiasmatic nucleus (SCN), a central modulator of mammalian circadian regulation, to lengthen the period of firing rate rhythms (Abe et al., 2000). Lithium has been shown to delay the peak times of biochemical rhythms such as calcium, potassium, and glucose levels in rats (Williams and Jope, 1995a; Subramanian et al., 1998). In numerous studies lithium was shown to modify the phase and period of circadian rhythms in a variety of other species, ranging from unicellular organisms and insects to mice and even humans (see (Klemfuss, 1992) for a complete review). These findings have led to a hypothesis that an evolutionarily conserved target of lithium may be both responsible for the actions of lithium on diverse the circadian rhythms of diverse species and the action of lithium as a bipolar disorder medication (Gould and Manji, 2002).
The effect of lithium on the circadian periods of behavioral activity, measured by means of locomotion or activity wheels, has been widely replicated in rodents (see Table 13). Lithium has generally been shown to lengthen these rhythms, delaying their onset when a light-dark cycle is in place, and lengthening their periods when the animal is in constant darkness (Kripke and Wyborney, 1980; Poirel and Larouche, 1989; Stewart et al., 1991b; Hafen and Wollnik, 1994; Nagayama, 1996; Iwahana et al., 2004). The drug also allows the animals to adapt to the artificial extension of the circadian period, which is induced by establishing a light-dark cycle of longer than twenty-four hours (McEachron et al., 1981). The chronobiological effects of lithium on behavior extend also to consumption patterns in rats (Reghunandanan et al., 1989; Nagayama, 1996).
Table 13.
Studies of the effect of lithium on behavioral circadian rhythms.
| Species, strain | Lithium | Experimental Design | Effect on circadian rhythm | Reference |
|---|---|---|---|---|
| Rat, Wistar | Li i.p. | Sleep/wake monitored via continuous EEG | Lengthened period | (Danguir et al., 1976) |
| Rat, SD | Li chow | Activity wheel | Lengthened period | (Kripke and Wyborney, 1980) |
| Rat, SD | Li chow | Activity wheel | Lengthened period | (McEachron et al., 1981) |
| Rat, Wistar | Li via cannula in bilateral SCN | Food consumption | Total intake unchanged; rhythm altered | (Reghunandanan et al., 1989) |
| Mouse, HaMICR | Li p.o. | OFT | Lengthened periods | (Poirel and Larouche, 1989) |
| Rat | Li chow | Activity wheel | Lengthened period | (Stewart et al., 1991b) |
| Rat, ACI/Ztm, BH/Ztm, LEW/Ztm | Li chow | Activity wheel | Lengthened period | (Hafen and Wollnik, 1994) |
| Rat, Wistar | Li or IMI i.p. | OFT, BT, and drinking behavior | Both lengthened period of activity in light-dark; Li alone lengthened periods of LOCO, BT, and drinking in continuous darkness | (Nagayama, 1996) |
| Mouse, C57BL/6 | Li chow | OFT | Lengthened period | (Iwahana et al., 2004) |
Abbreviations: BT: body temperature; EEG: electroencephalograph; IMI: imipramine; i.p.: intraperitoneally; Li: lithium; LOCO: locomotion; OFT: open field test; SCN: suprachiasmatic nucleus; SD: Sprague-Dawley.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
X. Aversive Effects of Lithium
Background
The aversive effects of lithium are those which cause the animal to avoid whatever substance is associated with the drug. These effects are not likely to be related to the therapeutic effects of lithium. They are described by some as possibly toxic effects, but, like the negative side effect profile seen in many lithium-treated bipolar patients, aversion to lithium can be elicited even when serum lithium levels are within the “therapeutic” range (Langham et al., 1975).
Lithium is frequently used in conditioned place or taste preference/aversion tests, which is an established measure to predict addictive qualities of drugs. In place and taste conditioning, distinctive cues—a particular flavor or location—are paired with an unconditioned stimulus. This stimulus might be a positive (e.g. morphine) or negative (e.g. naloxone) reinforcer. In these tasks lithium is an aversive cue. The test determines whether the animal exhibits preference, aversion, or neutrality towards the cue in the absence of the reinforcer. Thus a location previously paired with morphine will be frequented more, and a flavor associated with naloxone will be avoided. In addition to a reinforcing value, motivation for a particular location or flavor can be affected by hunger and thirst (Perks and Clifton, 1997). Drugs are often selective for either conditioned place or taste preference and aversion, and some lesion studies indicate that neuroanatomical regions might preferentially induce or inhibit one or the other (Persinger et al., 1994; Schalomon et al., 1994).
Effect of Lithium on Conditioned Aversion Models
Many data indicate the robust ability of lithium to induce both taste and place aversion, as well as to abolish the taste/place preferences induced by other drugs (See Table 14). Virtually all data on the effect of lithium in these tests has been accumulated from studies with rats, not mice. A particular flavor is associated with a negative physiological sensation, as can be induced by a high dose of lithium (generally administered intraperitoneally following consumption of its contingent flavor) (Crawley, 2000). In this way, lithium is capable of inducing taste aversion to saccharin, which would otherwise be associated with taste preference (Berg and Baenninger, 1974). It is also capable of reducing the reinforcing properties of sucrose in the conditioned place preference test: after twelve days of conditioning, sucrose place preference was instituted in Lister mice. After this time, sucrose was administered in the home cage, followed by lithium injections. The subsequent test date revealed that the pairing of lithium with the sucrose, effectively the induction of a taste aversion, was able to decrease the place preference induced by sucrose alone (Perks and Clifton, 1997).
Table 14.
Studies of the effect of lithium on conditioned preference and aversion tests in rats.
| Species, strain | Lithium | Experimental design | Results | Reference |
|---|---|---|---|---|
| Rat, Holtzmann | 20 experimental drugs tested | Muricide | All ADs, stimulants, and antihistaminics inhibited muricide. Amygdala lesion inhibited muricide | (Horovitz et al., 1966) |
| Rat, LE | Li i.p. | Muricide | Decreased | (Krames et al., 1973) |
| Rat, LE | Li i.p. | CTA | Li induced CTA | (Berg and Baenninger, 1974) |
| Rat, LE | Li i.g. | Muricide | Decreased | (Berg and Baenninger, 1974) |
| Rat, SD | Li i.p. | Muricide | Low-dose: no effect; high dose decreased, but maybe toxic | (Rush and Mendels, 1975) |
| Mouse, Harlan | Li i.p. | Cricket killing | Decreased | (Lowe and O’Boyle, 1976) |
| Rat, W | Li IV | CPA | Li produced CPA | (Mucha et al., 1982) |
| Rat, W | DMI, chloroipramine, AMI, CPZ, DZP, s.c. | Muricide | All decreased muricide, but CPZ and DZP also showed sedation | (Shibata et al., 1984) |
| Rat, Hooded | Li i.p. | CPA | Li dose-dependently institutes CPA | (White and Carr, 1985) |
| Rat, W | Li s.c. | Muricide | Decreased | (Yamamoto et al., 1985) |
| Rat, SD | Li chow | CPP/CPA | Li abolished MOR-induced CPP, naloxone-induced CPA, but not d-AMP-induced CPP or U-69593-induced CPA | (Shippenberg and Herz, 1991) |
| Rat, SD | Li i.p. | CPA/CTA | Rats conditioned for CPA and CTA showed strong CTA, but not CPA | (Schalomon et al., 1994) |
| Rat, SD | Li i.p. | CPP | Li did not affect COC-induced place preference | (Shippenberg and Heidbreder, 1995) |
| Rat, W | Li i.p. | CPA | CPA induced | (Frisch et al., 1995) |
| Rat, Lister | Li i.p. | CPP | Li decreases reinforcing value of sucrose | (Perks and Clifton, 1997) |
Abbreviations: AMI: amitryptiline; COC: cocaine; CPA: conditioned place aversion; CPP: conditioned place preference; CPZ: chlorpromazine; CTA: conditioned taste aversion; d-AMP: dexamphetamine; DZP: diazepam; i.p.: intraperitoneally; IV: intravenously; LE: Long-Evans; MOR: morphine; s.c.: subcutaneously; SD: Sprague-Dawley; W: Wistar.
Column “Lithium” is administration route of lithium or alternate mood stabilizer, if noted.
That the conditioned aversion of lithium arises from a negative physiological sensation is illustrated by the fact that conditioned place aversion can be blocked by pre-treatment with antiemetics (Frisch et al., 1995), or by lesioning the area postrema (Ritter et al., 1980). Such place aversion conditioned by lithium exposure is dose-dependent in rats, though there is some suggestion that the conditioned place aversion is not as strong as the conditioned taste aversion discussed above (White and Carr, 1985; Schalomon et al., 1994). Chronic lithium has been effective at abolishing the place preference induced by morphine, a mu-opioid receptor agonist; it has also been effective at abolishing place aversion induced by opioid receptor antagonist naloxone (Shippenberg and Herz, 1991). In this same study, Shippenberg and Herz found that lithium was unable to reverse amphetamine-induced place preference.
The aversive effect of lithium is also seen in models of “predatory” aggression, such as muricide in rats, or cricket-killing for mice. In the test of muricide, a mouse is placed inside the rat’s cage, and the attack and feeding behavior of the rat are evaluated. In this test, however, lithium is administered after—rather than before, as we see in the suppression of aggression—the behavior is elicited. The literature is very consistent, and leads to a clear conclusion that lithium prevents this behavior (Table 14). The aversive effect of lithium appears in some cases to be specific to the ingestion, rather than the attack; however, results do vary. Several investigators have looked more deeply into the specific parameters of this effect, but their details are outside the scope of this review.
XI. Possible Mechanisms
The behavioral effects of lithium as we describe are many and varied (Table 1), and a number hypotheses have been offered to describe the mechanism by which those effects may be mediated. While the purpose of this review is not to fully detail possible mechanisms for the behaviors we have described, this is an obvious critical step in understanding the relevant biochemical actions of lithium. As detailed in Figure 1, lithium has a number of direct inhibitory actions on cellular enzymes, as well as indirect effects on cell signaling pathways and brain physiological processes (see (Jope, 1999; Phiel and Klein, 2001; Shaldubina et al., 2001; Chuang, 2004; Gould et al., 2004; Williams et al., 2004) for extensive discussions).
Direct Targets of Lithium
While the administration of lithium can have an effect on a number of targets, those that are affected at therapeutic concentrations of lithium are more limited. By identifying which of these is responsible for the behavioral effects of lithium, we will be in a better position to develop more specific compounds, which mimic the mood-stabilizing properties of the drug, without the associated side effects.
Many of the direct targets of lithium of relevance to this review are likely magnesium-dependent intracellular enzymes. Several decades ago, lithium was shown to decrease brain inositol concentrations in rats (Allison and Stewart, 1971), and this effect was shown to occur through inhibition of inositol monophosphatase (IMPase) (Naccarato et al., 1974). Additional direct targets of lithium include: fructose 1,6-biphosphatase (FBPase), bisphosphate nucleotidase (BPNase), and inositol polyphosphate 1-phosphatase (IPPase) (York et al., 1995). Lithium also inhibits the metabolic enzyme phosphoglucomutase (PGM) (Ray et al., 1978) and glycogen synthase kinase (GSK-3) (Klein and Melton, 1996). Evidence also supports a role of magnesium competition to explain the effect of lithium in inhibition of G-protein function, in particular GTP binding and cyclic AMP production (see (Phiel and Klein, 2001; Gould et al., 2004) for reviews of direct lithium targets).
The inositol depletion hypothesis, supported by a large body of data, suggests that IMPase is a therapeutically relevant target of lithium and that the primary clinical effects of lithium are dependent upon a reduction in brain inositiol levels (Berridge et al., 1989); however, this hypothesis has long been debated. Some rodent biochemical studies have implicated inositol depletion in the induction of the lithium-pilocarpine seizures discussed earlier as a possible model of both status epilepticus and mania (Honchar et al., 1983; Honchar et al., 1990; Kofman et al., 1993; Kofman et al., 1995; Williams and Jope, 1995b), and in the effect of lithium on rearing (Kofman and Belmaker, 1993) and anxiety-like behaviors (Kofman et al., 2000; Youngs et al., 2006).
GSK-3 has attracted considerable attention since it was discovered, by Klein and Melton in 1996, to be directly inhibited by therapeutic levels of lithium (Klein and Melton, 1996). Substantial data implicates GSK-3 in the behavioral effects of lithium, including its attenuation of stimulant-induced hyperlocomotion, its actions in the forced swim test, and its alteration of circadian rhythms, three behavioral models relevant to the study of mood disorders and mood stabilization (see (Gould and Manji, 2005) for review). GSK-3 is involved in a number of signaling pathways, and the manipulation of signal transduction has gained favor recently as a means by which a single drug such as lithium might have such a broad range of effects.
Effects on Intracellular Signaling
Many of the direct targets of lithium are regulatory components of intracellular signaling pathways; thus, the fact that lithium has varied effects on a large number of signaling cascades is not surprising. Signaling pathways regulated by lithium include cyclic AMP, phosphoinositol/protein kinase C, the Wnt signaling pathway, ERK MAP kinase, PI3K/AKT/GSK-3, and arachidonic acid signaling pathway among others (see (Jope, 1999; Gould et al., 2004) for review). These pathways are also targets of other mood stabilizers, pointing to the possibility that their modulation is relevant to the therapeutic effects of the various treatments (Phiel and Klein, 2001; Gould et al., 2004). Signaling pathways are involved in the regulation of complex neurobiological functions such as cognition, appetite, sexual arousal, sleep patterns, weight, and response to hormones, all of which are altered in mood disorders and likely have effects on rodent behavior (Manji et al., 2003). The full clinical effects of psychotropic medications such as lithium are only observed after chronic treatment, implicating long-term processes, such as changes in the structure and connections of neurons and/or glia in the ultimate effects of treatment (Hyman and Nestler, 1996). Lithium-induced changes in signaling pathways likely regulate changes in protein and gene expression, leading to long-term changes in the plasticity of neurons. As we discuss in the section below, these effects on cellular signaling are likely related to the effects of lithium on cellular plasticity and physiology, which in turn might be related to its effects on behavior (Einat and Manji, 2006).
Physiological Actions of Lithium
Because the majority of antidepressants have been shown to enhance serotonergic and/or noradrenergic function, the antidepressant effect of lithium might also be associated with a similar action. Serotonergic neurotransmission has been shown to be altered by lithium; for example, some in vivo studies have shown increased 5-HT synthesis in the whole brain (Sheard and Aghajanian, 1970; Perez-Cruet et al., 1971; Berggren, 1985), and others have shown that lithium increases 5-HT turnover in certain brain regions (Eroglu and Hizal, 1987; Ghoshdastidar et al., 1989). The cholinergic, dopaminergic, and noradrenergic systems are also affected by lithium (Cassens and Mills, 1973; Singer and Rotenberg, 1973). Amphetamine-induced hyperlocomotion, for example, is likely related, at least in part, to the latter two systems. While lithium does not necessarily affect these neurotransmitter systems directly, it is likely that lithium modification of dopamine-mediated behavioral changes involves changes in receptor sensitivity or altered activation of downstream signaling pathways. The effects of lithium in the FST and TST (see section on Depression-like Behaviors) have been associated with the enhancement of serotonergic signaling (Nixon et al., 1994; Redrobe and Bourin, 1997; Petit-Demouliere et al., 2005).
Lithium also has robust neuroprotective effects in a number of paradigms. Significant regional impairments in cellular resilience (how cells respond to stressful stimuli) and synaptic plasticity have recently been associated with a multitude of psychiatric disorders, suggesting that neurochemical manipulation might be insufficient to treat such illnesses. Recent reviews have focused on the trophic effects of mood stabilizers, and the neuroprotective value of drug administration against neurotraumatic insults (Manji et al., 1999; Chuang, 2004). Lithium, which has known neuroprotective properties, has received favorable attention with the advent of the hypothesis that neuroplasticity and cellular resilience are important factors in mood disorder etiology and treatment (Manji et al., 1999). These studies, as well as further studies of the means by which lithium exerts its neurotrophic effects, may hold much promise for the development of future treatments.
XII. Future Applications of Lithium-Sensitive Animal Models
An understanding of both the immediate and the downstream effects of lithium is important for developing drugs that mimic its therapeutic actions, but this cannot be the sole purpose of such research. These models must also be investigated with a view toward understanding the underlying behavioral and biochemical dysfunctions characteristic of the illnesses it treats. Progress will require that a critical eye be cast on our work with behavioral models, so that we are not merely replicating effects that have been seen before (i.e. models that may be testing the same construct), or eliciting behaviors with a relationship to human disease that is tenuous at best. The endophenotype approach can provide basic research with new focus on the underlying disease process, so that animal models are no longer founded upon mere face validity, particularly for syndromes whose characteristic symptoms, such as mood alteration, are impossible to replicate in rodents. It is critical to examine the models discussed in this review to determine whether they represent models of symptom facets or endophenotypes, the latter of which likely are closer to the true underlying disease process and susceptibility genes than symptoms (Seong et al., 2002; Gottesman and Gould, 2003; Gould and Gottesman, 2006). Equipped with the molecular and cellular techniques that have developed relatively recently by comparison with many behavioral protocols, the use of behavioral models is prepared to enter a new phase, with greater relevance and a greater capacity to inform our understanding of human disorders. Known genes and pathways are being brought to our study of the models, as we use transgenic mice or pharmacological probes to investigate how the factors associated with clinical disorders are reflected in the behavior of laboratory animals. We are better equipped to unravel the mysteries of the molecular, cellular, and genetic underpinnings of the models that had until now been familiar but ill understood. Informed by clinical data, we would be well served to seek animal models of endophenotypes in complex disorders, to ensure that the behaviors we elicit have direct relevance to the underlying dysfunction of neurobiology found in the human condition. The animal models we have discussed illustrate the breadth of the effects of lithium; molecular techniques can penetrate the depth of those effects. The two avenues are complementary, and ought to be integrated with one another if we are to understand the meaning of our results and their relevance to human illness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe M, Herzog ED, Block GD. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport. 2000;11:3261–3264. doi: 10.1097/00001756-200009280-00042. [DOI] [PubMed] [Google Scholar]
- Al Banchaabouchi M, Pena de Ortiz S, Menendez R, Ren K, Maldonado-Vlaar CS. Chronic lithium decreases Nurr1 expression in the rat brain and impairs spatial discrimination. Pharmacol Biochem Behav. 2004;79:607–621. doi: 10.1016/j.pbb.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Alexander GJ, Alexander RB. Alcohol consumption in rats treated with lithium carbonate or rubidium chloride. Pharmacol Biochem Behav. 1978;8:533–536. doi: 10.1016/0091-3057(78)90383-0. [DOI] [PubMed] [Google Scholar]
- Allison JH, Stewart MA. Reduced brain inositol in lithium-treated rats. Nat New Biol. 1971;233:267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, Innis RB. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- Ananth J, Ghadirian AM, Engelsmann F. Lithium and memory: a review. Can J Psychiatry. 1987;32:312–316. doi: 10.1177/070674378703200414. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR. Oscillation follows drug sensitization: implications. Crit Rev Neurobiol. 1996;10:101–117. doi: 10.1615/critrevneurobiol.v10.i1.50. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR, Kucinski BJ, Fowler H, Gershon S, Edwards DJ, Austin MC, Stiller R, Kiss S, Kocan D. The effects of lithium on a potential cycling model of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:495–510. doi: 10.1016/s0278-5846(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Arban R, Maraia G, Brackenborough K, Winyard L, Wilson A, Gerrard P, Large C. Evaluation of the effects of lamotrigine, valproate and carbamazepine in a rodent model of mania. Behav Brain Res. 2005;158:123–132. doi: 10.1016/j.bbr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Arnt J. Differential effects of classical and newer antipsychotics on the hypermotility induced by two dose levels of D-amphetamine. Eur J Pharmacol. 1995;283:55–62. doi: 10.1016/0014-2999(95)00292-s. [DOI] [PubMed] [Google Scholar]
- Austin MP, Souza FG, Goodwin GM. Lithium augmentation in antidepressant-resistant patients. A quantitative analysis. Br J Psychiatry. 1991;159:510–514. doi: 10.1192/bjp.159.4.510. [DOI] [PubMed] [Google Scholar]
- Barros HM, Leite JR. Effects of acute and chronic carbamazepine administration on apomorphine-elicited stereotypy. Eur J Pharmacol. 1986;123:345–349. doi: 10.1016/0014-2999(86)90707-7. [DOI] [PubMed] [Google Scholar]
- Bauer M, Forsthoff A, Baethge C, Adli M, Berghofer A, Dopfmer S, Bschor T. Lithium augmentation therapy in refractory depression-update 2002. Eur Arch Psychiatry Clin Neurosci. 2003;253:132–139. doi: 10.1007/s00406-003-0430-9. [DOI] [PubMed] [Google Scholar]
- Belmaker HR, Bersudsky Y. Lithium-pilocarpine seizures as a model for lithium action in mania. Neurosci Biobehav Revs. 2007 doi: 10.1016/j.neubiorev.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Berg D, Baenninger R. Predation: separation of aggressive and hunger motivation by conditioned aversion. J Comp Physiol Psychol. 1974;86:601–606. doi: 10.1037/h0036174. [DOI] [PubMed] [Google Scholar]
- Berggren U. Effects of chronic lithium treatment on brain monoamine metabolism and amphetamine-induced locomotor stimulation in rats. J Neural Transm. 1985;64:239–250. doi: 10.1007/BF01256470. [DOI] [PubMed] [Google Scholar]
- Berggren U, Tallstedt L, Ahlenius S, Engel J. The effect of lithium on amphetamine-induced locomotor stimulation. Psychopharmacology (Berl) 1978;59:41–45. doi: 10.1007/BF00428028. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Bersudsky Y, Shaldubina A, Belmaker RH. Lithium’s effect in forced-swim test is blood level dependent but not dependent on weight loss. Behav Pharmacol. 2007;18:77–80. doi: 10.1097/FBP.0b013e32801416ed. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Dev Psychopathol. 2005;17:865–891. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Borison RL, Sabelli HC, Maple PJ, Havdala HS, Diamond BI. Lithium prevention of amphetamine-induced ‘manic’ excitement and of reserpine-induced ‘depression’ in mice: possible role of 2-phenylethylamine. Psychopharmacology (Berl) 1978;59:259–262. doi: 10.1007/BF00426631. [DOI] [PubMed] [Google Scholar]
- Bourin M, Colombel MC, Malinge M, Bradwejn J. Clonidine as a sensitizing agent in the forced swimming test for revealing antidepressant activity. J Psychiatry Neurosci. 1991;16:199–203. [PMC free article] [PubMed] [Google Scholar]
- Bourin M, Hascoet M, Colombel MC, Redrobe JP, Baker GB. Differential effects of clonidine, lithium and quinine in the forced swimming test in mice for antidepressants: possible roles of serotoninergic systems. Eur Neuropsychopharmacol. 1996;6:231–236. doi: 10.1016/0924-977x(96)00025-9. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Brain PF, Al-Maliki S. Effects of lithium chloride injections on rank-related fighting, maternal aggression and locust-killing responses in naive and experienced ‘TO’ strain mice. Pharmacol Biochem Behav. 1979;10:663–669. doi: 10.1016/0091-3057(79)90318-6. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Molecular clock genes in man and lower animals: possible implications for circadian abnormalities in depression. Neuropsychopharmacology. 2000;22:335–345. doi: 10.1016/S0893-133X(99)00145-1. [DOI] [PubMed] [Google Scholar]
- Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196:326–328. doi: 10.1126/science.847477. [DOI] [PubMed] [Google Scholar]
- Campbell M, Adams PB, Small AM, Kafantaris V, Silva RR, Shell J, Perry R, Overall JE. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34:445–453. [PubMed] [Google Scholar]
- Cao BJ, Peng NA. Magnesium valproate attenuates hyperactivity induced by dexamphetamine-chlordiazepoxide mixture in rodents. Eur J Pharmacol. 1993;237:177–181. doi: 10.1016/0014-2999(93)90266-k. [DOI] [PubMed] [Google Scholar]
- Cappeliez P, Moore E. Effects of lithium on latent inhibition in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:431–443. doi: 10.1016/0278-5846(88)90103-0. [DOI] [PubMed] [Google Scholar]
- Cappeliez P, Moore E. Effects of lithium on an amphetamine animal model of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:347–358. doi: 10.1016/0278-5846(90)90023-a. [DOI] [PubMed] [Google Scholar]
- Cappeliez P, White N. Lithium induces dose-related increases and decreases in activity levels in the rat. Psychopharmacology (Berl) 1981;73:34–38. doi: 10.1007/BF00431097. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Sharp PT. Rubidium and lithium: opposite effects on amine-mediated excitement. Science. 1971;172:1355–1357. doi: 10.1126/science.172.3990.1355. [DOI] [PubMed] [Google Scholar]
- Cassens GP, Mills AW. Lithium and amphetamine: opposite effects on threshold of intracranial reinforcement. Psychopharmacologia. 1973;30:283–290. doi: 10.1007/BF00422875. [DOI] [PubMed] [Google Scholar]
- Cha BH, Silveira DC, Liu X, Hu Y, Holmes GL. Effect of topiramate following recurrent and prolonged seizures during early development. Epilepsy Res. 2002;51:217–232. doi: 10.1016/s0920-1211(02)00157-2. [DOI] [PubMed] [Google Scholar]
- Charney DS, Barlow DH, Botteron KN, Cohen JD, Goldman D, Gur RC, Lin KM, Lopez JF, J.H. M-W, S.O. M, Nestler EJ, Watson SJ, Zalcman SJ. Neuroscience research agenda to guide development of a pathophysiologically based classification system. In: Kupfer DJ, First MB, Regier DA, editors. A research agenda for DSMV. American Psychiatric Association; Washington, D.C.: 2002. pp. xxiii–307. [Google Scholar]
- Chuang DM. Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases? Crit Rev Neurobiol. 2004;16:83–90. doi: 10.1615/critrevneurobiol.v16.i12.90. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Olney JW, Maniotis A, Collins RC, Zorumski CF. The functional anatomy and pathology of lithium-pilocarpine and high-dose pilocarpine seizures. Neuroscience. 1987;23:953–968. doi: 10.1016/0306-4522(87)90171-0. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Impulsive aggression: a behavior in search of clinical definition. Harv Rev Psychiatry. 1998;5:336–339. doi: 10.3109/10673229809003583. [DOI] [PubMed] [Google Scholar]
- Connor DF, Steingard RJ. A clinical approach to the pharmacotherapy of aggression in children and adolescents. Ann N Y Acad Sci. 1996;794:290–307. doi: 10.1111/j.1749-6632.1996.tb32529.x. [DOI] [PubMed] [Google Scholar]
- Cornell DG, Warren J, Hawk G, Stafford E, Oram G, Pine D. Psychopathy in instrumental and reactive violent offenders. J Consult Clin Psychol. 1996;64:783–790. doi: 10.1037//0022-006x.64.4.783. [DOI] [PubMed] [Google Scholar]
- Cox C, Harrison-Read PE, Steinberg H, Tomkiewicz M. Lithium attenuates drug-induced hyperactivity in rats. Nature. 1971;232:336–338. doi: 10.1038/232336a0. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse?: behavioral phenotyping of transgenic and knockout mice. Wiley-Liss; New York: 2000. [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. Animal models of mood disorders: Recent developments. Curr Opin Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- Cryns K, Shamir A, Shapiro J, Daneels G, Goris I, Van Craenendonck H, Straetemans R, Belmaker RH, Agam G, Moechars D, Steckler T. Lack of Lithium-Like Behavioral and Molecular Effects in IMPA2 Knockout Mice. Neuropsychopharmacology. 2007;32:881–891. doi: 10.1038/sj.npp.1301154. [DOI] [PubMed] [Google Scholar]
- Dalen P. Lithium therapy in Huntington’s chorea and tardive dyskinesia. Lancet. 1973;1:107–108. doi: 10.1016/s0140-6736(73)90510-2. [DOI] [PubMed] [Google Scholar]
- Danguir J, Nicolaidis S, Perino-Martel MC. Effects of lithium chloride on sleep patterns in the rat. Pharmacol Biochem Behav. 1976;5:547–550. doi: 10.1016/0091-3057(76)90267-7. [DOI] [PubMed] [Google Scholar]
- Davies C, Sanger DJ, Steinberg H, Tomkiewicz M, U’Prichard DC. Lithium and alpha-methyl-p-tyrosine prevent “manic” activity in rodents. Psychopharmacologia. 1974;36:263–274. doi: 10.1007/BF00421808. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Eliashar S, Belmaker RH, Ben-Uriah Y, Yehuda S. Chronic lithium treatment and dopamine-mediated behavior. Biol Psychiatry. 1980;15:459–467. [PubMed] [Google Scholar]
- Edelson A, Gottesfeld Z, Samuel D, Yuwiler A. Effect of lithium and other alkali metals on brain chemistry and behavior. II. Intracranial self-stimulation behavior. Psychopharmacologia. 1976;45:233–237. doi: 10.1007/BF00421133. [DOI] [PubMed] [Google Scholar]
- Eichelman B, Barchas J. Facilitated shock-induced aggression following antidepressive medication in the rat. Pharmacol Biochem Behav. 1975;3:601–604. doi: 10.1016/0091-3057(75)90180-x. [DOI] [PubMed] [Google Scholar]
- Eichelman B, Thoa NB, Perez-Cruet J. Alkali metal cations: effects on aggression and adrenal enzymes. Pharmacol Biochem Behav. 1973;1:121–123. doi: 10.1016/0091-3057(73)90066-x. [DOI] [PubMed] [Google Scholar]
- Einat H, Manji HK. Cellular Plasticity Cascades: Genes-To-Behavior Pathways in Animal Models of Bipolar Disorder. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.11.004. [DOI] [PubMed] [Google Scholar]
- El-Mallakh RS, El-Masri MA, Huff MO, Li XP, Decker S, Levy RS. Intracerebroventricular administration of ouabain as a model of mania in rats. Bipolar Disord. 2003;5:362–365. doi: 10.1034/j.1399-5618.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Eroglu L, Hizal A. Antidepressant action of lithium in behavioral despair test. Pol J Pharmacol Pharm. 1987;39:667–673. [PubMed] [Google Scholar]
- Faria MS, Teixeira NA. Reversal of learned helplessness by chronic lithium treatment at a prophylactic level. Braz J Med Biol Res. 1993;26:1201–1212. [PubMed] [Google Scholar]
- Fava M. Psychopharmacologic treatment of pathologic aggression. Psychiatr Clin North Am. 1997;20:427–451. doi: 10.1016/s0193-953x(05)70321-x. [DOI] [PubMed] [Google Scholar]
- Ferrie L, A HY, McQuade R. Effect of lithium and lithium withdrawal on potassium-evoked dopamine release and tyrosine hydroxylase expression in the rat. Int J Neuropsychopharmacol. 2005:1–7. doi: 10.1017/S1461145705006243. [DOI] [PubMed] [Google Scholar]
- Fessler RG, Sturgeon RD, London SF, Meltzer HY. Effects of lithium on behaviour induced by phencyclidine and amphetamine in rats. Psychopharmacology (Berl) 1982;78:373–376. doi: 10.1007/BF00433745. [DOI] [PubMed] [Google Scholar]
- Flemenbaum A. Lithium and amphetamine hyperactivity in rats. Differential effect on d and l isomers? Neuropsychobiology. 1975;1:325–334. doi: 10.1159/000117509. [DOI] [PubMed] [Google Scholar]
- Flemenbaum A. Lithium inhibition of norepinephrine and dopamine receptors. Biol Psychiatry. 1977;12:563–572. [PubMed] [Google Scholar]
- Frances H, Marion MH, Simon P. Antidepressant action of lithium: proposed mechanism based upon behavioral analysis in the mouse. Prog Neuropsychopharmacol. 1981a;5:357–361. doi: 10.1016/0364-7722(81)90086-2. [DOI] [PubMed] [Google Scholar]
- Frances H, Maurin Y, Lecrubier Y, Puech AJ, Simon P. Effect of chronic lithium treatment on isolation-induced behavioral and biochemical effects in mice. Eur J Pharmacol. 1981b;72:337–341. doi: 10.1016/0014-2999(81)90572-0. [DOI] [PubMed] [Google Scholar]
- Frisch C, Hasenohrl RU, Mattern CM, Hacker R, Huston JP. Blockade of lithium chloride-induced conditioned place aversion as a test for antiemetic agents: comparison of metoclopramide with combined extracts of Zingiber officinale and Ginkgo biloba. Pharmacol Biochem Behav. 1995;52:321–327. doi: 10.1016/0091-3057(95)00073-6. [DOI] [PubMed] [Google Scholar]
- Gallager DW, Pert A, Bunney WE., Jr. Haloperidol-induced presynaptic dopamine supersensitivity is blocked by chronic lithium. Nature. 1978;273:309–312. doi: 10.1038/273309a0. [DOI] [PubMed] [Google Scholar]
- Gambarana C, Ghiglieri O, Masi F, Scheggi S, Tagliamonte A, De Montis MG. The effects of long-term administration of rubidium or lithium on reactivity to stress and on dopamine output in the nucleus accumbens in rats. Brain Res. 1999;826:200–209. doi: 10.1016/s0006-8993(99)01286-x. [DOI] [PubMed] [Google Scholar]
- Geoffroy M, Tvede K, Christensen AV, Schou JS. The effect of imipramine and lithium on “learned helplessness” and acetylcholinesterase in rat brain. Pharmacol Biochem Behav. 1991;38:93–97. doi: 10.1016/0091-3057(91)90594-r. [DOI] [PubMed] [Google Scholar]
- George B, Kulkarni SK. Dopaminergic modulation of lithium/pilocarpine-induced status epilepticus in rats. Methods Find Exp Clin Pharmacol. 1997a;19:481–488. [PubMed] [Google Scholar]
- George B, Kulkarni SK. Modulation of lithium-pilocarpine-induced status epilepticus by adenosinergic agents. Methods Find Exp Clin Pharmacol. 1997b;19:329–333. [PubMed] [Google Scholar]
- Gerlach J, Thorsen K, Munkvad I. Effect of lithium on neuroleptic-induced tardive dyskinesia compared with placebo in a double-blind cross-over trial. Pharmakopsychiatr Neuropsychopharmakol. 1975;8:51–56. doi: 10.1055/s-0028-1094443. [DOI] [PubMed] [Google Scholar]
- Ghoshdastidar D, Dutta RN, Poddar MK. In vivo distribution of lithium in plasma and brain. Indian J Exp Biol. 1989;27:950–954. [PubMed] [Google Scholar]
- Gianutsos G, Moore KE. Dopaminergic supersensitivity in striatum and olfactory tubercle following chronic administration of haloperidol or clozapine. Life Sci. 1977;20:1585–1591. doi: 10.1016/0024-3205(77)90452-0. [DOI] [PubMed] [Google Scholar]
- Goedhard LE, Stolker JJ, Heerdink ER, Nijman HL, Olivier B, Egberts TC. Pharmacotherapy for the treatment of aggressive behavior in general adult psychiatry: A systematic review. J Clin Psychiatry. 2006;67:1013–1024. doi: 10.4088/jcp.v67n0702. [DOI] [PubMed] [Google Scholar]
- Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O’Donnell KC, Picchini AM, Schloesser RJ, Manji HK. [beta]-Catenin Overexpression in the Mouse Brain Phenocopies Lithium-Sensitive Behaviors. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Gould TD, Gottesman Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. The wnt signaling pathway in bipolar disorder. Neuroscientist. 2002;8:497–511. doi: 10.1177/107385802237176. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Picchini AM, Manji HK. Strain Differences in Lithium Attenuation of d-Amphetamine-Induced Hyperlocomotion: A Mouse Model for the Genetics of Clinical Response to Lithium. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry. 2004;9:734–755. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Keith RA, Bhat RV. Differential sensitivity to lithium’s reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav Brain Res. 2001;118:95–105. doi: 10.1016/s0166-4328(00)00318-1. [DOI] [PubMed] [Google Scholar]
- Gray P, Solomon J, Dunphy M, Carr F, Hession M. Effects of lithium on open field behavior in “stressed” and “unstressed” rats. Psychopharmacology (Berl) 1976;48:277–281. doi: 10.1007/BF00496861. [DOI] [PubMed] [Google Scholar]
- Guo WY, Todd KG, Bourin M, Hascoet M. The additive effects of quinine on antidepressant drugs in the forced swimming test in mice. Psychopharmacology (Berl) 1995;121:173–179. doi: 10.1007/BF02245627. [DOI] [PubMed] [Google Scholar]
- Hafen T, Wollnik F. Effect of lithium carbonate on activity level and circadian period in different strains of rats. Pharmacol Biochem Behav. 1994;49:975–983. doi: 10.1016/0091-3057(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Hamburger-Bar R, Robert M, Newman M, Belmaker RH. Interstrain correlation between behavioural effects of lithium and effects on cortical cyclic AMP. Pharmacol Biochem Behav. 1986;24:9–13. doi: 10.1016/0091-3057(86)90036-5. [DOI] [PubMed] [Google Scholar]
- Hascoet M, Bourin M, Khimake S. Additive effect of lithium and clonidine with 5-HT1A agonists in the forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:381–396. doi: 10.1016/0278-5846(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Healy D, Waterhouse JM. The circadian system and the therapeutics of the affective disorders. Pharmacol Ther. 1995;65:241–263. doi: 10.1016/0163-7258(94)00077-g. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Charney DS, Sternberg DE. Lithium carbonate augmentation of antidepressant treatment. An effective prescription for treatment-refractory depression. Arch Gen Psychiatry. 1983;40:1335–1342. doi: 10.1001/archpsyc.1983.01790110077013. [DOI] [PubMed] [Google Scholar]
- Hilal Karakucuk E, Yamanoglu T, Demirel O, Bora N, Zengil H. Temporal variation in drug interaction between lithium and morphine-induced analgesia. Chronobiol Int. 2006;23:675–682. doi: 10.1080/07420520600650745. [DOI] [PubMed] [Google Scholar]
- Hines G. Effects of lithium and rubidium on shock-induced changes in open-field activity. Psychopharmacology (Berl) 1986a;88:209–212. doi: 10.1007/BF00652242. [DOI] [PubMed] [Google Scholar]
- Hines G. Lithium effects on adjunctive alcohol consumption. I: Comparison with adjunctive water consumption. Pharmacol Biochem Behav. 1986b;25:1159–1162. doi: 10.1016/0091-3057(86)90104-8. [DOI] [PubMed] [Google Scholar]
- Hines G. Lithium effects on adjunctive alcohol consumption. III: FT-shock as the inducing schedule. Pharmacol Biochem Behav. 1989;34:591–593. doi: 10.1016/0091-3057(89)90563-7. [DOI] [PubMed] [Google Scholar]
- Hines G, Poling TH. Lithium effects on active and passive avoidance behavior in the rat. Psychopharmacology (Berl) 1984;82:78–82. doi: 10.1007/BF00426385. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Baram TZ, Snead OC., 3rd Ontogenic study of lithium-pilocarpine-induced status epilepticus in rats. Brain Res. 1992;583:120–126. doi: 10.1016/s0006-8993(10)80015-0. [DOI] [PubMed] [Google Scholar]
- Ho AK, Tsai CS. Effects of lithium on alcohol preference and withdrawal. Ann N Y Acad Sci. 1976;273:371–377. doi: 10.1111/j.1749-6632.1976.tb52902.x. [DOI] [PubMed] [Google Scholar]
- Honchar MP, Olney JW, Sherman WR. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983;220:323–325. doi: 10.1126/science.6301005. [DOI] [PubMed] [Google Scholar]
- Honchar MP, Vogler GP, Gish BG, Sherman WR. Evidence that phosphoinositide metabolism in rat cerebral cortex stimulated by pilocarpine, physostigmine, and pargyline in vivo is not changed by chronic lithium treatment. J Neurochem. 1990;55:1521–1525. doi: 10.1111/j.1471-4159.1990.tb04934.x. [DOI] [PubMed] [Google Scholar]
- Horovitz ZP, Piala JJ, High JP, Burke JC, Leaf RC. Effects of drugs on the mouse-killing (muricide) test and its relationship to amygdaloid function. Int J Neuropharmacol. 1966;5:405–411. doi: 10.1016/0028-3908(66)90005-0. [DOI] [PubMed] [Google Scholar]
- Huey LY, Janowsky DS, Judd LL, Abrams A, Parker D, Clopton P. Effects of lithium carbonate on methylphenidate-induced mood, behavior, and cognitive processes. Psychopharmacology (Berl) 1981;73:161–164. doi: 10.1007/BF00429209. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry. 1996;153:151–162. doi: 10.1176/ajp.153.2.151. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Akiyama M, Miyakawa K, Uchida A, Kasahara J, Fukunaga K, Hamada T, Shibata S. Effect of lithium on the circadian rhythms of locomotor activity and glycogen synthase kinase-3 protein expression in the mouse suprachiasmatic nuclei. Eur J Neurosci. 2004;19:2281–2287. doi: 10.1111/j.0953-816X.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Nutt JG, Haertzen CA, Griffith JD, Bunney WE. Lithium: effects on subjective functioning and morphine-induced euphoria. Science. 1977;195:582–584. doi: 10.1126/science.319532. [DOI] [PubMed] [Google Scholar]
- Jensen J. The effect of prolonged lithium ingestion on morphine actions in the rat. Acta Pharmacol Toxicol (Copenh) 1974;35:395–402. doi: 10.1111/j.1600-0773.1974.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Johnson FN. Dissociation of vertical and horizontal components of activity in rats treated with lithium chloride. Experientia. 1972a;28:533–535. doi: 10.1007/BF01931862. [DOI] [PubMed] [Google Scholar]
- Johnson FN. Effects of alkali metal chlorides on activity in rats. Nature. 1972b;238:333–334. doi: 10.1038/238333b0. [DOI] [PubMed] [Google Scholar]
- Johnson FN. Lithium effects upon components of activity in rats. Experientia. 1976;32:212–214. doi: 10.1007/BF01937771. [DOI] [PubMed] [Google Scholar]
- Johnson FN. The influence of alkali metal chlorides on environmentally-linked behavioural stereotypies in the rat. Int J Neurosci. 1981;13:199–204. doi: 10.3109/00207458108985802. [DOI] [PubMed] [Google Scholar]
- Johnson FN, Wormington S. Effect of lithium on rearing activity in rats. Nat New Biol. 1972;235:159–160. doi: 10.1038/newbio235159a0. [DOI] [PubMed] [Google Scholar]
- Johnsson A, Pflug B, Engelmann W, Klemke W. Effect of lithium carbonate on circadian periodicity in humans. Pharmakopsychiatr Neuropsychopharmakol. 1979;12:423–425. doi: 10.1055/s-0028-1094638. [DOI] [PubMed] [Google Scholar]
- Johnston IN, Westbrook RF. Inhibition of morphine analgesia by lithium: role of peripheral and central opioid receptors. Behav Brain Res. 2004;151:151–158. doi: 10.1016/j.bbr.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Jope RS. Anti-bipolar therapy: mechanism of action of lithium. Mol Psychiatry. 1999;4:117–128. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- Judd LL, Hubbard B, Janowsky DS, Huey LY, Attewell PA. The effect of lithium carbonate on affect, mood, and personality of normal subjects. Arch Gen Psychiatry. 1977a;34:346–351. doi: 10.1001/archpsyc.1977.01770150104012. [DOI] [PubMed] [Google Scholar]
- Judd LL, Hubbard B, Janowsky DS, Huey LY, Takahashi KI. The effect of lithium carbonate on the cognitive functions of normal subjects. Arch Gen Psychiatry. 1977b;34:355–357. doi: 10.1001/archpsyc.1977.01770150113013. [DOI] [PubMed] [Google Scholar]
- Judd LL, Hubbard RB, Huey LY, Attewell PA, Janowsky DS, Takahashi KI. Lithium carbonate and ethanol induced “highs” in normal subjects. Arch Gen Psychiatry. 1977c;34:463–467. doi: 10.1001/archpsyc.1977.01770160097008. [DOI] [PubMed] [Google Scholar]
- Kelley AE. In: Measurement of rodent stereotyped behavior, in Current protocols in neuroscience. Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, Wray S, editors. John Wiley & Sons; New York, NY: 2005. pp. 8.8.1–8.8.13. [DOI] [PubMed] [Google Scholar]
- Kitaichi Y, Inoue T, Nakagawa S, Izumi T, Koyama T. Effect of co-administration of subchronic lithium pretreatment and acute MAO inhibitors on extracellular monoamine levels and the expression of contextual conditioned fear in rats. Eur J Pharmacol. 2006;532:236–245. doi: 10.1016/j.ejphar.2005.12.078. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemfuss H. Rhythms and the pharmacology of lithium. Pharmacol Ther. 1992;56:53–78. doi: 10.1016/0163-7258(92)90037-z. [DOI] [PubMed] [Google Scholar]
- Kline NS, Wren JC, Cooper TB, Varga E, Canal O. Evaluation of lithium therapy in chronic and periodic alcoholism. Am J Med Sci. 1974;268:15–22. doi: 10.1097/00000441-197407000-00002. [DOI] [PubMed] [Google Scholar]
- Kofman O, Belmaker RH. Ziskind-Somerfeld Research Award 1993. Biochemical, behavioral, and clinical studies of the role of inositol in lithium treatment and depression. Biol Psychiatry. 1993;34:839–852. doi: 10.1016/0006-3223(93)90052-f. [DOI] [PubMed] [Google Scholar]
- Kofman O, Bersudsky Y. Behavioural effects of NKH-477, a forskolin analogue, on locomotion and rearing in rats. Int J Neuropsychopharmacol. 2000;3:27–33. doi: 10.1017/S146114579900173X. [DOI] [PubMed] [Google Scholar]
- Kofman O, Einat H, Cohen H, Tenne H, Shoshana C. The anxiolytic effect of chronic inositol depends on the baseline level of anxiety. J Neural Transm. 2000;107:241–253. doi: 10.1007/s007020050020. [DOI] [PubMed] [Google Scholar]
- Kofman O, Levin U, Alpert C. Lithium attenuates hypokinesia induced by immobilization stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:1081–1090. doi: 10.1016/0278-5846(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Kofman O, Sherman WR, Katz V, Belmaker RH. Restoration of brain myo-inositol levels in rats increases latency to lithium-pilocarpine seizures. Psychopharmacology (Berl) 1993;110:229–234. doi: 10.1007/BF02246978. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L, Anisman H. Amphetamine psychosis and schizophrenia: a dual model. Neurosci Biobehav Rev. 1981;5:449–461. doi: 10.1016/0149-7634(81)90015-4. [DOI] [PubMed] [Google Scholar]
- Krames L, Milgram NW, Christie DP. Predatory aggression: differential suppression of killing and feeding. Behav Biol. 1973;9:641–647. doi: 10.1016/s0091-6773(73)80059-8. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Wyborney VG. Lithium slows rat circadian activity rhythms. Life Sci. 1980;26:1319–1321. doi: 10.1016/0024-3205(80)90091-0. [DOI] [PubMed] [Google Scholar]
- Kropf D, Muller-Oerlinghausen B. Changes in learning, memory, and mood during lithium treatment. Approach to a research strategy. Acta Psychiatr Scand. 1979;59:97–124. doi: 10.1111/j.1600-0447.1979.tb06951.x. [DOI] [PubMed] [Google Scholar]
- Kucinski BJ, Antelman SM, Caggiula AR, Fowler H, Gershon S, Edwards DJ. Cocaine-induced oscillation is conditionable. Pharmacol Biochem Behav. 1999;63:449–455. doi: 10.1016/s0091-3057(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Langham RJ, Syme GJ, Syme LA. Voluntary lithium intake, antidotal thirst’ and concurrent behavior of rats. Br J Pharmacol. 1975;55:409–413. doi: 10.1111/j.1476-5381.1975.tb06945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautar SL, Rojas C, Slusher BS, Wozniak KM, Wu Y, Thomas AG, Waldon D, Li W, Ferraris D, Belyakov S. DPP IV inhibitor blocks mescaline-induced scratching and amphetamine-induced hyperactivity in mice. Brain Res. 2005;1048:177–184. doi: 10.1016/j.brainres.2005.04.069. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Blair RJ, Charney DS, Pine DS. Irritability in pediatric mania and other childhood psychopathology. Ann N Y Acad Sci. 2003;1008:201–218. doi: 10.1196/annals.1301.022. [DOI] [PubMed] [Google Scholar]
- Lejoyeux M, Ades J. Evaluation of lithium treatment in alcoholism. Alcohol Alcohol. 1993;28:273–279. [PubMed] [Google Scholar]
- Lerer B, Ebstein RP, Felix A, Belmaker RH. Lithium amelioration of reserpine-induced hypoactivity in rats. Int Pharmacopsychiatry. 1980;15:338–343. doi: 10.1159/000468460. [DOI] [PubMed] [Google Scholar]
- Lerer B, Globus M, Brik E, Hamburger R, Belmaker RH. Effect of treatment and withdrawal from chronic lithium in rats on stimulant-induced responses. Neuropsychobiology. 1984;11:28–32. doi: 10.1159/000118046. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kissling W, McGrath J. Lithium for schizophrenia revisited: a systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry. 2004;65:177–186. [PubMed] [Google Scholar]
- Li JX, Han R, Deng YP, Chen SQ, Liang JH. Different effects of valproate on methamphetamine- and cocaine-induced behavioral sensitization in mice. Behav Brain Res. 2005;161:125–132. doi: 10.1016/j.bbr.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Li R, el-Mallakh RS, Harrison L, Changaris DG, Levy RS. Lithium prevents ouabain-induced behavioral changes. Toward an animal model for manic depression. Mol Chem Neuropathol. 1997;31:65–72. doi: 10.1007/BF02815161. [DOI] [PubMed] [Google Scholar]
- Liebman JM, Segal DS. Lithium differentially antagonises self-stimulation facilitated by morphine and (+)-amphetamine. Nature. 1976;260:161–163. doi: 10.1038/260161a0. [DOI] [PubMed] [Google Scholar]
- Looney SW, el-Mallakh RS. Meta-analysis of erythrocyte Na,K-ATPase activity in bipolar illness. Depress Anxiety. 1997;5:53–65. doi: 10.1002/(sici)1520-6394(1997)5:2<53::aid-da1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lowe WC, O’Boyle M. Suppression of cricket killing and eating in laboratory mice following lithium chloride injections. Physiol Behav. 1976;17:427–430. doi: 10.1016/0031-9384(76)90102-5. [DOI] [PubMed] [Google Scholar]
- Maj M, Pirozzi R, Bartoli L, Magliano L. Long-term outcome of lithium prophylaxis in bipolar disorder with mood-incongruent psychotic features: a prospective study. J Affect Disord. 2002;71:195–198. doi: 10.1016/s0165-0327(01)00350-0. [DOI] [PubMed] [Google Scholar]
- Maj M, Pirozzi R, Magliano L, Bartoli L. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30–35. doi: 10.1176/ajp.155.1.30. [DOI] [PubMed] [Google Scholar]
- Malinge M, Bourin M, Colombel MC, Larousse C. Additive effects of clonidine and antidepressant drugs in the mouse forced-swimming test. Psychopharmacology (Berl) 1988;96:104–109. doi: 10.1007/BF02431541. [DOI] [PubMed] [Google Scholar]
- Malone RP, Bennett DS, Luebbert JF, Rowan AB, Biesecker KA, Blaney BL, Delaney MA. Aggression classification and treatment response. Psychopharmacol Bull. 1998;34:41–45. [PubMed] [Google Scholar]
- Mamelak M. An amphetamine model of manic depressive illness. Int Pharmacopsychiatry. 1978;13:193–208. doi: 10.1159/000468341. [DOI] [PubMed] [Google Scholar]
- Manji HK, Gottesman, Gould TD. Signal transduction and genes-to-behaviors pathways in psychiatric diseases. Sci STKE. 20032003:pe49. doi: 10.1126/stke.2003.207.pe49. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Lithium at 50: have the neuroprotective effects of this unique cation been overlooked? Biol Psychiatry. 1999;46:929–940. doi: 10.1016/s0006-3223(99)00165-1. [DOI] [PubMed] [Google Scholar]
- Marinho MM, de Bruin VM, de Sousa FC, Aguiar LM, de Pinho RS, Viana GS. Inhibitory action of a calcium channel blocker (nimodipine) on seizures and brain damage induced by pilocarpine and lithium-pilocarpine in rats. Neurosci Lett. 1997;235:13–16. doi: 10.1016/s0304-3940(97)00687-3. [DOI] [PubMed] [Google Scholar]
- Matthews K, Christmas D, Swan J, Sorrell E. Animal models of depression: navigating through the clinical fog. Neurosci Biobehav Rev. 2005;29:503–513. doi: 10.1016/j.neubiorev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- McEachron DL, Kripke DF, Wyborney VG. Lithium promotes entrainment of rats to long circadian light-dark cycles. Psychiatry Res. 1981;5:1–9. doi: 10.1016/0165-1781(81)90055-x. [DOI] [PubMed] [Google Scholar]
- McGlone JJ, Ritter S, Kelley KW. The antiaggressive effect of lithium is abolished by area postrema lesion. Physiol Behav. 1980;24:1095–1100. doi: 10.1016/0031-9384(80)90053-0. [DOI] [PubMed] [Google Scholar]
- McIntyre IM, Kuhn C, Demitriou S, Fucek FR, Stanley M. Modulating role of lithium on dopamine turnover, prolactin release, and behavioral supersensitivity following haloperidol and reserpine. Psychopharmacology (Berl) 1983;81:150–154. doi: 10.1007/BF00429010. [DOI] [PubMed] [Google Scholar]
- McKinney WT. Animal models of depression: an overview. Psychiatr Dev. 1984;2:77–96. [PubMed] [Google Scholar]
- Meng ZH, Feldpaush DL, Merchant KM. Clozapine and haloperidol block the induction of behavioral sensitization to amphetamine and associated genomic responses in rats. Brain Res Mol Brain Res. 1998;61:39–50. doi: 10.1016/s0169-328x(98)00196-x. [DOI] [PubMed] [Google Scholar]
- Merry J, Reynolds CM, Bailey J, Coppen A. Prophylactic treatment of alcoholism by lithium carbonate. A controlled study. Lancet. 1976;1:481–482. doi: 10.1016/s0140-6736(76)90784-4. [DOI] [PubMed] [Google Scholar]
- Miyauchi T, Kikuchi K, Satoh S. Further studies on the potentiating effect of lithium chloride on methamphetamine-induced stereotypy in mice. Jpn J Pharmacol. 1981;31:61–68. doi: 10.1254/jjp.31.61. [DOI] [PubMed] [Google Scholar]
- Mogilnicka E, Przewlocka B. Facilitated shock-induced aggression after chronic treatment with antidepressant drugs in the rat. Pharmacol Biochem Behav. 1981;14:129–132. doi: 10.1016/0091-3057(81)90231-8. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Jope RS, Snead OC., 3rd Effects of drugs on the initiation and maintenance of status epilepticus induced by administration of pilocarpine to lithium-pretreated rats. Exp Neurol. 1987a;97:193–200. doi: 10.1016/0014-4886(87)90293-7. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Jope RS, Snead OC., 3rd Status epilepticus is produced by administration of cholinergic agonists to lithium-treated rats: comparison with kainic acid. Exp Neurol. 1987b;98:594–605. doi: 10.1016/0014-4886(87)90268-8. [DOI] [PubMed] [Google Scholar]
- Morrison JM, Jr., Pritchard HD, Braude MC, D’Aguanno W. Plasma and brain lithium levels after lithium carbonate and lithium chloride administration by different routes in rats. Proc Soc Exp Biol Med. 1971;137:889–892. doi: 10.3181/00379727-137-35687. [DOI] [PubMed] [Google Scholar]
- Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Mukherjee BP, Bailey PT, Pradhan SN. Correlation of lithium effects on motor activity with its brain concentrations in rats. Neuropharmacology. 1977;16:241–244. doi: 10.1016/0028-3908(77)90101-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee BP, Pradhan SN. Effects of lithium on foot shock-induced aggressive behavior in rats. Arch Int Pharmacodyn Ther. 1976;222:125–131. [PubMed] [Google Scholar]
- Mukherjee S, Rosen AM, Caracci G, Shukla S. Persistent tardive dyskinesia in bipolar patients. Arch Gen Psychiatry. 1986;43:342–346. doi: 10.1001/archpsyc.1986.01800040052008. [DOI] [PubMed] [Google Scholar]
- Muraki I, Inoue T, Hashimoto S, Izumi T, Ito K, Ohmori T, Koyama T. Effect of subchronic lithium carbonate treatment on anxiolytic-like effect of citalopram and MKC-242 in conditioned fear stress in the rat. Eur J Pharmacol. 1999;383:223–229. doi: 10.1016/s0014-2999(99)00572-5. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Brodie HK, Goodwin FK, Bunney WE., Jr. Regular induction of hypomania by L-dopa in “bipolar” manic-depressive patients. Nature. 1971;229:135–136. doi: 10.1038/229135a0. [DOI] [PubMed] [Google Scholar]
- Naccarato WF, Ray RE, Wells WW. Biosynthesis of myo-inositol in rat mammary gland. Isolation and properties of the enzymes. Arch Biochem Biophys. 1974;164:194–201. doi: 10.1016/0003-9861(74)90022-8. [DOI] [PubMed] [Google Scholar]
- Nagayama H. Chronic administration of imipramine and lithium changes the phase-angle relationship between the activity and core body temperature circadian rhythms in rats. Chronobiol Int. 1996;13:251–259. doi: 10.3109/07420529609020905. [DOI] [PubMed] [Google Scholar]
- Namima M, Sugihara K, Watanabe Y, Sasa H, Umekage T, Okamoto K. Quantitative analysis of the effects of lithium on the reverse tolerance and the c-Fos expression induced by methamphetamine in mice. Brain Res Brain Res Protoc. 1999;4:11–18. doi: 10.1016/s1385-299x(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Dube C, Koning E. Status epilepticus induced by lithium-pilocarpine in the immature rat does not change the long-term susceptibility to seizures. Epilepsy Res. 2002;51:189–197. doi: 10.1016/s0920-1211(02)00125-0. [DOI] [PubMed] [Google Scholar]
- Nixon MK, Hascoet M, Bourin M, Colombel MC. Additive effects of lithium and antidepressants in the forced swimming test: further evidence for involvement of the serotoninergic system. Psychopharmacology (Berl) 1994;115:59–64. doi: 10.1007/BF02244752. [DOI] [PubMed] [Google Scholar]
- O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill HC, Schmitt MP, Stevens KE. Lithium alters measures of auditory gating in two strains of mice. Biol Psychiatry. 2003;54:847–853. doi: 10.1016/s0006-3223(03)00184-7. [DOI] [PubMed] [Google Scholar]
- Oehler J, Jahkel M, Schmidt J. The influence of chronic treatment with psychotropic drugs on behavioral changes by social isolation. Pol J Pharmacol Pharm. 1985;37:841–849. [PubMed] [Google Scholar]
- Okada K, Oishi R, Saeki K. Inhibition by antimanic drugs of hyperactivity induced by methamphetamine-chlordiazepoxide mixture in mice. Pharmacol Biochem Behav. 1990;35:897–901. doi: 10.1016/0091-3057(90)90377-t. [DOI] [PubMed] [Google Scholar]
- Ong JC, Brody SA, Large CH, Geyer MA. An investigation of the efficacy of mood stabilizers in rodent models of prepulse inhibition. J Pharmacol Exp Ther. 2005;315:1163–1171. doi: 10.1124/jpet.105.090845. [DOI] [PubMed] [Google Scholar]
- Ozawa H, Miyauchi T. Potentiating effect of lithium chloride on methamphetamine-induced stereotypy in mice. Eur J Pharmacol. 1977;41:213–216. doi: 10.1016/0014-2999(77)90211-4. [DOI] [PubMed] [Google Scholar]
- Ozawa H, Miyauchi T, Sugawara K. Potentiating effect of lithium chloride on aggressive behavior induced in mice by nialamide plus L-DOPA and by clonidine. Eur J Pharmacol. 1975;34:169–179. doi: 10.1016/0014-2999(75)90237-x. [DOI] [PubMed] [Google Scholar]
- Pachet AK, Wisniewski AM. The effects of lithium on cognition: an updated review. Psychopharmacology (Berl) 2003;170:225–234. doi: 10.1007/s00213-003-1592-x. [DOI] [PubMed] [Google Scholar]
- Perez-Cruet J, Tagliamonte A, Tagliamonte P, Gessa GL. Stimulation of serotonin synthesis by lithium. J Pharmacol Exp Ther. 1971;178:325–330. [PubMed] [Google Scholar]
- Perks SM, Clifton PG. Reinforcer revaluation and conditioned place preference. Physiol Behav. 1997;61:1–5. doi: 10.1016/s0031-9384(96)00243-0. [DOI] [PubMed] [Google Scholar]
- Persinger MA, Bureau YR, Peredery O. Dissociation between conditioned taste aversion and radial maze learning following seizure-induced multifocal brain damage: quantitative tests of serial vs. parallel circuit models of memory. Physiol Behav. 1994;56:225–235. doi: 10.1016/0031-9384(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Pert A, Rosenblatt JE, Sivit C, Pert CB, Bunney WE., Jr. 1978 [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Klein PS. Molecular targets of lithium action. Annu Rev Pharmacol Toxicol. 2001;41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- Poirel C, Larouche B. Circadian patterns of basic emotional reactivity and stress related events revisited in mice treated with lithium: behavioral rhythmometric analyses. Chronobiologia. 1989;16:229–239. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977a;229:327–336. [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977b;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Post RM, Rubinow DR, Ballenger JC. Conditioning and sensitisation in the longitudinal course of affective illness. Br J Psychiatry. 1986;149:191–201. doi: 10.1192/bjp.149.2.191. [DOI] [PubMed] [Google Scholar]
- Post RM, Weiss SR, Pert A. Differential effects of carbamazepine and lithium on sensitization and kindling. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:425–434. [PubMed] [Google Scholar]
- Prasad V, Sheard MH. Effect of lithium upon desipramine enhanced shock-elicited fighting in rats. Pharmacol Biochem Behav. 1982;17:377–378. doi: 10.1016/0091-3057(82)90097-1. [DOI] [PubMed] [Google Scholar]
- Quiroz JA, Singh J, Gould TD, Denicoff KD, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: clues from the molecular pathophysiology. Mol Psychiatry. 2004;9:756–776. doi: 10.1038/sj.mp.4001521. [DOI] [PubMed] [Google Scholar]
- Ramsey TA, Mendels J, Hamilton C, Frazer A. The effect of lithium carbonate on self-stimulating behavior in the rat. Life Sci I. 1972;11:773–779. doi: 10.1016/0024-3205(72)90211-1. [DOI] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Stereotyped behavior. Pharmacol Ther [B] 1975;1:757–768. doi: 10.1016/0306-039x(75)90027-6. [DOI] [PubMed] [Google Scholar]
- Ray WJ, Jr., Szymanki ES, Ng L. The binding of lithium and of anionic metabolites to phosphoglucomutase. Biochim Biophys Acta. 1978;522:434–442. doi: 10.1016/0005-2744(78)90076-1. [DOI] [PubMed] [Google Scholar]
- Reches A, Jackson-Lewis V, Fahn S. Lithium does not interact with haloperidol in the dopaminergic pathways of the rat brain. Psychopharmacology (Berl) 1984;82:330–334. doi: 10.1007/BF00427680. [DOI] [PubMed] [Google Scholar]
- Reches A, Wagner HR, Jackson V, Fahn S. Chronic lithium administration has no effect on haloperidol-induced supersensitivity of pre- and postsynaptic dopamine receptors in rat brain. Brain Res. 1982;246:172–177. doi: 10.1016/0006-8993(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Reda FA, Escobar JI, Scanlan JM. Lithium carbonate in the treatment of tardive dyskinesia. Am J Psychiatry. 1975;132:560–562. doi: 10.1176/ajp.132.5.560. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M. Effects of pretreatment with clonidine, lithium and quinine on the activities of antidepressant drugs in the mouse tail suspension test. Fundam Clin Pharmacol. 1997;11:381–386. doi: 10.1111/j.1472-8206.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M, Colombel MC, Baker GB. Dose-dependent noradrenergic and serotonergic properties of venlafaxine in animal models indicative of antidepressant activity. Psychopharmacology (Berl) 1998;138:1–8. doi: 10.1007/s002130050638. [DOI] [PubMed] [Google Scholar]
- Reghunandanan V, Badgaiyan RD, Marya RK, Reghunandanan R, Maini BK. Lithium chloride SCN injection alters the circadian rhythm of food intake. Chronobiol Int. 1989;6:123–129. doi: 10.3109/07420528909064622. [DOI] [PubMed] [Google Scholar]
- Rifkin A, Karajgi B, Dicker R, Perl E, Boppana V, Hasan N, Pollack S. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154:554–555. doi: 10.1176/ajp.154.4.554. [DOI] [PubMed] [Google Scholar]
- Ritter S, McGlone JJ, Kelley KW. Absence of lithium-induced taste aversion after area postrema lesion. Brain Res. 1980;201:501–506. doi: 10.1016/0006-8993(80)91061-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Moore CJ, Castaneda E, Mittleman G. Enduring enhancement in frontal cortex dopamine utilization in an animal model of amphetamine psychosis. Brain Res. 1985;343:374–377. doi: 10.1016/0006-8993(85)90760-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Wooten GF. The behavioral and biochemical effects of lithium on dopaminergic agonist-induced supersensitivity. Psychopharmacology (Berl) 1984;84:217–220. doi: 10.1007/BF00427448. [DOI] [PubMed] [Google Scholar]
- Rush J, Mendels J. Effects of lithium chloride on muricidal behavior in rats. Pharmacol Biochem Behav. 1975;3:795–797. doi: 10.1016/0091-3057(75)90109-4. [DOI] [PubMed] [Google Scholar]
- Saarnivaara L, Mannisto PT. Effects of lithium and rubidium on antinociception and behaviour in mice. I. Studies on narcotic analgesics and antagonists. Arch Int Pharmacodyn Ther. 1976;222:282–292. [PubMed] [Google Scholar]
- Sanghvi I, Gershon S. Rubidium and lithium: evaluation as antidepressant and anti-manic agents. Res Commun Chem Pathol Pharmacol. 1973;6:293–300. [PubMed] [Google Scholar]
- Sato M. Acute exacerbation of methamphetamine psychosis and lasting dopaminergic supersensitivity--a clinical survey. Psychopharmacol Bull. 1986;22:751–756. [PubMed] [Google Scholar]
- Sato M, Chen CC, Akiyama K, Otsuki S. Acute exacerbation of paranoid psychotic state after long-term abstinence in patients with previous methamphetamine psychosis. Biol Psychiatry. 1983;18:429–440. [PubMed] [Google Scholar]
- Schalomon PM, Robertson AM, Laferriere A. Prefrontal cortex and the relative associability of taste and place cues in rats. Behav Brain Res. 1994;65:57–65. doi: 10.1016/0166-4328(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Seong E, Seasholtz AF, Burmeister M. Mouse models for psychiatric disorders. Trends Genet. 2002;18:643–650. doi: 10.1016/s0168-9525(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Shaldubina A, Agam G, Belmaker RH. The mechanism of lithium action: state of the art, ten years later. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:855–866. doi: 10.1016/s0278-5846(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Shaldubina A, Johanson RA, O’Brien WT, Buccafusca R, Agam G, Belmaker RH, Klein PS, Bersudsky Y, Berry GT. SMIT1 haploinsufficiency causes brain inositol deficiency without affecting lithium-sensitive behavior. Mol Genet Metab. 2006;88:384–388. doi: 10.1016/j.ymgme.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A. 1975;72:3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M. Opiate-dependent modulation of adenylate cyclase. Proc Natl Acad Sci U S A. 1977;74:3365–3369. doi: 10.1073/pnas.74.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard M. Effect of lithium on human aggression. Nature. 1971;230:113–114. doi: 10.1038/230113a0. [DOI] [PubMed] [Google Scholar]
- Sheard MH. Effect of lithium on foot shock aggression in rats. Nature. 1970;228:284–285. doi: 10.1038/228284a0. [DOI] [PubMed] [Google Scholar]
- Sheard MH, Aghajanian GK. Neuronally activated metabolism of brain serotonin: effect of lithium. Life Sci. 1970;9:285–290. doi: 10.1016/0024-3205(70)90030-5. [DOI] [PubMed] [Google Scholar]
- Sheard MH, Marini JL, Bridges CI, Wagner E. The effect of lithium on impulsive aggressive behavior in man. Am J Psychiatry. 1976;133:1409–1413. doi: 10.1176/ajp.133.12.1409. [DOI] [PubMed] [Google Scholar]
- Shibata S, Nakanishi H, Watanabe S, Ueki S. Effects of chronic administration of antidepressants on mouse-killing behavior (muricide) in olfactory bulbectomized rats. Pharmacol Biochem Behav. 1984;21:225–230. doi: 10.1016/0091-3057(84)90219-3. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. The delta-opioid receptor antagonist naltrindole prevents sensitization to the conditioned rewarding effects of cocaine. Eur J Pharmacol. 1995;280:55–61. doi: 10.1016/0014-2999(95)00185-n. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Influence of chronic lithium treatment upon the motivational effects of opioids: alteration in the effects of mu- but not kappa-opioid receptor ligands. J Pharmacol Exp Ther. 1991;256:1101–1106. [PubMed] [Google Scholar]
- Singer I, Rotenberg D. Mechanisms of lithium action. N Engl J Med. 1973;289:254–260. doi: 10.1056/NEJM197308022890508. [DOI] [PubMed] [Google Scholar]
- Sivam SP, Smith DR, Takeuchi K, Hong JS. Lithium and haloperidol differentially alter the dynorphin A (1-8) and enkephalin levels in the neurointermediate lobe of rat pituitary. Neuropeptides. 1987;10:291–298. doi: 10.1016/0143-4179(87)90079-5. [DOI] [PubMed] [Google Scholar]
- Sivam SP, Strunk C, Smith DR, Hong JS. Proenkephalin-A gene regulation in the rat striatum: influence of lithium and haloperidol. Mol Pharmacol. 1986;30:186–191. [PubMed] [Google Scholar]
- Skuza G, Rogoz Z, Wieczorek A. Neuropsychopharmacological profile of remoxipride in comparison with clozapine. Pol J Pharmacol. 1997;49:5–15. [PubMed] [Google Scholar]
- Smith DF. Biogenic amines and the effect of short term lithium administration on open field activity in rats. Psychopharmacologia. 1975;41:295–300. doi: 10.1007/BF00428940. [DOI] [PubMed] [Google Scholar]
- Smith DF. Learned aversion and rearing movement in rats given LiCl, PbCl2 or NaCl. Experientia. 1978;34:1200–1201. doi: 10.1007/BF01922956. [DOI] [PubMed] [Google Scholar]
- Smith DF. Lithium and motor activity of animals: effects and possible mechanism of action. Int Pharmacopsychiatry. 1980;15:197–217. doi: 10.1159/000468440. [DOI] [PubMed] [Google Scholar]
- Smith DF, Smith HB. The effect of prolonged lithium administration on activity, reactivity, and endurance in the rat. Psychopharmacologia. 1973;30:83–88. doi: 10.1007/BF00422796. [DOI] [PubMed] [Google Scholar]
- Smith RC, Davis JM. Behavioral evidence for supersensitivity after chronic administration of haloperidol, clozapine, and thioridazine. Life Sci. 1976;19:725–731. doi: 10.1016/0024-3205(76)90170-3. [DOI] [PubMed] [Google Scholar]
- Souza FG, Goodwin GM. Lithium treatment and prophylaxis in unipolar depression: a meta-analysis. Br J Psychiatry. 1991;158:666–675. doi: 10.1192/bjp.158.5.666. [DOI] [PubMed] [Google Scholar]
- Squire LR, Judd LL, Janowsky DS, Huey LY. Effects of lithium carbonate on memory and other cognitive functions. Am J Psychiatry. 1980;137:1042–1046. doi: 10.1176/ajp.137.9.1042. [DOI] [PubMed] [Google Scholar]
- Staunton DA, Deyo SN, Shoemaker WJ, Ettenberg A, Bloom FE. Effects of chronic lithium on enkephalin systems and pain responsiveness. Life Sci. 1982;31:1837–1840. doi: 10.1016/0024-3205(82)90223-5. [DOI] [PubMed] [Google Scholar]
- Stengaard-Pedersen K, Schou M. In vitro and in vivo inhibition by lithium of enkephalin binding to opiate receptors in rat brain. Neuropharmacology. 1982;21:817–823. doi: 10.1016/0028-3908(82)90070-3. [DOI] [PubMed] [Google Scholar]
- Stengaard-Pedersen K, Schou M. Opioid peptides and receptors in relation to affective illness. Effects of desipramine and lithium on opioid receptors in rat brain. Acta Pharmacol Toxicol (Copenh) 1985;56(Suppl 1):170–179. doi: 10.1111/j.1600-0773.1985.tb02509.x. [DOI] [PubMed] [Google Scholar]
- Sternberg DE, Bowers MB, Jr., Heninger GR, Charney DS. Lithium prevents adaptation of brain dopamine systems to haloperidol in schizophrenic patients. Psychiatry Res. 1983;10:79–86. doi: 10.1016/0165-1781(83)90106-3. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stewart KT, McEachron DL, Rosenwasser AM, Adler NT. Lithium lengthens circadian period but fails to counteract behavioral helplessness in rats. Biol Psychiatry. 1991a;30:515–518. doi: 10.1016/0006-3223(91)90315-d. [DOI] [PubMed] [Google Scholar]
- Stewart KT, McEachron DL, Rosenwasser AM, Adler NT. Lithium lengthens circadian period but fails to counteract behavioral helplessness in rats. Biol Psychiatry. 1991b;30:515–518. doi: 10.1016/0006-3223(91)90315-d. [DOI] [PubMed] [Google Scholar]
- Subramanian P, Menon VP, Arokiam FV, Rajakrishnan V, Balamurugan E. Lithium modulates biochemical circadian rhythms in Wistar rats. Chronobiol Int. 1998;15:29–38. doi: 10.3109/07420529808998667. [DOI] [PubMed] [Google Scholar]
- Suchomelova L, Kubova H, Haugvicova R, Druga R, Mares P. Are acute changes after status epilepticus in immature rats persistent? Physiol Res. 2002;51:185–192. [PubMed] [Google Scholar]
- Takigawa M, Fukuzako H, Ueyama K, Tominaga H. Intracranial self-stimulation and locomotor traces as indicators for evaluating and developing antipsychotic drugs. Jpn J Psychiatry Neurol. 1994;48:127–132. doi: 10.1111/j.1440-1819.1994.tb03006.x. [DOI] [PubMed] [Google Scholar]
- Tarsy D, Baldessarini RJ. Pharmacologically induced behavioural supersensitivity to apomorphine. Nat New Biol. 1973;245:262–263. doi: 10.1038/newbio245262a0. [DOI] [PubMed] [Google Scholar]
- Teixeira NA, Pereira DG, Hermini AH. Chronic but not acute Li+ treatment prevents behavioral depression in rats. Braz J Med Biol Res. 1995;28:1003–1007. [PubMed] [Google Scholar]
- Tenk CM, Kavaliers M, Ossenkopp KP. Dose response effects of lithium chloride on conditioned place aversions and locomotor activity in rats. Eur J Pharmacol. 2005;515:117–127. doi: 10.1016/j.ejphar.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Tenn CC, Fletcher PJ, Kapur S. A putative animal model of the “prodromal” state of schizophrenia. Biol Psychiatry. 2005;57:586–593. doi: 10.1016/j.biopsych.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Mague SD, Cohen BM, Carlezon WA., Jr. Behavioral effects of short-term administration of lithium and valproic acid in rats. Brain Res. 2006 doi: 10.1016/j.brainres.2006.03.102. [DOI] [PubMed] [Google Scholar]
- Tomkiewicz M, Steinberg H. Lithium treatment reduces morphine self-adminstration in addict rats. Nature. 1974;252:227–229. doi: 10.1038/252227a0. [DOI] [PubMed] [Google Scholar]
- Tort AB, Dall’Igna OP, de Oliveira RV, Mantese CE, Fett P, Gomes MW, Schuh J, Souza DO, Lara DR. Atypical antipsychotic profile of flunarizine in animal models. Psychopharmacology (Berl) 2005;177:344–348. doi: 10.1007/s00213-004-1955-y. [DOI] [PubMed] [Google Scholar]
- Treiman DM, Walton NY, Kendrick C. A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res. 1990;5:49–60. doi: 10.1016/0920-1211(90)90065-4. [DOI] [PubMed] [Google Scholar]
- Tsaltas E, Kontis D, Boulougouris V, Papakosta VM, Giannou H, Poulopoulou C, Soldatos C. Enhancing effects of chronic lithium on memory in the rat. Behav Brain Res. 2006 doi: 10.1016/j.bbr.2006.11.003. [DOI] [PubMed] [Google Scholar]
- U’Prichard DC, Steinberg H. Proceedings: Selective effects of lithium on two forms of spontaneous activity. Br J Pharmacol. 1972;44:349P–350P. [PMC free article] [PubMed] [Google Scholar]
- Umeda K, Suemaru K, Todo N, Egashira N, Mishima K, Iwasaki K, Fujiwara M, Araki H. Effects of mood stabilizers on the disruption of prepulse inhibition induced by apomorphine or dizocilpine in mice. Eur J Pharmacol. 2006 doi: 10.1016/j.ejphar.2006.09.050. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP, Docherty JP, Marder SR, Rosenblatt JE, Bunney WE., Jr. Lithium attenuates the activation-euphoria but not the psychosis induced by d-amphetamine in schizophrenia. Psychopharmacology (Berl) 1985;87:111–115. doi: 10.1007/BF00431789. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia. 1975;44:215–224. doi: 10.1007/BF00428897. [DOI] [PubMed] [Google Scholar]
- Vasconcellos AP, Tabajara AS, Ferrari C, Rocha E, Dalmaz C. Effect of chronic stress on spatial memory in rats is attenuated by lithium treatment. Physiol Behav. 2003;79:143–149. doi: 10.1016/s0031-9384(03)00113-6. [DOI] [PubMed] [Google Scholar]
- Venugopal M, Persinger MA. Conditioned taste aversion is reduced in rats with a history of lithium/pilocarpine-induced limbic seizures. Neurosci Lett. 1988;90:177–180. doi: 10.1016/0304-3940(88)90807-5. [DOI] [PubMed] [Google Scholar]
- Verimer T, Goodale DB, Long JP, Flynn JR. Lithium effects on haloperidol-induced pre- and postsynaptic dopamine receptor supersensitivity. J Pharm Pharmacol. 1980;32:665–666. doi: 10.1111/j.2042-7158.1980.tb13033.x. [DOI] [PubMed] [Google Scholar]
- Wajda IJ, Banay-Schwartz M, Manigault I, Lajtha A. Effect of lithium and sodium ions on opiate- and dopamine-receptor binding. Neurochem Res. 1981;6:321–331. doi: 10.1007/BF00964047. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Wirz-Justice A. Circadian rhythm mechanisms in affective illness and in antidepressant drug action. Pharmacopsychiatria. 1982;15:31–39. doi: 10.1055/s-2007-1019506. [DOI] [PubMed] [Google Scholar]
- White NM, Carr GD. The conditioned place preference is affected by two independent reinforcement processes. Pharmacol Biochem Behav. 1985;23:37–42. doi: 10.1016/0091-3057(85)90127-3. [DOI] [PubMed] [Google Scholar]
- Wielosz M. The effect of lithium chloride on the activity of some psychotropic drugs. Pol J Pharmacol Pharm. 1976;28:189–198. [PubMed] [Google Scholar]
- Wielosz M, Mlynarczyk M, Kleinrok Z. Effect of nifedipine on lithium-pilocarpine-induced seizures in the rats. Pol J Pharmacol. 1995;47:279–284. [PubMed] [Google Scholar]
- Williams MB, Jope RS. Circadian variation in rat brain AP-1 DNA binding activity after cholinergic stimulation: modulation by lithium. Psychopharmacology (Berl) 1995a;122:363–368. doi: 10.1007/BF02246267. [DOI] [PubMed] [Google Scholar]
- Williams MB, Jope RS. Modulation by inositol of cholinergic- and serotonergic-induced seizures in lithium-treated rats. Brain Res. 1995b;685:169–178. doi: 10.1016/0006-8993(95)00395-7. [DOI] [PubMed] [Google Scholar]
- Williams R, Ryves WJ, Dalton EC, Eickholt B, Shaltiel G, Agam G, Harwood AJ. A molecular cell biology of lithium. Biochem Soc Trans. 2004;32:799–802. doi: 10.1042/BST0320799. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Goodwin GM, De Souza R, Green AR. The pharmacokinetic profile of lithium in rat and mouse; an important factor in psychopharmacological investigation of the drug. Neuropharmacology. 1986;25:1285–1288. doi: 10.1016/0028-3908(86)90149-8. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci U S A. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CL, Huang LT, Liou CW, Wang TJ, Tung YR, Hsu HY, Lai MC. Lithium-pilocarpine-induced status epilepticus in immature rats result in long-term deficits in spatial learning and hippocampal cell loss. Neurosci Lett. 2001;312:113–117. doi: 10.1016/s0304-3940(01)02202-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Araki H, Abe Y, Ueki S. Effects of chronic LiCl and RbCl on muricide induced by midbrain raphe lesions in rats. Pharmacol Biochem Behav. 1985;22:559–563. doi: 10.1016/0091-3057(85)90275-8. [DOI] [PubMed] [Google Scholar]
- Yang P, Beasley A, Swann A, Dafny N. Valproate modulates the expression of methylphenidate (ritalin) sensitization. Brain Res. 2000;874:216–220. doi: 10.1016/s0006-8993(00)02500-2. [DOI] [PubMed] [Google Scholar]
- Yang P, Singhal N, Modi G, Swann A, Dafny N. Effects of lithium chloride on induction and expression of methylphenidate sensitization. Eur J Pharmacol. 2001;426:65–72. doi: 10.1016/s0014-2999(01)01213-4. [DOI] [PubMed] [Google Scholar]
- York JD, Ponder JW, Majerus PW. Definition of a metal-dependent/Li(+)-inhibited phosphomonoesterase protein family based upon a conserved three-dimensional core structure. Proc Natl Acad Sci U S A. 1995;92:5149–5153. doi: 10.1073/pnas.92.11.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZD, Li JH, Song CY, Lu CL, He C. Oxytocin mediates the inhibitory action of acute lithium on the morphine dependence in rats. Neurosci Res. 2001;41:143–150. doi: 10.1016/s0168-0102(01)00272-3. [DOI] [PubMed] [Google Scholar]
- Youngs RM, Chu MS, Meloni EG, Naydenov A, Carlezon WA, Jr., Konradi C. Lithium administration to preadolescent rats causes long-lasting increases in anxiety-like behavior and has molecular consequences. J Neurosci. 2006;26:6031–6039. doi: 10.1523/JNEUROSCI.0580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]