Abstract
Ark1p (actin regulating kinase 1) was identified as a yeast protein that binds to Sla2p, an evolutionarily conserved cortical actin cytoskeleton protein. Ark1p and a second yeast protein, Prk1p, contain NH2-terminal kinase domains that are 70% identical. Together with six other putative kinases from a number of organisms, these proteins define a new protein kinase family that we have named the Ark family.
Lack of both Ark1p and Prk1p resulted in the formation of large cytoplasmic actin clumps and severe defects in cell growth. These defects were rescued by wild-type, but not by kinase-dead versions of the proteins. Elevated levels of either Ark1p or Prk1p caused a number of actin and cell morphological defects that were not observed when the kinase-dead versions were overexpressed instead. Ark1p and Prk1p were shown to localize to actin cortical patches, making these two kinases the first signaling proteins demonstrated to be patch components. These results suggest that Ark1p and Prk1p may be downstream effectors of signaling pathways that control actin patch organization and function. Furthermore, results of double-mutant analyses suggest that Ark1p and Prk1p function in overlapping but distinct pathways that regulate the cortical actin cytoskeleton.
Keywords: protein kinases, Saccharomyces cerevisiae, cyclin-G–associated kinases (GAKs), endocytosis, actin
The actin cytoskeleton plays a pivotal role in governing cellular morphology and provides a structural framework for organelle transport and cytokinesis. The dynamic nature of actin filaments is essential for their ability to function; actin filaments turn over rapidly and therefore can rapidly be disassembled and reassembled in response to cues from the cell's environment. However, these dynamics imply instability, and instability necessitates tight regulation. Therefore, the actin cytoskeleton is controlled by a number of parallel and overlapping regulatory pathways. Small GTPases of the ras superfamily, such as Rho (Ridley and Hall, 1992), Rac (Ridley et al., 1992), and Cdc42 (Kozma et al., 1995; Nobes and Hall, 1995), are critical enzymes in these signaling pathways (for reviews see Hall, 1998; Tanaka and Takai, 1998). Kinases are often responsible for further amplifying and dispersing the signals necessary for actin modulation in response to the various needs of the cell. For example, the p21-activated kinases (PAK) and the LIM kinases, directly or indirectly, are activated by Rho subfamily GTPases and have been shown to phosphorylate components of the actin-based cytoskeleton (Ramos et al., 1997; Sells and Chernoff, 1997; Arber et al., 1998; Yang et al., 1998). The final targets of these signaling pathways include actin-binding proteins such as actin depolymerizing factors (ADFs), profilin, myosin heavy and light chains, and members of the ezrin, radixin, and moesin (ERM) family of proteins (for reviews see Sohn and Goldschmidt-Clermont, 1994; Tsukita and Yonemura, 1997; Lappalainen et al., 1998). Although many signaling proteins that are implicated in cytoskeleton regulation have been identified, the complex pathways regulating the actin cytoskeleton have not been elucidated fully. A more thorough dissection of known pathways and the identification of additional components of these pathways will be necessary before it will be possible to develop a complete understanding of how a cell changes its shape, membrane dynamics, organization, or motility in response to environmental and internal cues.
Several signal transduction proteins with homology to the above-mentioned mammalian GTPases and kinases have been implicated in the control of actin organization in the budding yeast Saccharomyces cerevisiae. These include the GTPases Cdc42p and Rho1, as well as downstream effectors of Cdc42 including the PAK kinases Cla4p and Ste20p (Adams et al., 1990; Johnson and Pringle, 1990; Eby et al., 1998). Therefore, budding yeast provides a more simple, genetically tractable model organism in which to identify new components of these pathways.
In yeast, filamentous actin is found in two forms, cables and patches. Actin cables are oriented along the mother– bud axis and are involved in processes such as organelle inheritance and vesicle targeting (Drubin et al., 1993; Simon et al., 1995). Actin patches are motile structures found at regions of cortical expansion where they appear to be involved in endocytosis (Novick and Botstein, 1985; Kubler and Riezman, 1993; Doyle and Botstein, 1996; Waddle et al., 1996; Mulholland et al., 1997). Much of our understanding of actin dynamics in yeast has resulted from genetic studies that have allowed the identification of actin-binding proteins and have revealed redundant regulatory mechanisms (for review see Ayscough and Drubin, 1996; Botstein et al., 1997).
SLA2 (synthetic lethal with Abp1) was identified in a screen for mutations that are synthetic lethal with a null allele of the gene encoding Abp1p, a nonessential actin-binding protein (Holtzman et al., 1993). In yeast, Sla2p is required for the nucleation of cortical actin assembly in permeabilized yeast cells (Li et al., 1995). This protein contains an actin-binding talin-like domain. A null allele of SLA2 results in severe disruption of the actin cytoskeleton and defects in endocytosis (Holtzman et al., 1993; Wesp et al., 1997). There appear to be homologues of Sla2p in all eukaryotes. A human homologue, HIP1 (huntingtin-interacting protein 1), associates with huntingtin, the protein that is mutated in patients with Huntington's disease (Kalchman et al., 1997; Wanker et al., 1997).
The ultimate function of the signaling pathways described above is to modulate the assembly and disassembly of actin-based complexes that mediate a variety of cellular processes. Sla2p is thus a candidate target of signals intended to modulate the actin cytoskeleton. Here we report that a screen for proteins that interact with Sla2p and a separate screen for proteins that disrupt the actin cytoskeleton when overexpressed have identified two novel protein kinases that have a critical role in the regulation of the actin cytoskeleton in budding yeast.
Materials and Methods
All plasmids used in this work are listed in Table I.
Table I.
Plasmids Used in This Study
| Name | Features* | Description | ||
|---|---|---|---|---|
| pTS395‡ | GFP(wt) | Allows expression of COOH-terminal GFP fusion proteins under the inducible GAL1,10 promoter. | ||
| PGAL1,10 | ||||
| pTS408‡ | GFP(wt) | Allows expression of NH2-terminal GFP fusion proteins under the inducible GAL1,10 promoter. | ||
| PGAL1,10 | ||||
| pDD371 | CEN, HIS3 | An EcoRI fragment containing the SLA2 ORF, but lacking sequence encoding amino acids 360–575, in pRS313. | ||
| pDD373 | CEN, TRP1 | Sla2p residues 503–968 fused to the DNA-binding domain of Gal4p at the BamHI site of pASI-CYH2. | ||
| pDD382 | CEN, LEU2 | A genomic fragment containing ARK1 plus 453 bp upstream and 250 bp downstream sequence cloned into the XbaI and SacI sites of pRS315. | ||
| pDD554 | Pgal GFP-Prk1p | The entire ORF of PRK1 fused to the COOH terminus of GFP (wild-type). Parent vector is pTS408. | ||
| CEN, URA | ||||
| pDD555 | Pgal GFP-Ark1p | The entire ORF of ARK1 fused to the NH2 terminus of GFP (wild-type). Parent vector is pTS395. | ||
| CEN, URA | ||||
| pDD556 | CEN, URA | PRK1 ORF, plus 385 bp of 5′ UTR and 284 bp of 3′ UTR between SacI and SalI sites of pRS316. | ||
| pDD557 | 6-myc source | 6-myc tag flanked by NotI and XmaI sites inserted into the Xmal site of pBluescript II SK+. | ||
| pDD558 | CEN, URA | 6-myc tagged PRK1 genomic fragment in pRS316. | ||
| pDD559 | CEN, LEU | Same as pDD382, except with the K56A kinase-dead mutation within the ARK1 ORF and a | ||
| silent mutation in the LEU2 gene eliminating a unique AflII site. | ||||
| pDD560 | CEN, URA | Same as pDD556, except with the K56A kinase-dead mutation within the PRK1 ORF and a | ||
| silent mutation in the URA3 gene eliminating a unique NotI site. | ||||
| pDD561 | Pgal GFP-Prk1p | Same as pDD554, except with the kinase-dead mutation K56A. | ||
| kinase-dead | ||||
| CEN, URA | ||||
| pDD562 | Pgal GFP-Ark1p | Same as pDD555, except with the kinase-dead mutation K56A. | ||
| kinase dead | ||||
| CEN, URA | ||||
| HL3 | CapR * | Marker-swap plasmid containing the HIS3 gene disrupted by the LEU2 ORF (Cross, 1997). Parent vector is pBCKS+ (Stratagene). |
All plasmids listed are ampicillin-resistant, except HL3, which is chloramphenicol-resistant.
A kind gift from T. Stearns.
Two-Hybrid Screen
Residues 503–968 of Sla2p were fused to the Gal4p DNA-binding domain in pAS1-CYH2 generating pDD373 (Yang, S., M.J.T.V. Cope, and D.G. Drubin, manuscript submitted for publication). This construct expresses a product of the expected size (71 kD), which is detected by anti-Sla2p antibody (data not shown). A yeast Y190 (Table II) strain containing pDD373 was transformed with a library containing random cDNA fragments fused to the activating domain of Gal4p (Durfee et al., 1993) and was selected on synthetic medium lacking tryptophan, leucine, and histidine, and containing 50 μM 3-amino-1,2,4-triazole. Healthy colonies that also displayed β-galactosidase activity by filter-lift assay were selected and the activation-domain fusion plasmids were isolated and sequenced from the primer “HA_internal” (GCTTACCCATACGATGTT).
Table II.
Yeast Strains Used in This Study
| Name | Genotype | Source | ||
|---|---|---|---|---|
| DDY130 | MAT a his3-Δ200 ura3-52 leu2-3,112 lys2-801 | 1 | ||
| DDY131 | MATα his3-Δ200 ura3-52 leu2-3,112 lys2-801 ade2-1 | 1 | ||
| DDY318 | MATα sac6Δ::LEU2 his3 ura3 leu2 lys2 | 2 | ||
| (AAY1046) | ||||
| DDY322 | MATα abp1Δ::LEU2 his3-Δ200 ura3-52 leu2-3,112 | 1 | ||
| DDY426 | MAT a/MATα ura3-52/ura3-52 leu2-3,112/leu2-3,112 lys2-801/lys2-801 his3-Δ200/his3-Δ200 ade2-1/+ | 1 | ||
| DDY545 | MAT a sla2Δ::URA3 his3-Δ200 ura3-52 leu2-3,112 | 1 | ||
| DDY950 | MATα rvs167Δ::TRP1 ura3 leu2 lys2 trp1 | 1 | ||
| DDY952 | MATα srv2Δ::HIS3 ura3 leu2 his3 lys2 | 1 | ||
| DDY1166 | MAT a sla2Δ::HIS3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 ade2-1 | 1 | ||
| DDY1407 | MAT a ark1Δ::HIS3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 ade2-1 | 3 | ||
| DDY1408 | MATα ark1Δ::HIS3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 | 3 | ||
| DDY1518 | MATα crn1Δ::LEU2 his3-11,15 ura3-52 leu2-3,112 ade2-1 trp1-1 can1-100 | 1 | ||
| DDY1521 | MAT a crn1Δ::URA3 ade3-130 ade2-101 ura3-52 leu2-3,112 | 1 | ||
| DDY1558 | MATα prk1Δ::LEU2 his3-Δ200 ura3-52 leu2-3,112 lys2-801 | 3 | ||
| DDY1559 | MAT a prk1Δ::LEU2 his3-Δ200 ura3-52 leu2-3,112 lys2-801 ade2-1 | 3 | ||
| DDY1560 | MATα prk1Δ::URA3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 | 3 | ||
| DDY1561 | MAT a prk1Δ::URA3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 ade2-1 | 3 | ||
| DDY1562 | DDY545 containing pDD371 | 3 | ||
| DDY1563 | MATα ark1Δ::HIS3 prk1Δ::URA3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 | 3 | ||
| DDY1564 | MAT a ark1Δ::HIS3 prk1Δ::LEU2 his3-Δ200 ura3-52 leu2-3,112 lys2-801 | 3 | ||
| DDY1565 | MAT a sac6Δ::LEU2 ark1Δ::HIS3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 | 3 | ||
| DDY1566 | MAT a abp1Δ::LEU2 ark1Δ::HIS3 his3-Δ200 leu2-3,112 ura3-52 | 3 | ||
| DDY1567 | MATα aip1Δ::URA3 ark1Δ::HIS3 his3-Δ200 ura3-52 leu2-3,112 | 3 | ||
| DDY1568 | MATα sla2Δ::URA3 ark1Δ::HIS3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 | 3 | ||
| DDY1569 | MATα aip1Δ::URA3 prk1Δ::LEU2 his3-Δ200 ura3-52 leu2-3,112 | 3 | ||
| DDY1570 | MAT a sac6Δ::LEU2 prk1Δ::URA3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 | 3 | ||
| DDY1571 | MATα abp1Δ::LEU2 prk1Δ::URA3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 | 3 | ||
| DDY1572 | MATα crnΔ::LEU2 ark1Δ::HIS3 his3-Δ200 ura3-52 leu2-3,112 lys2-801 trp1-1 ade2-1. Possibly canavanol resistant. | 3 | ||
| DDY1573 | MAT a ark1Δ::LEU2 his3 ura3-52 leu2-3,112 lys2-801 ade2-1 | 3 | ||
| DDY1574 | MATα crn1Δ::URA3 prk1Δ::LEU2 ura3-52 leu2-3,112 ade2-1 | 3 | ||
| DDY1575 | MAT a rvs167Δ::TRP1 ark1Δ::LEU2 ura3-52 leu2-3,112 trp1-1 lys2-801 his3-Δ200 | 3 | ||
| DDY1576 | MATα rvs167Δ::TRP1 prk1Δ::LEU2 ura3-52 leu2-3,112 trp1-1 lys2-801 his3-Δ200 | 3 | ||
| DDY1577 | MAT a srv2Δ::HIS3 prk1Δ::LEU2 ura3-52 leu2-3,112 lys2-801 his3-Δ200 | 3 | ||
| DDY1578 | MATα srv2Δ::HIS3 ark1Δ::LEU2 ura3-52 leu2-3,112 lys2-801 his3-Δ200 | 3 | ||
| DDY1579 | MATα ark1Δ::HIS3 sla1Δ::URA3 ura3-52 leu2-3,112 his3-Δ200 ade2-1 | 3 | ||
| DDY1580 | MATα prk1Δ::LEU2 sla1Δ::URA3 ura3-52 leu2-3,112 ade2-1 lys2-801 | 3 | ||
| DAY32 | MAT a aip1Δ::URA3 his3-Δ200 ura3-52 leu2Δ-1 trp1Δ63 | 4 | ||
| P190 | MAT a gal4 gal180 ura3-52 leu2-3,112 his3 trp1-901 ade2-101 + URA3::GAL→ lacZ, LYS2::GAL→ HIS3 cyhR | 5 |
Sources: 1, Drubin lab; 2, Alison Adams; 3, this study; 4, David Amberg; 5, Steve Elledge.
Sequence Alignment and Phylogenetic Analysis
Sequence alignments were performed using the ClustalW software package (Thompson et al., 1994), implemented at the European Bioinformatics Institute web site at http://croma.ebi.ac.uk/clustalw/. The phylogenetic tree was determined from the alignment data, again using ClustalW. An allowance was made for multiple substitutions (Kimura, 1983). Information from intervals in the alignment for which gaps are found in some sequences was not included, in order to avoid the inappropriate weighting of some sequences. The tree was tested (1,000 trials) for branching order confidence by bootstrapping (Felsenstein, 1985). Further information on this procedure (as applied to myosin motor domains) can be found on the World Wide Web at http://www.mrc-lmb.cam.ac.uk/myosin/myosin.html.
Disruption of the ARK1 and the PRK1 Genes
The ARK1 open reading frame (ORF)1 was precisely replaced by a DNA fragment containing the HIS3 gene. The primers SY40 (GAGAAAGAAATATTACTCTGCATAATTAGGTATTTTAAGCAACCAGATAAATCAACCTGTGCGGTATTTCACACCGC) and SY41 (CATGTTACCAGCCTCTTCAGAGATCGATCCGGTTCTGTTGAGCCAAATACTCAGATTGTACTGAGAGTGCACC) were used to amplify a HIS3-containing DNA fragment from pRS313 using PCR. DDY426 was transformed with this fragment and the resulting transformants were screened for His+ colonies. Replacement of the ARK1 ORF with the HIS3-containing fragment was verified by PCR using the primers SY51 (CGGAGCTCGGCAACCTTCATGCCTTATG) and SY52 (CGTCTAGAGGAGAGCACAATCCAGC). The heterozygous (ARK1/ark1Δ::HIS3) diploid was sporulated and ark1Δ::HIS3 MATa (DDY1407) and ark1Δ:: HIS3 MATα (DDY1408) haploid cells were isolated.
The PRK1 ORF was precisely replaced by a DNA fragment containing either the URA3 ORF or the LEU2 ORF. Primers SY44 (GTTGATCAAGATTATTTGTAACCTCCTATCTTTAGTTGAACTGATCCAAAAACACTGTGCGGTATTTCACACCGC) and SY45 (CATTTTGTATGACTTTTAATATTACATAGTCTATTATGTGTGAGAGCAAGTTTTAGATTGTACTGAGAGTGCACC) were used to PCR amplify URA3-containing fragments from pRS316 or LEU2-containing fragments from pRS315. Ura+ or Leu+ transformants of DDY426 were isolated and verified for replacement of the PRK1 gene by PCR using the primers SY61 (TGATGTGATAGTGGCACCAAAC) and SY62 (CGTATGCAGAGCGAAGGTCTT), or the primers SY45 and SY62. Diploids heterozygous at the PRK1 locus were sporulated and prk1Δ::URA3 MATa (DDY1561), prk1Δ::URA3 MATα (DDY1560), prk1Δ::LEU2 MATa (DDY1559), and prk1Δ::LEU2 MATα (DDY1558) haploid were isolated.
Finally, yeast in which the ARK1 ORF deletion mutation was marked with LEU2 was obtained by using the marker-swap method (Cross, 1997). The plasmid HL3 (a kind gift from F. Cross) was digested with ApaI and PstI to release the LEU2-disrupted HIS3 gene. The digested DNA was used to transform DDY1407. Leu+/His− yeast (DDY1573) were identified and the new LEU2-marked disruption of the ARK1 gene was verified by PCR using the primers SY63 and SY51.
Amplification of Genomic Fragments Containing the ARK1 and PRK1 Genes
A genomic fragment containing the ARK1 ORF, plus 453 upstream base pair and 250 downstream base pair, was amplified from the S. cerevisiae cosmid 70944 by high-fidelity PCR using the primers SY51 and SY52. This fragment was inserted between the XbaI and SacI sites of pRS315, creating the plasmid pDD382. This plasmid fully complemented the temperature-sensitive growth defects of ark1Δ prk1Δ double-null yeast.
A genomic fragment containing the PRK1 ORF, plus 385 upstream base pair and 284 downstream base pair, was amplified from the S. cerevisiae cosmid 70775 between primers JC_YIL095w_4 (TAGAGCTCGTACTGATAGAGATTTCCG) and JC_YIL095w_5 (CATGTAGTCGACCCACAACGAAGCTGCCCAAG). The PCR product was digested and inserted between the SacI and SalI sites of pRS316, creating pDD556. This plasmid fully complemented the temperature-sensitive growth defects of ark1Δ prk1Δ cells.
Myc-Tagging of Prk1p
A 6-myc epitope-tag flanked by XmaI sites (from pDD557) was inserted into the XmaI site present 11 codons into the PRK1 ORF in pDD556, forming pDD558. This plasmid fully complemented the temperature-sensitive growth defects of ark1Δ prk1Δ double-null mutant cells.
Indirect Immunofluorescence
For rhodamine-phalloidin staining of filamentous actin when preservation of green fluorescent protein (GFP) fluorescence was not required, cells were grown to log-phase in YPD (Guthrie and Fink, 1991). To 1.5 ml of cells in YPD, 200 μl of a 37% formaldehyde solution was added and the culture was incubated at room temperature for 30 min to 1 h. The cells were washed two times in PBS containing 1 mg/ml BSA (PBS-BSA) and then resuspended in 50 μl PBS-BSA, to which 10 μl of rhodamine-phalloidin (Molecular Probes) solution was added (300 U in 1.5 ml methanol). After a 30-min incubation, the cells were washed three times in PBS-BSA and resuspended in mounting medium (Pringle et al., 1991) before visualization. When preservation of GFP fluorescence was required, cells were fixed in 2% formaldehyde for no longer than 30 min.
For actin immunofluorescence, a 1:2,000 dilution of guinea pig anti– yeast actin serum was used (Mulholland et al., 1994). The myc epitope-tag was detected using a 1:50 dilution of rabbit polyclonal anti-myc antibodies (Santa Cruz Biotech.). Sla2p, Sac6p, and cofilin were detected using affinity-purified antibodies raised in rabbit, at dilutions of 1:50, 1:100, and 1:200, respectively (Yang, S., M.J.T.V. Cope, and D.G. Drubin, manuscript submitted for publication; Adams et al., 1989; Moon et al., 1993). Secondary antibodies were: 1:2,000 dilution of FITC-conjugated goat anti–guinea pig antibody (Cappel/Organon Technika Inc.), and 1:2,000 dilution of Cy3-conjugated goat anti–rabbit antibody (Sigma Chemical Co.). Fixation and permeabilization of yeast cells was performed as described by Ayscough and Drubin (1998).
Instruments
Conventional light microscopy of fixed and fluorescently labeled cells was performed using a Zeiss Axioskop fluorescence microscope equipped with a Zeiss 100×/1.3 Plan-Neofluar oil-immersion objective and a Sony CCD camera controlled by Phase-3 software (Phase-3 Imaging Systems). Microscopy of living cells expressing GFP fusion proteins was performed using a Nikon TE300 (Nikon) equipped with a 100× Plan-Apo/1.4 objective and an Orca-100 cooled-CCD camera (Hamamatsu) controlled by Phase-3 software.
Confocal light microscopy of rhodamine-phalloidin–stained yeast was performed using a Zeiss 510 laser-scanning confocal microscope.
GFP Fusion Constructs
The ORFs for YNL020c (ARK1) and YIL095w (PRK1) were cloned into GFP expression vectors under control of the GAL1,10 promoter as follows. ARK1 was amplified by PCR using the primers JC_YNL020c_1 (GCTCTAGACTTATCCAAGGATAACTTTCG) and SY50 (CGTCTAGAATGAATCAACCTCAAATTGG) with pDD382 as template. The product was cloned into the XbaI site of pTS395, creating a plasmid (pDD555) encoding a chimeric protein with GFP at the COOH terminus of Ark1p. (The plasmids pTS395 and pTS408 are derivatives YCp50 [Ma et al., 1987], containing additionally the GAL1,10 promoter, wild-type GFP, and ACT1 terminator sequences, and are kind gifts from Tim Stearns, Stanford University). PRK1 was amplified by PCR using the primers JC_YIL095w_1 (CGGGGATCCATGAATACTCCACAGATTAG) and JC_YIL095W_2 (GCTCTAGATTAAACTTTGCTGGGAAACC) with pDD556 as the template. The product was inserted between the BamHI and XbaI sites of pTS408 to create a plasmid (pDD554) encoding a chimeric protein with GFP at the NH2 terminus of Prk1p. All PCR reactions were performed with low numbers of cycles and with accurate DNA polymerases (Pfu from Stratagene or Vent from New England Biolabs). The Ark1p-GFP (pDD555) construct complemented the growth defects of ark1Δ prk1Δ double-mutant cells at both 15 and 37°C. The GFP-Prk1p construct (pDD554) was itself deleterious to growth when overexpressed, but slow-growing colonies could form when this fusion protein was overexpressed in ark1Δ prk1Δ double-null cells at 37°C, whereas a control plasmid lacking an insert does not allow growth at 37°C.
An Abp1p-GFP fusion construct, a kind gift from Tim Doyle (Stanford University), was also used in these studies (Doyle and Botstein, 1996).
Induction of Galactose-regulated Expression
Induction of GFP-tagged proteins from the GAL1,10 promoter was accomplished by first picking from solid media a single, isolated colony (containing the requisite vector) and inoculating it into liquid synthetic medium (SM) lacking uracil and containing 2% glucose. After growth overnight, a small sample was removed, washed in SM, and transferred to SM lacking uracil, but containing this time 2% raffinose and 2% galactose as carbon sources. After 8–10 h, the glucose repression is overcome and the GFP-tagged fusion constructs begin to be expressed. Cells were visualized 12–16 h after transfer to the raffinose-galactose–containing medium.
Creation of Kinase-dead Mutations in Ark1p and Prk1p
The codon encoding lysine 56 of Ark1p in pDD382 was converted to a codon encoding an alanine (K56A), using the primer JC_Ark_ded (GTTGCATGCTTGGCCAGAGTCATTGTTC), in conjunction with the primer JC_Afl2_mut (GTACGCCAACTTGAGACCATGTAAC) with the “Transformer” site-directed mutagenesis kit (Clontech). In addition to making the K56A mutation, JC_Ark_ded introduces a unique MscI site. JC_Afl2_mut creates a silent mutation within the LEU2 ORF that eliminates a unique AflII site. These mutations are incorporated in the plasmid pDD559. The kinase-dead ARK1 was amplified as described above using SY50 and JC_YNL020c_1 and inserted into the XbaI site of pTS395 for expression as a GFP fusion under the control of the GAL1,10 promoter, creating pDD562. This plasmid was sequenced across the XbaI site at the 5′ end of ARK1 and the K56A mutation verified.
The codon encoding lysine 56 of Prk1p in pDD556 was converted to a codon encoding an alanine (K56A) using the primer JC_Prk_ded (CATAGCATGCTTGGCCAGAGTCATTGTC), in conjunction with the primer JC_NcoI_mut (CTTGACTGATTTTTCAATGGAGGGCACAGTTAAG), again with the “Transformer” site-directed mutagenesis kit. In addition to making the K56A mutation, JC_Prk_ded introduces a unique MscI site. JC_NcoI_mut creates a silent mutation within the URA3 ORF that eliminates a unique NcoI site. These mutations are incorporated in the plasmid pDD560. The kinase-dead PRK1 was amplified as described above using JC_YIL095w_1 and JC_YIL095w_2 and inserted into the XbaI site of pTS408 for expression as a GFP fusion under the control of the GAL1,10 promoter, creating pDD561. This plasmid was sequenced across the BamHI site at the 5′ end of PRK1 and the K56A mutation verified.
The plasmids pDD561 and pDD562 were transformed into DDY130 and expression of the kinase-dead variants of Ark1p and Prk1p were induced as described above. The cells were examined by fluorescence microscopy to verify Ark1p and Prk1p expression and localization, by immunofluorescence microscopy for actin distribution, and by differential interference-contrast (DIC) microscopy for cell appearance and morphology.
Vital Staining Using FUN-1
Cells were tested for viability using the vital dye FUN-1 (Molecular Probes). The cells were grown to log-phase in minimal medium and FUN-1 was added to a final concentration of 1 μM. After rotation at 25°C for 30 min, the cells were washed once and then resuspended in 250 μl of minimal media for visualization. Living cells were able to internalize the stain to the vacuole, where it could be metabolized to form aggregates that fluoresced brightly when observed using a rhodamine filter set. Dead cells, by contrast, displayed a uniform distribution of dye throughout the cell and did not develop bright vacuolar aggregates.
Results
A Two-Hybrid Screen Reveals an Interaction between a Novel Protein Kinase and a Component of the Actin Cytoskeleton
Sla2p/End4p/Mop2p is a component of the yeast cortical actin cytoskeleton implicated in the control of actin organization, endocytosis, and the maintenance of an ATPase at the plasma membrane (Holtzman et al., 1993; Raths et al., 1993; Li et al., 1995; Na et al., 1995). To identify proteins that interact with Sla2p and thus might be involved in the assembly or function of cortical actin patches, a fusion of residues 503–968 of Sla2p to the DNA binding domain of Gal4p was used to screen a two-hybrid library of cDNA fragments fused to the activation domain of Gal4p (Durfee et al., 1993). Of the six clones identified, two were derived from the yeast gene YNL020c. One clone encoded residues 380–638, and the other encoded residues 218–552 of the protein. The remaining clones were single isolates and have not yet been pursued further. YNLO20c encodes a protein of 638 amino acids with a 300-residue region at the NH2 terminus containing many elements conserved in serine-threonine kinases (Fig. 1 A). Because of the findings presented below, we have named this gene ARK1, for actin regulating kinase 1.
Figure 1.

Sla2p-Ark1p two-hybrid interactions and sequence comparisons of kinase domains similar to that of Ark1p. (A) The COOH terminus of Sla2p (residues 581– 968) interacts with residues 380–553 of Ark1p in a two-hybrid assay. The kinase domain of Ark1p is 70% identical to the kinase domain of Prk1p. However, the tails lack significant similarity except for a small motif PxPPPKP, found near the COOH termini of both proteins, once in Prk1p and twice in Ark1p. (B) An alignment of the Ark1p and Prk1p kinase domains with closely related serine-threonine kinase domains from a number of organisms. All are found at the NH2 terminus of their respective proteins. Thus, the numbering at the side of the alignment corresponds to the residue number within the proteins, except for the S. pombe kinase, for which, in the interests of space, 13 residues (n) have been omitted after the initial methionine. Residues conserved in at least six of the eight proteins are shaded. 12 subdomains are indicated by Roman numerals and these correspond to those defined by Hardie and Hanks (1995). Below the subdomain delineators there is a consensus line which indicates residues conserved in an alignment of 400 kinases. The symbolism used here is the same as that used in Hardie and Hanks (1995): uppercase letters, invariant residues; lowercase letters, nearly invariant residues; o, positions occupied by nonpolar residues; *, positions occupied by polar residues; and +, positions occupied by residues with short side chains and with near neutral polarity. The so-called p-loop domain, involved in binding the nontransferable phosphates of ATP, is indicated by a dashed line. A bullet denotes an invariant lysine residue required for kinase activity (for review see Hanks et al., 1988). After the alignment was made, it was imported into SeqVu (Garvan Institute) for presentation and was annotated in Freehand (Aldus Corp.). (C) An unrooted phylogenetic tree showing how the Ark1p and the Prk1p kinase domains relate to representative kinases from other serine-threonine kinase families. Horizontal bars indicate evolutionary distances between the kinases. The length corresponding to 20% difference in identity is indicated. Note that >100% difference in identity is possible due to the correction for multiple substitutions, which tends to lengthen long branches. The Ark1p and Prk1p kinase domains, together with the kinase domains shown in B, form a newly identified family which we term the Ark family. GenBank accession numbers for the sequences are as follows: Ark1p: 1301849; Prk1p: 763251; YBR059c (S. cerevisiae): 585348; Spo kin (S. pombe kinase): 2894277; Cel kin (C. elegans kinase): 1066951; Ath kin (A. thaliana kinase): 2702277; Hs GAK (Homo sapiens GAK): 2506080; Rn GAK (Rattus norvegicus GAK): 2829846; YPK2: 140977; cAPKa: 125205; PKCa: 125549; AKT1: 400112; S6K: 125695; bARK1: 114151; PVPK1: 125568; DBF2: 1706307; SPK1: 134835; KIN1: 2507199; caMYII: 125285; CKIIa: 125257; MCK1: 126820; ERK1: 232067; SGV1: 134474; and CDC2Hs: 115922.
Ark1p Homologues
Examination of the S. cerevisiae genome indicated that the putative protein kinase domain of Ark1p is very similar in amino acid sequence to the kinase domain of a protein encoded by the ORF YIL095w. This protein was found in a genetic screen for modifiers of mammalian p53 activity in yeast and has been named p53-regulating kinase 1, or Prk1p (originally Pak1p; Thiagalingam et al., 1995). The kinase domains of the two proteins are 73% identical at the amino acid level. Furthermore, a third S. cerevisiae protein, encoded by the unstudied ORF YBR059c, a Schizosaccharomyces pombe protein, and proteins from Caenorhabditis elegans, Arabidopsis thaliana, rat, and human were also found to be similar to Ark1p and Prk1p within the kinase domains, having between 30 and 48% identity to the Ark1p kinase domain. This level of identity is significantly higher than that observed with any other kinases retrieved from the databases, according to the BLAST algorithm (Altschul et al., 1990). The rat and human proteins have been identified as cyclin-G–associated kinases (GAKs) and the human GAK has been shown to be a functional kinase in vitro (Kanaoka et al., 1997; Kimura et al., 1997). The kinase domains of these proteins were reported previously to be most similar to those of Nek1 and CDK2 (Kanaoka et al., 1997). However, our analysis now places them in a separate family. We propose to call this the Ark family, after Ark1p, the first member of this family to be named for a biological process.
A sequence alignment shows the similarities between the kinase domains of Ark1p, Prk1p, and six other Ark family members (Fig. 1 B). The residues that are absolutely conserved in all serine-threonine kinases are present, but there are significant variations from the norm that are distinguishing features of the eight kinase domains shown here (Hanks et al., 1988; Hardie and Hanks, 1995). Most notably, the largely invariant p-loop sequence (involved in binding the nontransferable phosphates of ATP) in other kinases conforms to the consensus GxGxxGxV, while the Ark family members have their own consensus S/EGGFA/SxVY (where x is any amino acid, S/E is serine or glutamate, and A/S is alanine or serine). There is a GxGxxG motif very near the NH2 terminus of the GAKs which has led to the suggestion that these residues might constitute part of a p-loop (Kanaoka et al., 1997). However, based on the alignment shown in Fig. 1 B, we feel that these residues are unlikely to comprise the true phosphate anchoring loop. This is because the first two glycines are separated by a proline, an amino acid never seen at this position in other kinases, and because other conserved features are present surrounding the S/EGGFA/SxVY motif, but not surrounding the GxGxxG motif in these kinases. A crystal structure will be required to be certain about this issue. Fig. 1 B also shows that several other residues are absolutely conserved in the kinase domains of the Ark family members but are rarely found in kinases not in this group.
To demonstrate objectively that these eight putative kinases are more closely related to each other than to other serine-threonine kinases, we performed a further multiple alignment. This time, we also included representative kinases from each of the classes defined in Hardie and Hanks (1995) (alignment not shown). The resulting phylogenetic tree indicates that the eight kinase domains shown in Fig. 1 B constitute a new family within the superfamily of serine-threonine kinases (Fig. 1 C).
The Nonkinase Domains of Ark1p and Prk1p Are Not Closely Related
Although the kinase domains of Ark1p and Prk1p are highly similar, their nonkinase COOH-terminal domains lack detectable similarity along most of their lengths. Ark1p encodes a protein of 638 amino acids with a nonkinase COOH-terminal domain of ∼340 amino acids, while Prk1p encodes a protein of 810 amino acids with a nonkinase 510–amino acid COOH-terminal domain with no significant extensive similarity to that of Ark1p. However, a short conserved motif does exist close to the COOH terminus of both proteins. Ark1p contains two copies, and Prk1p contains one copy, of the conserved motif PxPPPKP. Proline-rich regions are known to mediate protein– protein interactions and can be bound by, for example, Src-homology 3 (SH3) domains. Several interactions between proline-rich motifs and SH3 domains are found among proteins found in yeast cortical actin patches, and it is possible that Ark1p and Prk1p also participate in such associations (Lila and Drubin, 1997).
Lack of Both Ark1p and Prk1p Leads to Severe Actin Abnormalities and Inviability at Extreme Temperatures: Kinase-active Ark1p or Prk1p Are Required to Rescue These Defects
To determine whether deletion of either the ARK1 or the PRK1 gene has a detectable effect on the actin cytoskeleton or on the growth characteristics of the cell, we replaced each of these genes individually with auxotrophic markers. Deletion of these genes singly caused little or no detectable defects in the actin cytoskeleton (Fig. 2, D–I). However, growth rates were slightly lower than in the parental wild-type strain (1.8 h doubling time at 30°C for each single deletion mutant compared with 1.5 h for the wild-type [DDY130]).
Figure 2.

Phenotypes of single deletions of ARK1 and PRK1, and of ark1Δ prk1Δ double-mutant yeast. Deletions in the ARK1 and PRK1 genes were created as described in Materials and Methods. All strains were grown to log-phase in rich liquid medium (YPD) at 30°C before fixation and visualization. (A–C) DDY130 (wild-type); (D–F) DDY1558 (prk1Δ:: LEU2); (G–I) DDY1407 (ark1Δ::HIS3); (J–L) DDY1564 (ark1Δ::HIS3, prk1Δ:: LEU2). (A, D, G, and J) Actin localization, visualized by rhodamine-phalloidin staining; (B, E, H, and K) nuclear and mitochondrial DNA localization visualized by DAPI staining; (C, F, I, and L) DIC images. Bar, 5 μm.
Whereas the effects of genetically removing either Ark1p or Prk1p from the cell were negligible (Fig. 2, D–I), the effects of the removal of both proteins were profound (Fig. 2, J–L). ark1Δ prk1Δ double mutants were inviable at 15 and 37°C, and grew optimally at 30°C. At 30°C, many of the double-mutant cells were substantially larger than the parental cells and had thicker bud necks (Fig. 2 L). Moreover, the double-mutant cells displayed a severely abnormal actin cytoskeleton. Most cells in the population (85%) contained one or more large clumps of actin (Fig. 2 J). Similar clumps are not seen in wild-type cells. These clumps contain filamentous actin because they stain brightly with rhodamine-phalloidin. Actin cortical patches and actin cables were still present, although the cortical patches no longer appeared uniform in size and they were not polarized. In addition, fewer cables could be observed in the double-mutant cells than in wild-type cells (50% of mutant cells contained visible cables versus 75% in the wild-type cells). Despite the severity of the actin defects, these cells did manage to form buds and divide. The doubling time of ark1Δ prk1Δ haploid cells at 30°C was ∼3 h, however, compared with a doubling time of 90 min for the parental wild-type cells. An increase over wild-type in the number of multinucleate cells was not observed. Shifting cells from 30 to 37°C or to 15°C did not change actin distribution noticeably.
The phenotypes observed in the ark1Δ prk1Δ double-mutant cells were rescued when they were transformed with low-copy (CEN) plasmids bearing genomic fragments containing either ARK1 (pDD382) or PRK1 (pDD556) (data not shown). We then mutated in Ark1p and Prk1p a conserved lysine within the kinase domain that had been shown in a number of previous studies on other members of the protein kinase superfamily to result in a loss of protein kinase activity (Fig. 1 B; Hanks et al., 1988). For both Ark1p and Prk1p, a conserved lysine (K56) was changed to an alanine. When ark1Δ prk1Δ double-mutant cells were transformed with low-copy plasmids bearing the kinase-dead mutants of ARK1 (pDD559) and PRK1 (pDD560), the phenotypes described above were not rescued (data not shown). These data suggest that Ark1p and Prk1p are active kinases, and that their kinase activities are necessary for their in vivo function(s).
The Actin Clumps Found in ark1Δ prk1Δ Cells Also Contain Cofilin, Sla2p, Sac6p, and Abp1p
To determine whether the actin clumps observed by rhodamine-phalloidin staining in ark1Δ prk1Δ cells were merely abnormal aggregates of filamentous actin derived from cables, or whether they also contained proteins normally found in cortical actin patches, we performed indirect immunofluorescence on these cells using antibodies directed against Sac6p (yeast fimbrin), Sla2p, and cofilin. All of these proteins are normally found in actin patches, Sac6p also associates with actin cables. All three of these proteins associated with the actin clumps in ark1Δ prk1Δ cells (Fig. 3). Abp1p-GFP also localizes to these clumps in vivo (data not shown). In addition, all four proteins are found to be present in the cortical actin patches that remain in the ark1Δ prk1Δ cells. As has been observed in wild-type cells, the localization of cortical Sla2p patches was not always coincident with that of cortical actin patches (Yang, S., M.J.T.V. Cope, and D.G. Drubin, manuscript submitted for publication).
Figure 3.
Actin clumps found in ark1Δ prk1Δ double mutants also contain Sac6p, cofilin, and Sla2p. ark1Δ prk1Δ double mutants were fixed and stained for actin (detected by FITC-conjugated secondary antibody) and for either Sac6p, cofilin, or Sla2p (detected by CY3-conjugated secondary antibody). (A–C) Actin localization in ark1Δ prk1Δ double mutants. (D) Sac6p localization, (E) cofilin localization, and (F) Sla2p localization in the same cells shown in A–C. Bar, 5 μm.
The Actin Clumps Found in ark1Δ prk1Δ Cells Are Not Strictly Cortical
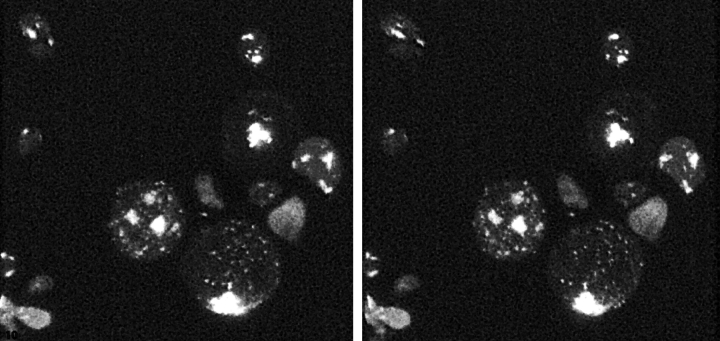
The presence of cofilin, Sla2p, Sac6p, and Abp1p in the actin clumps of ark1Δ prk1Δ double-mutant cells is a characteristic shared with actin cortical patches. To determine whether these clumps were also associated with the cell cortex, we used confocal microscopy. The actin clumps were found associated with the cell cortex in only a minority of cases (Fig. 4). Immuno-EM using anti-actin antibodies has verified this observation (data not shown). Furthermore, the confocal microscopy revealed that, while variable in size, the actin clumps could occupy a volume of the cytoplasm comparable to that of the nucleus. Thus, the clumps are not merely comprised of many actin patches aggregated at the cell surface. Rather, they are large masses of actin and other cortical patch proteins that accumulate in the absence of Ark1p and Prk1p.
Figure 4.
A stereo-pair showing a three-dimensional reconstruction of ark1Δ prk1Δ double-mutant yeast in which the actin has been labeled using rhodamine-phalloidin. The three-dimensional reconstruction from confocal serial sections was produced using Imageworks software running on a Silicon Graphics workstation.
Ark1p and Prk1p Are Associated with Cortical Actin Patches
Since Ark1p was found as a protein that interacts with Sla2p, and since Ark1p and Prk1p were shown to affect profoundly the organization of cortical actin cytoskeleton proteins, we determined the subcellular localization of Ark1p and Prk1p. First, we placed a 6-myc epitope-tag close to the NH2 terminus of each protein. In the case of Prk1p, this construct was capable of fully complementing the actin and growth phenotypes of the ark1Δ prk1Δ double-mutant cells when expressed from a low-copy plasmid under its own promoter. Indirect immunofluorescence of actin and of the myc epitope in cells expressing only the tagged version of Prk1p shows that this protein localizes to cortical actin patches (Fig. 5 A). These patches were appropriately organized according to the cell-cycle stage, indicating that expression of the myc-tagged protein was not causing the formation of abnormal structures or interfering with actin localization.
Figure 5.

Prk1p and Ark1p localize to cortical actin patches. (A) Indirect immunofluorescence of myc-tagged Prk1p and actin in prk1Δ cells. Epitope-tagged Prk1p colocalizes with cortical actin patches. The control cells show ark1Δ prk1Δ cells bearing vector with no insert, stained with the same combination of antibodies. (B) Indirect immunofluorescence of GFP-Ark1p and actin in wild-type cells. Cortical patches containing actin were found also to contain GFP-Ark1p. Two cells are not expressing Ark1p-GFP. They may be dead or they may have lost the plasmid. Cortical actin patches and actin bars occasionally survive after the death of the cells. (C) Indirect immunofluorescence of GFP-Prk1p and actin in wild-type cells. GFP-Prk1p is found to be associated with actin patches. The central cell shows a strongly staining actin bar, a feature often seen in cells that are dead or dying. This cell is dead since there is no longer any GFP fluorescence. An actin bar is also visible in the left-hand cell, but this cell is still alive and expressing GFP-Prk1p. GFP-Ark1p or GFP-Prk1p overexpression leads to delocalization of the cortical patches and actin bars. Bar, 5 μm.
Epitope-tagged constructs of Ark1p appeared to be unstable. As an alternative approach to localizing Ark1p and to examine the in vivo localization of both Ark1p and Prk1p, we placed ARK1 and PRK1 in vectors that would express them as GFP fusion proteins under the control of the strong, inducible GAL1,10 promoter. When expressed in the presence of galactose, Ark1p-GFP was visible in cortical patch structures very similar in appearance and behavior (i.e., motility) to actin patches. When GFP and actin were both localized by indirect immunofluorescence, they were found to be coincident in patches at the cell cortex (Fig. 5 B). Kinase-dead Ark1p-GFP, which does not perturb the actin cytoskeleton (see below), also localized to cortical actin patches. Thus, we conclude that Ark1p localizes to cortical actin patches. GFP-Prk1p was also found to colocalize with cortical actin patches (Fig. 5 C).
Effects of Elevated Levels of Ark1p and Prk1p on Cell Morphology
Elevated levels of Ark1p and Prk1p lead to the formation of delocalized actin patches and actin bars (Fig. 5, B and C). Actin bars are intracellular aggregates of actin monomers and are therefore not labeled by rhodamine-phalloidin, a compound that binds to filamentous but not monomeric actin. Actin bars are in this respect different from the actin clumps observed in ark1Δ prk1Δ cells. Continued overexpression of either Ark1p or Prk1p leads to a variety of further and more severe effects (Fig. 6, A and B). In the case of Prk1p, this ultimately leads to inviability of the cell population. The initial consequences of overexpression of either Ark1p or Prk1p included cells with abnormally shaped buds, and apparent septation defects, multiple buds, and/or severely abnormal internal structures. Cells with abnormal internal structures were determined to be dead using the vital dye FUN-1 (Fig. 6 C). 4–6 h after induction of Prk1p-GFP overexpression, ∼35% of budded cells had abnormal buds and/or multiple buds, and ∼25% of cells were dead. Most dead cells contained actin bars. Continued growth of Prk1p-GFP–expressing cells (24 h) in galactose-containing media resulted in lethality for the majority of cells, although some did survive and micro-colonies were formed after 5–7 d growth at 30°C (data not shown). While overexpression of Ark1p caused similar bud morphology, septation, and multibudded phenotypes after 4–6 h after derepression (Fig. 6 B), fewer dead cells were seen (15–20%) and this percentage did not exceed 25% after continued overexpression (24 h).
Figure 6.
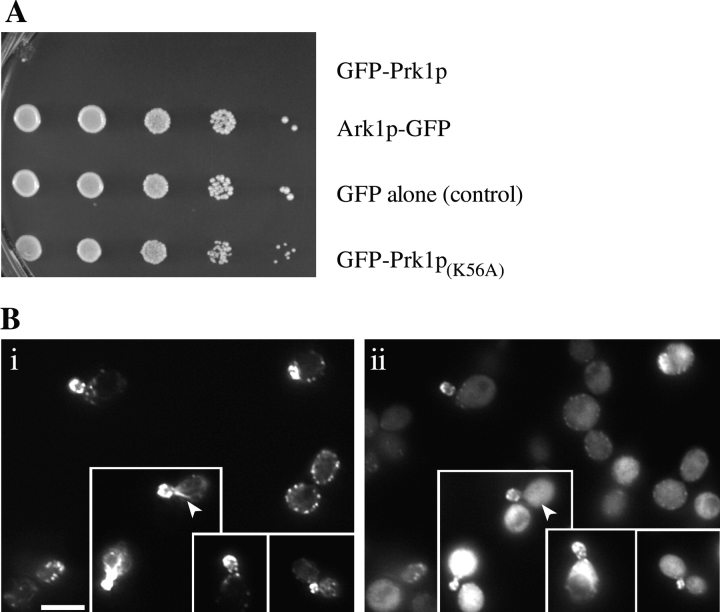
Effects of Ark1p and Prk1p overexpression. Elevated levels of either Ark1p or Prk1p result in a number of cellular abnormalities. These abnormalities include unusual bud morphologies, septation defects and multiple buds, and death. (A) Rhodamine-phalloidin (i) and DIC microscopy (ii) of selected fields from cultures overexpressing GFP-Prk1p (DDY130 transformed with pDD554) at 25°C for 8 h. Similar results were obtained when native Prk1p was overexpressed (data not shown). Cells that display abnormal internal morphology and that do not stain for actin are indicated by arrowheads. These cells are most likely dead. Continued overexpression of Prk1p ultimately leads to cell death in a majority of the cells. (B) Rhodamine-phalloidin (i) and DIC microscopy (ii) of selected fields from cultures overexpressing GFP-Ark1p (DDY130 transformed with pDD555) at 25°C for 8 h. (C) Cells with abnormal, brain-like, intracellular morphologies are dead. (i) DIC microscopy and (ii) FUN-1 staining of living and dead cells overexpressing Prk1p. In living cells, the FUN-1 is internalized to the vacuole where it is processed into an aggregate that fluoresces brightly (as in the two cells on the right-hand side). Dead cells display a diffuse pattern of fluorescence throughout the cell (as in the cell on the left-hand side).
The fact that Prk1p overexpression is lethal to most cells was independently demonstrated in this laboratory in a screen for proteins that cause death upon overexpression (DUO). The DUO screen was performed on yeast cells transformed with a galactose-inducible genomic library to select for transformants that grew on glucose plates (expression inhibited) and that died on galactose plates (expression induced). The PRK1 gene was identified in a subsequent visual screen as a gene that caused lethality and abnormal cell morphology when present at elevated levels. The overexpression phenotypes caused by this untagged Prk1p were identical to those described above.
As reported above, the kinase activities of Ark1p and of Prk1p are necessary for either protein to rescue the phenotypes of ark1Δ prk1Δ double-mutant yeast. To test whether the overexpression phenotypes were also dependent on kinase activity, we placed the kinase-dead variants of the proteins, also as GFP-containing chimeras, under the control of the GAL1,10 promoter. Overexpression of the kinase-dead variant of Prk1p did not cause inviability (Fig. 7 A; compare dilution series i to dilution series iv). However, the kinase-dead variant of Prk1p retained the ability to cause delocalization of actin patches (data not shown). As mentioned above, the kinase-dead variant of Ark1p localized to cortical patches, but when present at elevated levels no longer caused delocalization of actin patches (Fig. 7 B), nor did it cause abnormally budded or multibudded cells, death, or any of the phenotypes that were exhibited by cells containing elevated levels of wild-type Ark1p. Furthermore, using the kinase-dead mutant, the colocalization of Ark1p with cortical actin patches (and not actin cables) was confirmed under conditions where the actin cytoskeleton itself is unperturbed.
Figure 7.
(A) Dilution series of wild-type (DDY130) cells expressing various GFP fusion proteins under the control of the GAL1,10 promoter. (i) GFP-Prk1p (pDD554); (ii) Ark1p-GFP (pDD555); (iii) GFP alone (control; pTS408); (iv) GFP-Prk1p(K56A) (pDD561). Cells were grown on glucose-containing medium before dilution and subsequent growth on galactose-containing medium to induce overexpression of the constructs. The plate was photographed after 4 d of growth. (B) (i) Rhodamine-phalloidin staining of filamentous actin and (ii) GFP localization in DDY130 cells overexpressing Ark1p(K56A)-GFP. The actin patches are polarized normally. Ark1p(K56A)-GFP localizes to cortical actin patches, but not to actin cables (arrowhead). Bar, 5 μm.
Ark1p and Prk1p Localization in Cells Lacking Specific Cortical Actin Cytoskeleton Proteins
The COOH-terminal, nonkinase domain of Ark1p interacts with Sla2 in the two-hybrid assay (Fig. 1) and Sla2p partially colocalizes with actin in cortical patches (Yang, S., M.J.T.V. Cope, and D.G. Drubin, manuscript submitted for publication). Thus, it was of interest to determine whether Ark1p and Prk1p were capable of being localized to cortical patches in the absence of Sla2p. Interestingly, both Ark1p and Prk1p were still found in patches at the cortex in sla2Δ cells (Fig. 8). However, localization of both proteins to cortical patches was reduced dramatically in abp1Δ cells. Ark1p and Prk1p localize to cortical patches in sac6Δ, srv2Δ, and in rvs167Δ cells. Yeast containing sac6Δ, srv2Δ, or rvs167Δ mutations have abnormal actin cortical patch morphologies, whereas abp1Δ yeast do not (Adams et al., 1989; Holtzman et al., 1993; Lila and Drubin, 1997). Overexpression of Ark1p and Prk1p results in further disruption of actin in each of these null mutant strains (even in abp1Δ cells); however, it is clear that Ark1p and Prk1p remain colocalized with actin in all the mutants tested, except abp1Δ cells.
Figure 8.
Ark1p-GFP and Prk1p-GFP localization to cortical patches is reduced in abp1Δ cells, but not in sla2Δ, sac6Δ, srv2Δ, or rvs167Δ cells. (A) Actin and (B) Prk1p-GFP localization in (i) sac6Δ, (ii) abp1Δ, (iii) rvs167Δ, (iv) srv2Δ, and (v) sla2Δ cells. (C) Actin and (D) Ark1p-GFP localization in (i) sac6Δ, (ii) abp1Δ, (iii) rvs167Δ, (iv) srv2Δ, and (v) sla2Δ cells. sac6Δ (DDY318), abp1Δ (DDY322), rvs167Δ (DDY950), srv2Δ (DDY952), or sla2Δ (DDY1166) cells were transformed with either pDD554 (GFP-Prk1p) or pDD555 (GFP-Ark1p), expression was induced for 8–12 h, and actin and GFP localization was examined. GFP-Ark1p and GFP-Prk1p are mostly found in the cytoplasm when expressed in abp1Δ cells. In only a small population of cells (<10%) can faint cortical patches of GFP-Ark1p or GFP-Prk1p be detected. Bar, 5 μm.
Genetic Interactions Involving ARK1 and PRK1 Provide Evidence for Parallel Pathways in Actin Cytoskeleton Regulation
The synthetic lethality that results when ark1Δ and prk1Δ are combined suggests that these proteins function in parallel to regulate an essential process or processes. To gain deeper insight into how Ark1p and Prk1p might regulate the actin cytoskeleton, we constructed a number of double mutants containing a null allele of ARK1 or of PRK1, together with a null allele of a gene implicated in cortical actin cytoskeleton function. The genes chosen for testing were ABP1, SAC6, SLA1, SLA2, SRV2, RVS167, CRN1, and AIP1. Abp1p is an actin-binding protein that localizes to actin patches (Drubin et al., 1990). Sac6p, the yeast fimbrin homologue, bundles actin filaments and localizes to both actin patches and to actin cables (Adams et al., 1989, 1991). Sla1p is an SH3 domain containing protein found in cortical actin patches (Holtzman et al., 1993). Null alleles of the SLA1 gene are synthetic lethal with null alleles of the ABP1 and RVS167 genes (Holtzman et al., 1993; Lila and Drubin, 1997). SLA2 encodes a cortical actin-binding protein and mutant alleles of this gene are synthetic lethal with ABP1 null alleles. Mutations in the SLA2 gene lead to actin and endocytosis defects (Holtzman et al., 1993; Wesp et al., 1997). Srv2p, the yeast homologue of CAP, binds to adenylyl cyclase, Abp1p, and actin monomers (Field et al., 1988; Freeman et al., 1995, 1996). Rvs167p is necessary for a normal actin cytoskeleton morphology and for endocytosis (Bauer et al., 1993; Munn et al., 1995). Yeast coronin (Crn1p) binds tightly to actin and localizes to actin patches (Goode et al., 1999). Aip1p interacts with actin and cofilin, and localizes to actin patches (Rodal, A.A., J. Tetrault, P. Lappalainen, D.G. Drubin, and D.C. Amberg, manuscript submitted for publication).
Table III summarizes the effects of combining null mutations in the above genes with null mutations in the ARK1 and PRK1 genes. In addition to showing a negative synergism with prk1Δ, ark1Δ also shows a synthetic genetic interaction with sac6Δ. This was the only additional genetic interaction detected involving ARK1. By contrast, prk1Δ, as well as showing negative synergism with ark1Δ and with sac6Δ, shows synthetic genetic interactions with sla2Δ and with abp1Δ. From two separate crosses between different sla2Δ and prk1Δ strains (32 tetrads were dissected), >50% of predicted prk1Δ sla2Δ double-mutant spores were viable. Surviving double mutants were extremely temperature and cold sensitive and could not reliably be streaked to single colonies. Thus, we consider the prk1Δ sla2Δ combination to be lethal.
Table III.
Synthetic Genetic Interactions Involving ARK1 and PRK1
| Mutation(s) | Temperature growth range* | Actin organization | Negative growth synergism | |||
|---|---|---|---|---|---|---|
| ark1Δ | 15°C–37°C | Normal | — | |||
| prk1Δ | 15°C–37°C | Normal | — | |||
| sac6Δ | 15°C–34°C | Depolarized patches, | — | |||
| fewer cables | ||||||
| sla1Δ | 15°C–34°C | Fewer, larger | — | |||
| patches, depolarized | ||||||
| sla2Δ | 15°C–30°C‡ | Larger number of | — | |||
| patches, depolarized | ||||||
| abp1Δ | 15°C–37°C | Normal | — | |||
| srv2Δ | 15°C–37°C§ | Depolarized patches, | — | |||
| occasional actin bars | ||||||
| rvs167Δ | 15°C–37°C | Delocalized actin | — | |||
| crn1Δ | 15°C–37°C | Normal | — | |||
| aip1Δ | 15°C–37°C | Normal | — | |||
| ark1Δ prk1Δ | 20°C–34°C‡ | Severly disrupted: | Yes | |||
| actin “clumps” and | ||||||
| depolarized patches, | ||||||
| fewer cables | ||||||
| ark1Δ sac6Δ | 15°C‡–34°C‡ | Larger number of | Yes | |||
| depolarized patches | ||||||
| prk1Δ sac6Δ | 15°C–30°C‡ | Larger number of | Yes | |||
| depolarized patches | ||||||
| ark1Δ sla1Δ | 15°C–34°C | Same as sla1Δ | No | |||
| prk1Δ sla1Δ | 15°C–34°C | Same as sla1Δ | No | |||
| ark1Δ sla2Δ | 15°C–30°C‡ | Same as sla2Δ | No | |||
| prk1Δ sla2Δ | Lethal‖ | Yes | ||||
| ark1Δ abp1Δ | 15°C–37°C | Normal | No | |||
| prk1Δ abp1Δ | 15°C–34°C‡ | Severely disrupted: | Yes | |||
| actin “clumps” and | ||||||
| depolarized patches | ||||||
| ark1Δ srv2Δ | 15°C–37°C§ | Same as srv2Δ | No | |||
| prk1Δ srv2Δ | 15°C–37°C§ | Same as srv2Δ | No | |||
| ark1Δ rvs167Δ | 15°C–37°C | Same as rvs167Δ | No | |||
| prk1Δ rvs167Δ | 15°C–37°C | Same as rvs167Δ | No | |||
| ark1Δ crn1Δ | 15°C–37°C | Normal | No | |||
| prk1Δ crn1Δ | 15°C–37°C | Normal | No | |||
| ark1Δ aip1Δ | 15°C–37°C | Normal | No | |||
| prk1Δ aip1Δ | 15°C–37°C | Normal | No |
Growth was tested at 15, 20, 25, 30, 34, and 37°C.
Very slow growing at the indicated extremes of temperature.
7 out of the 12 predicted double-mutant progeny were inviable.
Slow growing at the indicated extremes of temperature.
Discussion
We have identified a putative serine-threonine kinase that, in the yeast two-hybrid system, binds to the evolutionarily conserved, cortical actin-associated protein, Sla2p. We have subsequently assigned the gene name ARK1 (for actin-regulating kinase 1) to this locus.
Ark1p and another S. cerevisiae protein, encoded by the PRK1 gene, have at their NH2 termini predicted serine-threonine kinase domains of ∼300 amino acids that are >70% identical. Searches of sequence databases identified six other kinases that are highly similar, within their kinase domains, to Ark1p and Prk1p, but are dissimilar outside the kinase domains. Together, these eight proteins define a new family of serine-threonine kinases, which we have termed the Ark family. Two mammalian members of the Ark family were identified as cyclin-G–associated proteins (Kanaoka et al., 1997; Kimura et al., 1997), although there are no proteins with significant homology to cyclin-G in budding yeast. A third predicted serine-threonine kinase from S. cerevisiae, encoded by the ORF YBR059c, is a member of the Ark family of kinases and has been implicated in the yeast pheromone response pathway (Caponigro et al., 1998). The kinase domain of this protein is less similar to those of Ark1p and Prk1p than they are to each other. Since Ark1p and Prk1p are similar primarily only in their kinase domains, but both regulate the actin cytoskeleton organization in S. cerevisiae, it is also possible that Ark family kinases present in other organisms have a related role.
Deletion of either the ARK1 or the PRK1 gene individually had no observed consequences with respect to actin distribution, cell morphology, or growth. By contrast, when deletions in the ARK1 and the PRK1 genes were combined, the effect was strongly deleterious. Yeast lacking both Ark1p and Prk1p were sensitive to high and low temperatures, were slow growing, and had severely disrupted actin cytoskeletons. It appeared that the majority of the filamentous actin in these cells was present in large clumps. These actin clumps also contain other proteins normally found in cortical actin patches, suggesting that the clumps result from a loss of actin patch regulation. The clumps are typically not cortical, although electron microscopy (not shown) suggests that they might maintain connection with the cortex. Therefore, the clumps might be formed by the inappropriate aggregation of many cortical patches/patch proteins at the cell cortex. Since cortical actin cytoskeleton proteins seem to be part of the endocytic machinery, actin patch aggregation at the cell cortex might in turn lead to invagination of the cell surface such that large clumps of cortical actin cytoskeleton proteins become inappropriately localized to the cytoplasm. Alternatively, the clumps might form as a result of detachment of patches from the cortex, or they might form as a result of inappropriate nucleation of patch assembly in the cytoplasm. The latter scenario would be possible if patch components are themselves recycled between an endocytic compartment and the plasma membrane, and loss of Ark1p and Prk1p function caused proteins responsible for nucleating patch assembly to be trapped in an endocytic compartment.
The cellular defects caused by loss of both Ark1p and Prk1p are alleviated by expressing wild-type Ark1p or Prk1p, but not by expressing Ark1p or Prk1p carrying a mutation in a residue required for kinase activity. Overexpression of either wild-type kinase results in a number of actin and growth defects, but these defects are absent when kinase-dead Ark1p is overexpressed, and reduced when kinase-dead Prk1p is overexpressed. Thus, the function(s) of Ark1p and Prk1p appear to depend upon their ability to function as kinases.
Ark1 and Prk1 fusion proteins localize to cortical actin patches. These two kinases are the first signaling proteins known to localize to actin patches. Because of their localization, they are candidates for downstream effectors of signaling pathways controlled by proteins such as Cdc42p that, although not localized in patches, appear to regulate the cortical actin cytoskeleton.
Since Ark1p and Prk1p both need to be eliminated before significant defects in the actin cytoskeleton are observed, these kinases seem to have redundant functions. To gain deeper insights into the functional relationships between these kinases, we made a series of double mutants between the ark1Δ or prk1Δ mutants and mutants of genes encoding actin cortical cytoskeleton proteins. Any synthetic effects involving the ARK1 or the PRK1 genes are likely to be significant since ark1Δ and prk1Δ single-mutant cells are very similar to wild-type cells in terms of appearance and growth. Null mutants in ARK1 or in PRK1 both show synthetic defects in combination with null mutants in the SAC6 gene, encoding yeast fimbrin, an actin filament bundling protein (Table III and Fig. 9 A). No other synthetic interactions involving ARK1 were observed. By contrast, prk1Δ showed severe negative synergy in combination with sla2Δ and with abp1Δ. prk1Δ and sla2Δ were synthetic lethal. prk1Δ abp1Δ double-mutant cells were large and contained actin clumps very similar to those found in ark1Δ prk1Δ cells. abp1Δ cells, like ark1Δ and prk1Δ single-mutant cells, are very healthy and normal in appearance. The pronounced synthetic phenotype of the prk1Δ abp1Δ double mutants thus reflects an extreme negative synergism. We conclude that Prk1p and Abp1p contribute to a critical process in a redundant manner, and that Abp1p may function with Ark1p because both proteins are redundant with Prk1p but not with each other. ark1Δ sac6Δ and prk1Δ sac6Δ cells were very large and temperature sensitive, but did not contain actin clumps (data not shown). The genetic interactions with sac6Δ may reflect general additive effects of cytoskeleton mutants rather than specific functional relationships in patch regulation because the sac6Δ mutation shows synthetic effects with a large number of mutant alleles of genes encoding components of the actin cytoskeleton (Holtzman et al., 1993; Botstein et al., 1997; Lila and Drubin, 1997), and because Sac6p is an actin filament bundling and stabilizing protein (Adams et al., 1989, 1991).
Figure 9.
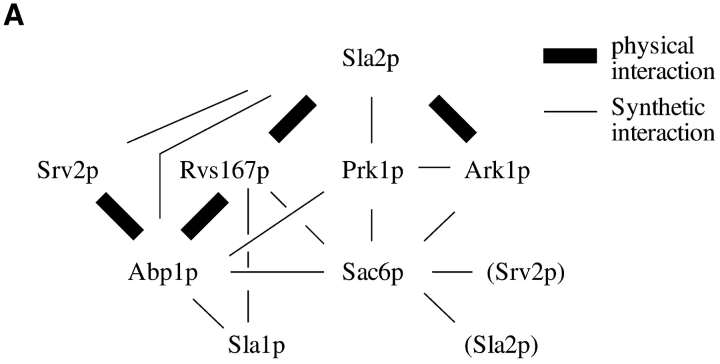
(A) A summary of the physical and synthetic genetic interactions between the proteins investigated in this study. (B) A potential signal-flow diagram for actin regulation involving Ark1p and Prk1p in budding yeast.
A pronounced phenotypic synergy between mutant alleles of two genes can reflect at least two functional relationships between the products of the genes. First, it may indicate that they act in separate pathways that can each perform the same essential function. Second, it may indicate that the two gene products are components of an essential protein complex which can retain functionality in the absence of one, but not both, proteins. abp1Δ, for example, is synthetic lethal with sla2Δ. This could be interpreted to suggest that Sla2p and Abp1p function in separate pathways towards a common, essential process. However, there is evidence suggesting that Abp1p and Sla2p might function as part of the same protein complex. Abp1p interacts with Rvs167p and Srv2p via SH3 domain– poly-proline interactions (Lila and Drubin, 1997), and Rvs167p interacts with Sla2p in a two-hybrid assay (Wesp et al., 1997). Both proteins localize to cortical actin patches, and a specific domain in Sla2p performs an endocytosis function that is redundant with a function performed by Abp1p (Wesp et al., 1997). Thus Abp1p, Sla2p, Rvs167p, and Srv2p have the potential to form a complex that may interact with and be regulated by Ark1p.
Similarly, the genetic interactions involving Prk1p and Ark1p (summarized in Fig. 9 A) may reflect synergistic contributions to the integrity of a single protein complex, or, alternatively, parallel pathways (Fig. 9 B). The lack of synthetic interactions between ark1Δ and sla2Δ, or between ark1Δ and abp1Δ, suggests formally that Ark1p, Sla2p, and Abp1p function in the same pathway. The fact that Ark1p and Sla2p interact in the two-hybrid assay supports this assessment. However, abp1Δ and sla2Δ are synthetically lethal, as mentioned above. Therefore, a more satisfactory interpretation of the current results would place Ark1p in a cortical complex together with Abp1p and Sla2p. In the absence of Sla2p, Ark1p is still capable of localizing to cortical patches, so Ark1p is obviously capable of interacting with other cortical patch components, possibly via its proline-rich motifs. Abp1p, on the other hand, is important for the localization of Ark1p to patches, so perhaps its SH3 domain is also capable of interacting with the proline-rich motif in Ark1p.
prk1Δ, in contrast to ark1Δ, shows synthetic effects with both sla2Δ and with abp1Δ. Therefore, Prk1p may either function in a separate pathway that operates in parallel to the Ark1p pathway to regulate the cortical actin cytoskeleton, or it may impinge upon the same complex in a different manner. The fact that combining null mutations in the SLA2 and PRK1 genes leads to more deleterious effects than result from combining null mutations in the ARK1 and PRK1, or in the ABP1 and PRK1 genes, suggests that Sla2p has additional functions to those that involve Ark1p and Abp1p. As mentioned above, placing Prk1p in a separate pathway from Ark1p, however, does not exclude the possibility that Prk1p interacts with a potential Abp1p/ Rvs167p/Srv2p/Sla2p complex. Prk1p localizes to cortical actin patches, and, as with Ark1p, it shows a dependency on Abp1p (but not Sla2p, Rvs167p, Sac6p, or Srv2p) for normal localization to these patches, implying that it too may associate with such a complex. However, since deletion of the ABP1 gene does not result in a phenocopy of the ark1Δ prk1Δ double deletion and does not eliminate the effects of Ark1p or Prk1p overexpression, it is reasonable to assume that localization at a reduced level allowed by interaction with, for example, Sla2p, is sufficient for Ark1p and Prk1p function. Alternatively, it might be that localization of these kinases to cortical patches is not required for Ark1p and Prk1p to fulfill their cellular functions.
What might be the phosphorylation targets of Ark1p and Prk1p? Our evidence strongly suggests that both Ark1p and Prk1p are functional kinases in vivo (see above). Other Ark family kinases have also been shown to be functional protein kinases in vitro (Kimura et al., 1997). One putative target is Sla2p, because it interacts with Ark1p in the two-hybrid system. Sla2p is a phosphoprotein in vivo, but phosphorylation of Sla2p is not eliminated in ark1Δ prk1Δ cells (data not shown). Results published while this manuscript was under revision indicate that Prk1p regulates by phosphorylation the activity of Pan1p (Zeng and Cai, 1999). Pan1p is a yeast homologue of the mammalian Eps15 proteins that play an important role in endocytosis (Wendland and Emr, 1998). Prk1p is also capable of phosphorylating the cortical actin patch protein, Sla1p (Zeng and Cai, 1999). Pan1p is an essential protein, a temperature-sensitive mutation (pan1-4) which causes the appearance of actin clumps similar to those observed in ark1Δ prk1Δ cells under nonpermissive conditions (Tang and Cai, 1996). The similar phenotypes of pan1-4 and ark1Δ prk1Δ cells suggest that Pan1p might be regulated by Ark1p and by Prk1p. Since actin clumps do not appear in ark1Δ or prk1Δ single-mutant cells, Pan1p might be phosphorylated by Ark1p in the absence of Prk1p and vice versa (Fig. 9 B). While our studies, together with those of Zhang and Cai, suggest that both Ark1p and Prk1p play important roles in Pan1p regulation, our genetic analysis (Fig. 9 A) is most consistent with the possibility that Prk1p but not Ark1p regulates Sla1p. We base this conclusion on the observation that null mutations in the PRK1 gene, but not in the ARK1 gene, have similar genetic interactions as those exhibited by null alleles of the SLA1 gene.
Ark1p and Prk1p clearly play a critical role in regulating actin distribution in vivo. This conclusion is based on several different criteria: effects of null mutations, localization, overexpression effects, and genetic interactions with other genes encoding proteins known to be involved in modulating actin distribution in yeast. Elucidation of the signaling pathways in which Ark1p and Prk1p are involved and the identification of upstream and downstream components of those pathways are now important goals.
Acknowledgments
This work was supported by grants from the Human Frontier Science Program to M.J.T.V. Cope and from the National Institutes of Health (GM50399) to D.G. Drubin.
Abbreviations used in this paper
- DIC
differential interference-contrast (Nomarski)
- GAK
cyclin-G–associated kinase
- GFP
green fluorescent protein
- ORF
open reading frame
- SM
synthetic medium
Footnotes
Confocal microscopy was performed in the College of Natural Resources (CNR) Biological Imaging Facility at U.C. Berkeley. Drs. Tim Stearns, Fred Cross, Steve Elledge, and David Amberg are gratefully acknowledged for their provision of plasmids and yeast strains used in this work. We also thank Avital Rodal and Drs. Bruce Goode, Georjana Barnes, Rachel Dent, and Amy Wolven for critical review of the manuscript and constructive discussion.
Shirley Yang's present address is Baylor College of Medicine, Department of Microbiology and Immunology, Houston, TX 77030.
References
- Adams AE, Botstein D, Drubin DG. A yeast actin-binding protein is encoded by SAC6, a gene found by suppression of an actin mutation. Science. 1989;243:231–233. doi: 10.1126/science.2643162. [DOI] [PubMed] [Google Scholar]
- Adams AE, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. . J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AE, Botstein D, Drubin DG. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature. 1991;354:404–408. doi: 10.1038/354404a0. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Ayscough, K.R., and D.G. Drubin. 1996. ACTIN: general principles from studies in yeast. In Annual Review of Cell and Developmental Biology. Vol. 12. J.A. Spudich, editor. Annual Reviews Inc., Palo Alto, CA. 129–160. [DOI] [PubMed]
- Ayscough, K.R., and D.G. Drubin. 1998. Immunofluorescence microscopy of yeast cells. In Cell Biology. Vol. 2. 2nd edition. J.E. Celis, editor. Academic Press, Inc., San Diego, CA. 477–485.
- Bauer F, Urdaci M, Aigle M, Crouzet M. Alteration of a yeast SH3 protein leads to conditional viability with defects in cytoskeletal and budding patterns. Mol Cell Biol. 1993;13:5070–5084. doi: 10.1128/mcb.13.8.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein, D., D. Amberg, J. Mulholland, T. Huffaker, A. Adams, D. Drubin, and T. Stearns. 1997. The yeast cytoskeleton. In Cold Spring Harbor Monograph Series, 21. The Molecular and Cellular Biology of the Yeast Saccharomyces. Vol. 3. Cell Cycle and Cell Biology. J.R. Pringle, J.R. Broach, and E.W. Jones, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 1–90.
- Caponigro G, Abedi MR, Hurlburt AP, Maxfield A, Judd W, Kamb A. Transdominant genetic analysis of a growth control pathway. Proc Natl Acad Sci USA. 1998;95:7508–7513. doi: 10.1073/pnas.95.13.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross FR. “Marker Swap” plasmids: convenient tools for budding yeast molecular genetics. Yeast. 1997;13:647–653. doi: 10.1002/(SICI)1097-0061(19970615)13:7<647::AID-YEA115>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Doyle T, Botstein D. Movement of yeast cortical actin cytoskeleton visualized in vivo. Proc Natl Acad Sci USA. 1996;93:3886–3891. doi: 10.1073/pnas.93.9.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Mulholland J, Zhu ZM, Botstein D. Homology of a yeast actin-binding protein to signal transduction proteins and myosin-I. Nature. 1990;343:288–290. doi: 10.1038/343288a0. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Jones HD, Wertman KF. Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol Biol Cell. 1993;4:1277–1294. doi: 10.1091/mbc.4.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee T, Becherer K, Chen PL, Yeh SH, Yang Y, Kilburn AE, Lee WH, Elledge SJ. The retinoblastoma protein associated with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Eby JJ, Holly SP, Van Drogen F, Grishin AV, Peter M, Drubin DG, Blumer KJ. Actin cytoskeleton organization regulated by the PAK family of protein kinases. Curr Biol. 1998;8:967–970. doi: 10.1016/s0960-9822(98)00398-4. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence-limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson IA, Lerner RA, Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiaeby use of an epitope addition method. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman NL, Chen Z, Horenstein J, Weber A, Field J. An actin monomer binding activity localizes to the carboxyl-terminal half of the Saccharomyces cerevisiaecyclase-associated protein. J Biol Chem. 1995;270:5680–5685. doi: 10.1074/jbc.270.10.5680. [DOI] [PubMed] [Google Scholar]
- Freeman NL, Lila T, Mintze RKA, Chen Z, Pahk AJ, Ren R, Drubin DG, Field J. A conserved proline-rich region of the Saccharomyces cerevisiaecyclase-associated protein binds SH3 domains and modulates cytoskeletal localization. Mol Cell Biol. 1996;16:548–556. doi: 10.1128/mcb.16.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Wong JJ, Butty A-C, Peter M, McCormack AL, Yates JR, Drubin DG, Barnes G. Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J Cell Biol. 1999;144:83–98. doi: 10.1083/jcb.144.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and G.R. Fink. 1991. Guide to Yeast Genetics and Molecular Biology. Academic Press, San Diego, CA. 194.
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hardie, D., and S. Hanks. 1995. The Protein Kinase FactsBook. In FactsBook. Vol. 2. Academic Press, London. 246.
- Holtzman DA, Yang S, Drubin DG. Synthetic-lethal interactions identify two novel genes Sla1 and Sla2 that control membrane cytoskeleton assembly in Saccharomyces cerevisiae. . J Cell Biol. 1993;122:635–644. doi: 10.1083/jcb.122.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DI, Pringle JR. Molecular characterization of CDC42, a Saccharomyces cerevisiaegene involved in the development of cell polarity. J Cell Biol. 1990;111:143–152. doi: 10.1083/jcb.111.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalchman MA, Koide HB, McCutcheon K, Graham RK, Nichol K, Nishiyama K, Kazemi-Esfarjani P, Lynn FC, Wellington C, Metzler M, et al. HIP1, a human homologue of S. cerevisiaeSla2p, interacts with membrane associated huntingtin in the brain. Nat Genet. 1997;16:44–53. doi: 10.1038/ng0597-44. [DOI] [PubMed] [Google Scholar]
- Kanaoka Y, Kimura SH, Okazaki I, Ikeda M, Nojima H. GAK: a cyclin G associated kinase contains a tensin-auxilin-like domain. FEBS Lett. 1997;402:73–80. doi: 10.1016/s0014-5793(96)01484-6. [DOI] [PubMed] [Google Scholar]
- Kimura, M. 1983. The neutral theory of molecular evolution. Cambridge University Press, Cambridge.
- Kimura SH, Tsuruga H, Yabuta N, Endo Y, Nojima H. Structure, expression, and chromosomal localization of human GAK. Genomics. 1997;44:179–187. doi: 10.1006/geno.1997.4873. [DOI] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO (Eur Mol Biol Organ) J. 1993;12:2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen P, Kessels MK, Cope MJTV, Drubin DG. The ADF homology (ADF-H) domain: a highly exploited actin-binding module. Mol Biol Cell. 1998;9:1951–1959. doi: 10.1091/mbc.9.8.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zheng Y, Drubin DG. Regulation of cortical actin cytoskeleton assembly during polarized cell growth in budding yeast. J Cell Biol. 1995;128:599–615. doi: 10.1083/jcb.128.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lila T, Drubin DG. Evidence for physical and functional interactions among two Saccharomyces cerevisiaeSH3 domain proteins, an adenylyl cyclase-associated protein an the actin cytoskeleton. Mol Biol Cell. 1997;8:367–385. doi: 10.1091/mbc.8.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Kunes S, Schatz PJ, Botstein D. Plasmid construction by homologous recombination in yeast. Gene. 1987;58:201–216. doi: 10.1016/0378-1119(87)90376-3. [DOI] [PubMed] [Google Scholar]
- Moon AL, Janmey PA, Louie KA, Drubin DG. Cofilin is an essential component of the yeast cortical cytoskeleton. J Cell Biol. 1993;120:421–435. doi: 10.1083/jcb.120.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J, Wesp A, Riezman H, Botstein D. Yeast actin cytoskeleton mutants accumulate a new class of Golgi-derived secretory vesicle. Mol Biol Cell. 1997;8:1481–1499. doi: 10.1091/mbc.8.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn AL, Stevenson BJ, Geli MI, Riezman H. end5, end6 and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. . Mol Biol Cell. 1995;6:1721–1742. doi: 10.1091/mbc.6.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na S, Hincapie M, McCusker JH, Haber JE. MOP2 (SLA2) affects the abundance of the plasma membrane H(+)-ATPase of Saccharomyces cerevisiae. . J Biol Chem. 1995;270:6815–6823. doi: 10.1074/jbc.270.12.6815. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Novick P, Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Adams AE, Drubin DG, Haarer BK. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- Ramos E, Wysolmerski RB, Masarcacchia RA. Myosin phosphorylation by human cdc42-dependent S6-H4 kinase-gamma-PAK from placenta and lymphoid cells. Receptors Signal Transduct. 1997;7:99–110. [PubMed] [Google Scholar]
- Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. . J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Sells MA, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 1997;7:162–167. doi: 10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- Simon VR, Swayne TC, Pon LA. Actin-dependent mitochondrial motility in mitotic yeast and cell-free systems: identification of a motor activity on the mitochondrial surface. J Cell Biol. 1995;130:345–354. doi: 10.1083/jcb.130.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn RH, Goldschmidt-Clermont PJ. Profilin: at the crossroads of signal transduction and the actin cytoskeleton. Bioessays. 1994;16:465–472. doi: 10.1002/bies.950160705. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Takai Y. Control of reorganization of the actin cytoskeleton by Rho family small GTP-binding proteins in yeast. Curr Opin Cell Biol. 1998;10:112–116. doi: 10.1016/s0955-0674(98)80093-8. [DOI] [PubMed] [Google Scholar]
- Tang HY, Cai M. The EH-domain-containing protein Pan1 is required for normal organization of the actin cytoskeleton in Saccharomyces cerevisiae. . Mol Cell Biol. 1996;16:4897–4914. doi: 10.1128/mcb.16.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagalingam S, Kinzler KW, Vogelstein B. PAK1, a gene that can regulate p53 activity in yeast. Proc Natl Acad Sci USA. 1995;92:6062–6066. doi: 10.1073/pnas.92.13.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Yonemura S. ERM (ezrin-radixin-moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- Waddle JA, Karpova TS, Waterston RH, Cooper JA. Movement of cortical actin patches in yeast. J Cell Biol. 1996;132:861–870. doi: 10.1083/jcb.132.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanker EE, Rovira C, Scherzinger E, Hasenbank R, Walter S, Tait D, Colicelli J, Lehrach H. HIP-I: a huntingtin interacting protein isolated by the yeast two-hybrid system. Hum Mol Genet. 1997;6:487–495. doi: 10.1093/hmg/6.3.487. [DOI] [PubMed] [Google Scholar]
- Wendland B, Emr SD. Pan1p, yeast eps15, functions as a multivalent adaptor that coordinates protein interactions essential for endocytosis. J Cell Biol. 1998;141:71–84. doi: 10.1083/jcb.141.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesp A, Hicke L, Palecek J, Lombardi R, Aust T, Munn AL, Riezman H. End4p-S1a2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. . Mol Biol Cell. 1997;8:2291–2306. doi: 10.1091/mbc.8.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Zeng G, Cai M. Regulation of the actin cytoskeleton organization in yeast by a novel serine/threonine kinase Prk1p. J Cell Biol. 1999;144:71–82. doi: 10.1083/jcb.144.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]