Abstract
We report here the genetic, molecular, and functional characterization of the Drosophila melanogaster minifly (mfl) gene. Genetic analysis shows that mfl is essential for Drosophila viability and fertility. While P-element induced total loss-of-function mutations cause lethality, mfl partial loss-of-function mutations cause pleiotropic defects, such as extreme reduction of body size, developmental delay, hatched abdominal cuticle, and reduced female fertility. Morphological abnormalities characteristic of apoptosis are found in the ovaries, and a proportion of eggs laid by mfl mutant females degenerates during embryogenesis. We show that mfl encodes an ubiquitous nucleolar protein that plays a central role in ribosomal RNA processing and pseudouridylation, whose known eukaryotic homologues are yeast Cfb5p, rat NAP57 and human dyskerin, encoded by the gene responsible for the X-linked dyskeratosis congenita disease. mfl genetic analysis represents the first in vivo functional characterization of a member of this highly conserved gene family from higher eukaryotes. In addition, we report that mfl hosts an intron encoded box H/ACA snoRNA gene, the first member of this class of snoRNAs identified so far from Drosophila.
Keywords: Drosophila, rRNA, ribosome, nucleolus, snoRNA
In eukaryotic cells, synthesis, maturation and modification of rRNA take place in the nucleolus, and RNP composed of a variety of nucleolar proteins and small nucleolar RNAs (snoRNAs)1 are known to be responsible for these essential cellular processes (reviewed by Melese and Xue, 1995). Almost 100 different snoRNAs species have been identified so far in yeast and mammalian cells. Recently, it became evident that most of these snoRNAs can be classified into two major distinct families, each defined by common associated proteins and by the presence of conserved sequences, designated as either C/D or H/ACA boxes (reviewed by Balakin et al., 1996; Smith and Steitz, 1997). The C and D box–containing snoRNAs display extensive sequence complementarity to conserved rRNA regions and are associated with a conserved nucleolar protein, fibrillarin or, in yeast, with the fibrillarin homologue Nop1p (reviewed by Bachellerie and Cavaille, 1997). Some fibrillarin-associated snoRNAs are required for rRNA processing, but most of them function as a guide in site-specific ribose methylation of rRNA (Kiss-Laszlo et al., 1996; Nicoloso et al., 1996).
Members of the other large class of snoRNAs share H and ACA elements and have only short rRNA complementary motifs, brought together by a conserved stem-loop secondary structure (Ganot et al., 1997b). This structure, composed of two hairpins connected and followed by short single-stranded regions containing the H and ACA elements, directs the site-specific pseudouridylation event with the short (5–9 nucleotide [nt]) regions of snoRNA/ rRNA complementarity flanking both sides of the target site (Ganot et al., 1997a; Ni et al., 1997).
In yeast, members of the box H/ACA class of snoRNAs are specifically associated with two essential nucleolar proteins, Gar1p and Cbf5p (Balakin et al., 1996; Ganot et al., 1997b; Lafontaine et al., 1998). Gar1p, a glycine–arginine-rich protein required for accumulation of mature 18S rRNA (Balakin et al., 1996; Girard et al., 1992) and for rRNA pseudouridylation (Bousquet-Antonelli et al., 1997), is thought to play a crucial role in structuring box H/ACA sno-RNPs and favoring association of H/ACA snoRNAs to the pre-rRNA. In a two hybrid yeast assay, Gar1p interacts with Cbf5p which, in turn, coprecipitates with box H/ACA snoRNAs and is required for their stability (Lafontaine et al., 1998). Interestingly, Cbf5p is the yeast member of a highly conserved protein family that includes homologues from at least 18 organisms. Among eukaryotes, genetic analysis has so far been restricted to two members of this family: the yeast Cbf5 (Jiang et al., 1993) and the DKC1 human gene, whose mutations cause the X-linked dyskeratosis congenita disease (Heiss et al., 1998). Whereas little information is available on human dyskerin, Cbf5p and its rat homologue, NAP57, are known to be proteins with prevalent nucleolar localization (Cadwell et al., 1997; Meier and Blobel, 1994). However, whereas it has been proposed that NAP57 may be involved in nucleo-cytoplasmatic shuttling (Meier and Blobel, 1994), the yeast protein has been shown to be required for transcription, processing and efficient rRNA pseudouridylation (Cadwell et al., 1997; Lafontaine et al., 1998). This last finding raises the possibility that Cbf5p might act as eukaryotic rRNA pseudouridine synthase, a role originally suggested (Cadwell et al., 1997) by its homology with E. coli TruB/ P35 synthase. Considering the multiple, essential functions played by Cbf5p in yeast cells, the definition of the roles played by members of this family in multicellular organisms appears to be a relevant issue that deserves extensive investigation.
Here we describe the cloning of the Drosophila member of the Cbf5/Nap57/DKC1 gene family, that we called minifly (mfl), and report a detailed genetic, molecular, and functional analysis of its expression. With the isolation and the characterization of mfl mutants reported in this paper, we provide the first animal model system for the study of the molecular basis of the DKC human disease. Our data also reveal that mfl has an intriguing molecular organization, hosting an intron-encoded box H/ACA snoRNA that represents the first member of this class thus far described in Drosophila. We named this RNA snoH1 and suggest that it may be functionally equivalent to the human U70 snoRNA.
Materials and Methods
P-Element Mutagenesis/Enhancer-Trap Schemes, P-Cytogenetic Mapping, Construction of Transformed Lines, and Lethal Phase Analysis
The genetic markers and chromosomes used for mutagenesis and mapping are described in Lindsley and Zimm (1992). Most stocks were from the Bloomington Drosophila Stock Center, while the l(2)k06308 and l(2)k05318 strains were provided by the Berkeley Drosophila Genome Project Stock Center. The mfl1 allele was isolated in a small-scale P-element mutagenesis screen performed essentially according to the “reversion jumping” scheme (Tower et al., 1993). In our experiments, tocl(2)01361, a lethal P[LacZ, ry+] (O'Kane and Gehring, 1987) insertion at the toucan locus, was mobilized by the P[ry+, Δ(2-3)99B] element (Laski et al., 1986; Robertson et al., 1988) as a source of transposase. Males carrying both the Δ(2-3) and tocl(2)01361 elements were crossed to females carrying a lethal toc allele that lost the ry+ marker. This allele, named tocΔ01361, was generated in our laboratory from tocl(2)01361 by P imprecise excision. In the next generation, flies lacking the CyO chromosome balancer (reversion event of the tocl(2)01361 allele) but marked with ry+ were recovered, and second chromosomes carrying these new insertions were balanced and retained for further study. Single P-element insertions were verified by genomic Southern blot analyses with PZ-derived probes. Wild-type P-element excised revertants were generated by crossing homozygous mfl1 males to w1118; CyO/L2; Sb, P[ry+,(Δ2-3)99B]/TM6B,Tb virgin females and by individual mating of disgenic F1 males to 5-10 CyO/Sp; ry506 virgin females. Individual non-Stubble males that lost the ry+ marker were collected from the F2 progeny and balanced over the CyO chromosome. The resulting stocks were checked for the presence of homozygous revertant flies in which P-element excision was verified by PCR amplification and DNA sequence analysis. In situ hybridization to salivary gland polytene chromosomes was performed with a DIG-labeled probe derived from the PZ element, essentially as described in Ashburner (1989).
The P-element hsp70:mfl construct (P[hs:mfl]) used for P-element– mediated transformation (Rubin and Spradling, 1982) was prepared by inserting a 1833 bp cDNA sequence containing the complete mfl ORF into the EcoRI site of the pCaSpeR-hs-act vector (Thummel et al., 1988). Transgenic flies carrying the P[hs:mfl] on the X or third chromosome were used to introduce the transposon in mfl mutant background. Lethal phase analysis was performed according to Fletcher et al. (1995). As control, lethal phases of mfl/Df(2R)Px4 transheterozygous were also determined. To identify homozygotes carrying mfl lethal alleles we generated y w; mfl/ CyO y + and y w P[hs:mfl]; mfl/CyO y + stocks in which homozygous mutant larvae were distinguished from their mfl/CyO y + heterozygous siblings by the yellow phenotype of mouth hooks and denticle belts.
Cloning Techniques
Basic cloning techniques, DNA and RNA extraction, manipulation and labeling, screening and sequencing techniques were carried out according to Sambrook et al. (1989).
RNA and Protein Analysis
For Northern blot analysis, 5 μg of poly(A)+ or 10 μg of total RNA were electrophoresed and transferred to Hybond-NX (Amersham) filters for hybridization. The 5′ end of SnoH1 RNA was determined by primer extension analyses, using 50 μg of total RNA together with primers complementary to nucleotides 96–135 and 149–189 of the fourth mfl intron. rRNA processing was studied by [3H]uridine (1 mCi/ml, 22.4 Ci/nmol) incorporation in Drosophila larvae. After 48 h, total RNA was extracted and analyzed by agarose electrophoresis followed by fluorography, as described by Tollervey (1987).
In rRNA northern blot analyses, probe I corresponds to oligonucleotide 5′-GTTAAAATCTTTTTATGAGGTTGCCAAGCCCCACAC-3′; probe II to oligonucleotide 5′-CACCATTTTACTGGCATATATCAATTCCTTCAATAAATG-3′; probe III to oligonucleotide 5′-CTATTTCCGAATCATTAATAAGAGACAATTCTAGATG-3′. Mapping of Drosophila ribosomal pseudouridines was performed essentially as described by Bakin and Ofengand (1993) using as primer the oligonucleotides: 5′-AATCAAGTTCGGTCAACTTTTGCGAAACAACCGTAACAC-3′ for 18S U1820, U1821, and U1822; 5′-GCGTCGTAATACTAATGCCCCCAAACTGCTTC-3′ for 18S U830/U831, U840, U841, and U885; 5′-CCATTCATGCGCGTCACTAATTAGATGACGAG-3′ for 28S U2442, U2444, and U2499. Western blots were analyzed with a 1:1,000 dilution of an affinity-purified rabbit anti-MFL antibody, kindly provided by S. Poole (University of California, Santa Barbara, CA).
In Situ Analysis
Whole mount ovaries in situ hybridization, using single-stranded DIG-labeled probes, obtained by PCR, and immunohistochemical staining of ovaries were performed essentially as described in Ashburner (1989). The rabbit primary anti-MFL antibody, kindly provided by S. Poole, was diluted 1:400 and detected with a biotin-conjugated secondary antibody and a horseradish peroxidase–biotin–avidin complex (ABC Elite Kit; Vector Labs).
Computer Analysis
Sequence comparisons were performed using the BLAST search algorithms available at the National Center for Biotechnology Information Web pages; multiple alignments were performed using the CLUSTAL and BOXSHADE programs. The snoH1 RNA putative secondary structure was established using the MFOLD program.
Results
Isolation, Genetic, and Phenotypical Analysis of mfl Mutants
The first minifly allele, mfl1, was isolated in our laboratory in the course of a PZ-element mutagenesis screen on the second chromosome (see Materials and Methods) as a viable, recessive mutation causing a variety of phenotypic abnormalities. The mfl1 pleiotropic phenotype included an extreme reduction of body size (Fig. 1, a and b), developmental delay, essentially due to a 4–5-d prolongation of the larval life, defects in the abdominal cuticle (Fig. 1 c), strong reduction in the length and thickness of abdominal bristles, and reduced female fertility. Most traits of the mfl1 phenotype largely overlapped those caused by the Drosophila Minute (Kay and Jacobs-Lorena, 1985), mini (Procunier and Tartof, 1975) or bobbed mutations (Boncinelli et al., 1972) that affect, respectively, the synthesis of ribosomal proteins, 5S, or 18S and 28S rRNAs. This similarity suggested for mfl a possible role in ribosome biogenesis, encouraging us to attempt the molecular cloning of the gene.
Figure 1.
mfl1 phenotype. Comparing to wild-type, flies mfl1 females (a) and males (b) are both characterized by strong reduction of the body size, reduction in the number of abdominal bristles and abdominal cuticular defects; this last aspect is more marked in females (c). (d) Hybridization of a P-element probe to polytene chromosomes from mfl1 heterozygous larvae. The hybridization signal (arrowhead) is restricted to mfl1 parental chromosome of heterozygous larvae, allowing us to map the single P-element insertion at the 60B-60C polytene subdivisions boundary, on chromosome arm 2R.
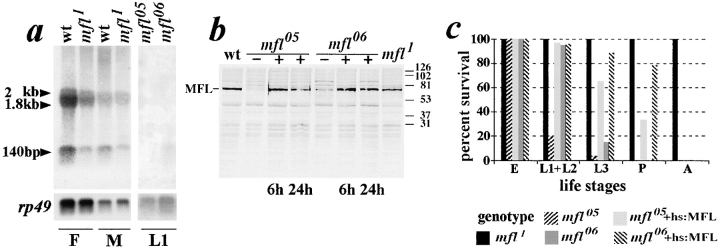
mfl1 mutation was caused by a single P-element insertion, which, by in situ hybridization of a P-specific probe to salivary gland polytene chromosomes of mfl1 heterozygous larvae (Fig. 1 d), was mapped on the chromosome arm 2R, at the 60B-60C polytene subdivisions boundary. Given that wild-type revertants could be recovered from dysgenic crosses after precise excision of the element (see Materials and Methods), mfl1mutation appeared to be directly caused by this single PZ insertion. Complementation analysis assigned the gene to the region covered by the Df(2R)Px4 deficiency. Among a number of P-induced lethal mutations recovered by Török et al. (1993) and subsequently deposited as part of the Berkeley Drosophila Genome Project, five mapped at the 60B-60C polytene subdivisions boundary. These mutations were all tested in a complementation analysis, by crossing each of them to mfl1 heterozygous flies. Two lines, l(2)k05318 and l(2)k06308, yielded transheterozygous flies with a strong mfl phenotype at the expected ratio, leading us to conclude that they belonged to the mfl complementation group and represented lethal mfl alleles. Accordingly, these lines were renamed, respectively, mfl05and mfl06. Previous cytological mapping by the Berkeley Drosophila Genome Project assigned these two mfl alleles to the polytene interval 60B11-C2, in good agreement with our results. By lethal phase analysis (Fletcher et al., 1995; see below) we observed that mfl05 homozygotes die mainly as first instar larvae, while most of the mfl06 animals die later, either as second or mainly as young third-instar larvae. Both mfl05 and mfl06 animals fail to increase their size as compared with their wild-type heterozygous siblings and survive for an additional 4–5 d as first or third instar larvae, respectively.
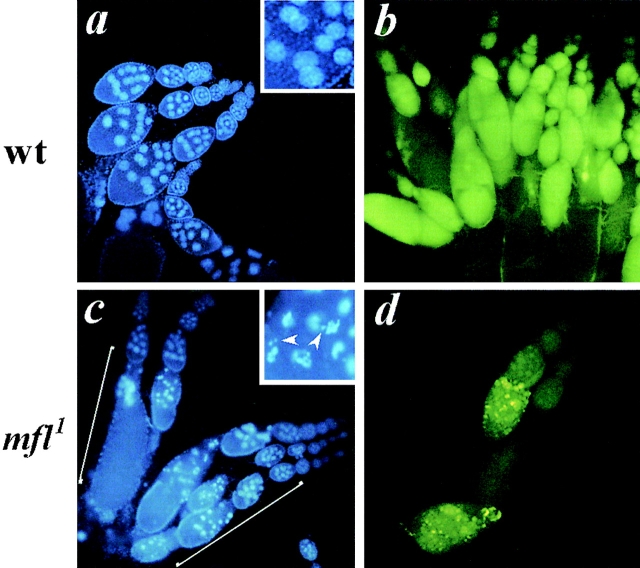
Since a feature of the mfl1 pleiotropic phenotype was represented by reduced female fertility, we looked at the structure of mutant ovaries. Morphological abnormalities were often observed, with some of the egg chambers beginning to degenerate beyond approximately stage 7 (according to King, 1970) of oogenesis (Fig. 2 c, see brackets). In the degenerating egg chambers, fragmented or condensed nurse cell nuclei with irregular shape are frequently found (Fig. 2 c, boxed). These observations raise the possibility that apoptotic cell death may occur in mfl1 abnormal ovaries. This possibility was investigated by staining egg chambers with acridine orange (AO). AO is a vital dye that is known to selectively stain apoptotic cells in insects (Spreij, 1971; Abrams et al., 1993) and has successfully been used to study the distribution of apoptosis in Drosophila ovaries (Foley and Cooley, 1998). In our experiments, wild-type ovaries exhibit a diffuse green fluorescence (Fig. 2 b), whereas highly fluorescent yellow spots are detected in mfl1 degenerating egg chambers (Fig. 2 d). These yellow spots are known to correspond to apoptotic, AO highly positive nuclei (Foley and Cooley, 1998), thus confirming the occurrence of apoptosis in mfl1 ovaries.
Figure 2.
Structure of mfl1 mutant ovaries. In the upper panel, as control, ovaries from wild-type females were stained with DAPI (a), or with the vital dye acridine orange (b). In the lower panel, ovaries from mfl1 homozygous females were stained with DAPI (c). Egg chambers, morphological abnormalities are observed beyond stage 7 of oogenesis (in brackets). Fragmented or condensed nurse cell nuclei with irregular shape are indicated by arrowheads in the boxed high magnification. (d) Acridine orange staining of mfl1 degenerating ovaries reveals highly fluorescent yellow spots, which correspond to apoptotic cells (Foley and Cooley, 1998).
As a consequence of the gonadic abnormalities observed, mfl1 homozygous females lay a reduced number of mature eggs, and ∼15% of the embryos produced failed to hatch. Such degenerating embryos show asynchronous and atypical development, invariantly accompanied by diffuse apoptotic cell death (data not shown). Many mutations causing partial loss-of-function of vital genes interfere with the proper development of the egg, causing female sterility. Inadequate rate of protein synthesis is also known to affect Drosophila oogenesis, by slowing the level of yolk production and retarding egg chamber progression into vitellogenesis, beginning at stage 8 (reviewed by Spradling, 1993). This effect is common to mutants unable to produce large amount of proteins, having reduced levels of either ribosomal proteins, 18S, 28S, or 5S rRNAs.
Molecular Organization, Coding Properties, and Developmental Expression Profile of the Minifly Gene
The genomic region adjacent to the PZ transposon was cloned from the mfl1 stock by plasmid rescue (Wilson et al., 1989) and used to isolate the sequences encompassing the PZ insertion site. Genomic probes spanning a region of ∼4 kb surrounding PZ insertion identified on Northern blots of poly(A)+ RNA two main transcripts of 1.8 and 2.0 kb in length, whose expression was affected in each mfl mutant line (see next section). While the 1.8-kb species was constitutively expressed throughout the life cycle, the 2.0-kb RNA was specifically found in adult female and embryonic RNA preparations, in which a further transcript of ∼4.0 kb was also occasionally detected (Fig. 3 b). However, no cDNA representative of this mRNA subform was isolated after extensive screening of an adult female cDNA library, so that it remains unclear whether it actually derives from the mfl gene. In contrast, several cDNAs representative of the 1.8 and 2.0 kb were isolated from adult female and larval libraries. The longest cDNAs of each class, respectively, of 1,833 and 2,034 bp, including the poly(A) tail, represented almost full-length transcripts and allowed us to define the mfl gene structure by Southern blot hybridization and alignment with nucleotide sequence of the genomic region. In each mfl mutant line, a copy of P was inserted at the 5′ common end of 1.8- and 2.0-kb transcription units: in mfl06, the insertion site was mapped 18 nt upstream from the 5′ end of the longest cDNAs obtained, in mfl118 nt downstream, within the 5′ leader sequence, while in mfl05 the insertion occurred within the first intron of the gene (see Fig. 3 a). The 1.8- and 2.0-kb mfl mRNA subforms share a common coding region and differ from each other only at their alternatively spliced 3′ untranslated region, where two additional exons (7 and 8) are specifically included in the 2.0-kb mRNA. When used on northern blots, a probe derived from these two exons (probe 2, depicted below the genomic map) detects exclusively the 2.0-kb subform, specifically present in embryos and adult female RNAs (Fig. 3 c). Hybridization of this probe to whole mount preparations of wild-type ovaries reveals that the female transcript accumulates in germ line cells from the early germarial till last oogenesis stages (Fig. 3 d). We then followed the accumulation profile of both mfl mRNAs during embryogenesis by developmental northern blot analysis of carefully synchronized embryos. As depicted in Fig. 3 e, both mfl mRNAs are detected in very early, 0–2 h embryos. However, while the zygotic 1.8-kb mRNA persists at later stages, the level of female transcript drops subsequently, and becomes very low in 4–6 h embryos. This developmental pattern is very similar to that of other stable maternally supplied RNAs, which persist from early stages up to gastrulation.
Figure 3.
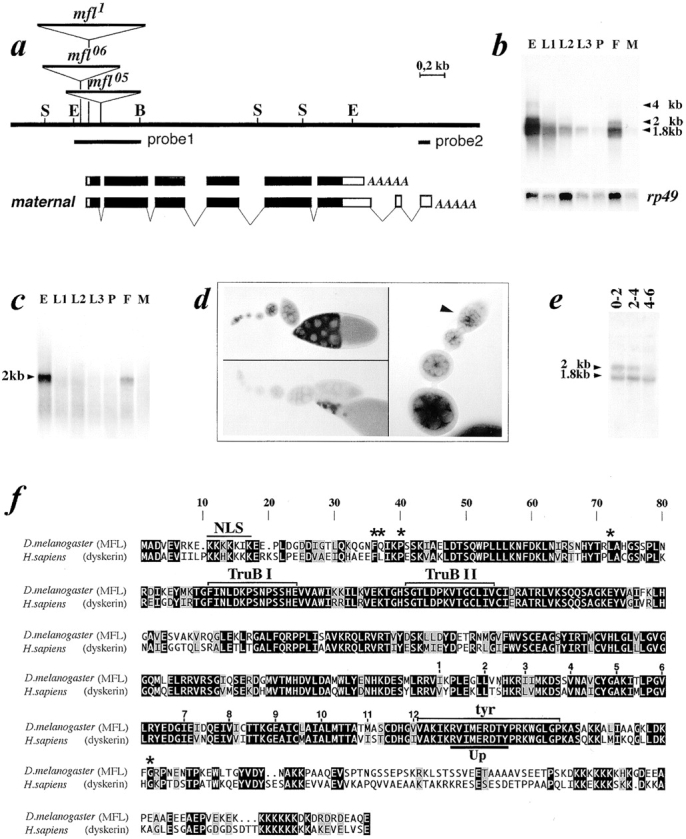
Molecular characterization of the minifly gene. (a) Restriction map of the genomic region encompassing the minifly gene (S, SalI; E, EcoRI; B, BamHI). Genomic DNA sequence can be obtained from GenBank (accession number AF097634). On the top, position of P-element insertions. Below, organization of the 1.8- and 2.0-kb mfl transcription units. Exonic regions spanned by mfl ORF are depicted in black. Nucleotide sequence of the mfl maternal transcript can be obtained from GenBank (accession number AF089837). (b) Developmental Northern blot analysis of the mfl gene. Poly(A)+ RNA was hybridized to a genomic probe, depicted as probe 1 below the map of the region. E, 0–20 h embryos; L1, L2, and L3, first, second, and third instar larvae; P, pupae; F and M, female and male adult flies. The relative amount of RNA loaded in each lane was checked by hybridization with a probe derived from the gene coding for the Drosophila ribosomal protein rp49 (O'Connell and Rosbash, 1984). (c) Hybridization of the same RNA panel shown in b with probe 2 (depicted below the map), specific to mfl maternal mRNA. (d) In situ hybridization of whole mount wild-type ovaries with probe 2. On the left, hybridization with the mfl RNA anti-sense strand (top) and with the sense strand as negative control (bottom); on the right, enlargement of the tip of an ovariole in which the hybridization signal starts to be detected from the early oogenesis stages, within the germarial region (marked by the arrowhead). (e) Northern blot hybridization of total RNA preparations obtained from 0–2, 2–4, and 4–6 h staged embryos with genomic probe 1. (f) Alignment of MFL and dyskerin amino acid sequences. Black boxed letters highlight identical amino acids, different yet conserved amino acids are on a gray backgrounds; block letters on a white background indicate different and nonconserved amino acids. Lines above the sequences indicate putative functional domains; NLS, nuclear localization signal; TruBI and TruBII, regions having homology with bacterial and yeast tRNA pseudouridine synthases; tyr, tyrosine domain; Up, putative uracile binding pocket. Asterisks on the top indicate the positions of missense mutations so far identified in dyskerin from DKC patients (Heiss et al., 1998).
The mfl open reading frame (ORF), identically present in both mRNA subforms, encodes a predicted protein of 508 amino acids with a calculated molecular mass of 56 kD. Database searches revealed that this protein belongs to the Cbf5p/NAP57/dyskerin family (Fig. 3 f). The MFL polypeptide shows a significant degree of conservation to other members of the family, particularly with the two very similar rat and human proteins (66% identity, 79% similarity to human dyskerin). The conservation increases remarkably within several specific domains, strongly underlining that their function has been preserved during evolution. As depicted in Fig. 3 f, total identity exists among Drosophila and human proteins within the two TruB motifs which have homology with bacterial and yeast tRNA pseudouridine synthases (Heiss et al., 1998). A repeated hydrophobic domain, possibly involved in the nucleo-cytoplasmatic shuttling postulated for the rat protein is also highly conserved. This domain is immediately followed by a block of >20 amino acids having a central tyr that is identical in Drosophila, rat, and human proteins. Although no function has been suggested so far for this domain, its conservation suggests that it might play a relevant role in protein activity. Within the tyr domain, we noticed a RX-x(2,3)-DE-x(2,3)-Y central core motif highly conserved among uracil-DNA glycosylases from different organisms as part of the rigid uracil-binding pocket (Up) present in these repair enzymes. Within the pocket, the tyrosine residue has been shown to be directly involved in uracil recognition (Kavli et al., 1996; Slupphaug et al., 1996). By analogy, it is reasonable to suggest that the highly conserved tyr motif might play a similar role in uracil recognition. A highly charged lysin-rich COOH-terminal region containing a nuclear localization signal is found in MFL, as in NAP57 and dyskerin, and the NH2-terminal nuclear localization signal observed in rat and human proteins is also preserved. Finally, it is interesting to note that all five missense mutations thus far identified in DKC patients fall into regions that are conserved between the human and the Drosophila gene (see positions of asterisks in Fig. 3 f).
mfl Is an Essential Drosophila Gene Playing a Key Role in rRNA Processing
When mfl mutants were checked for gene expression, we found that they were all characterized by reduced levels of mfl mRNAs. While the viable, hypomorphic mfl1 allele showed only a modest reduction, mfl expression was strongly disrupted in mfl05 and mfl06 (Fig. 4 a), the two alleles causing larval lethality. MFL protein accumulation strictly paralleled the level of mfl mRNAs, so that it was strongly reduced in mfl06 and nearly null in mfl05 (Fig. 4 b). Remarkably, the developmental time at which lethality is achieved in these two mutants correlates well with MFL level since, as mentioned above, mfl05 homozygotes die mainly as first instar larvae, while mfl06 animals as second or early third-instar larvae (see Fig. 4 c). Note that, considering the timing of persistence of maternal rRNA (Winkles et al., 1985), mortality at the first instar is that which may be expected for mutations causing severe loss of function of a gene essential for rRNA processing. Taken together, all these data indicated that MFL level may be critical for Drosophila viability. We then attempted to rescue mfl lethal phenotype by ectopically expressing MFL from the heat-inducible hsp70 promoter. mfl05 and mfl06 transgenic animals were then obtained and daily treated at 37°C for 30 min. These heat-shock conditions usually produce amounts of the ectopically expressed protein that largely exceed the wild-type level. However, in our experiments they produced a MFL level just comparable to that present in wild-type flies, even though the induced protein remains quite stable from 6 h to as long as 24 h from the heat-shock pulse (Fig. 4 b). Nevertheless, the level of induced MFL is sufficient to allow mfl05 and mfl06 transformed animals to developed synchronously with their wild-type siblings and to show a normal increase in their size. Moreover, 30% of the mfl05 and 80% of the mfl06 transgenic animals develop up to the pupal stage, although these transgenic pupae all failed to eclose adult flies (see Fig. 4 c). A possible explanation for this partial rescue of the mfl mortality is that the level of ectopically expressed MFL may be inadequate with respect to the rate of protein synthesis required in specific cell types during metamorphosis. An alternative possibility is suggested by the observation that, in yeast, Cbf5p is required for the stability of other components of the H/ACA class of RNPs, such as Gar1p and box H/ACA snoRNAs (Lafontaine et al., 1998). It is thus possible that the MFL level reached under heat-shock conditions may not be constant and this affects the stability of other essential RNP components. However, since no member of the H/ACA class of RNPs has yet been described in Drosophila, this hypothesis cannot be tested at present.
Figure 4.
Molecular and functional characterization of mfl mutants. (a) Northern blot analysis of total RNA extracted from wild-type or mfl animals with a genomic probe including the fourth mfl intron. Female (F) and male (M) adult flies carrying the hypomorphic mfl1 allele or first-instar larvae (L1) carrying the mfl05 and mfl06 alleles were analyzed. (b) Western blot analysis of extracts obtained from wild-type or mfl animals, carrying (+) or not carrying (−) a MFL coding transgene. An affinity-purified rabbit polyclonal anti-MFL antibody, kindly provided by S. Poole, was used. Both wt and mfl homozygous animals were grown under heat shock regimen (30 min/d). As shown, MFL level is reduced in all mfl mutants (lanes −) but reaches nearly the wild-type amount in mfl05 and mfl06 transformed larvae (lanes +) at 6 or 24 h from the heat-shock pulse. (c) Lethal phase (see Materials and Methods) of mfl mutants is compared with that of mfl05 and mfl06 transgenic lines in which MFL was overexpressed from the heat-inducible hsp70 promoter. While most of mfl05 or mfl06 homozygotes develop only until the first or the third-larval instar, respectively, 30% of mfl05 and 80% of mfl06 transgenic animals reach the pupal stage when grown under daily heat-shock treatment. Moreover, these transgenic animals develop synchronously with their wild-type siblings and show a normal increase in their size.
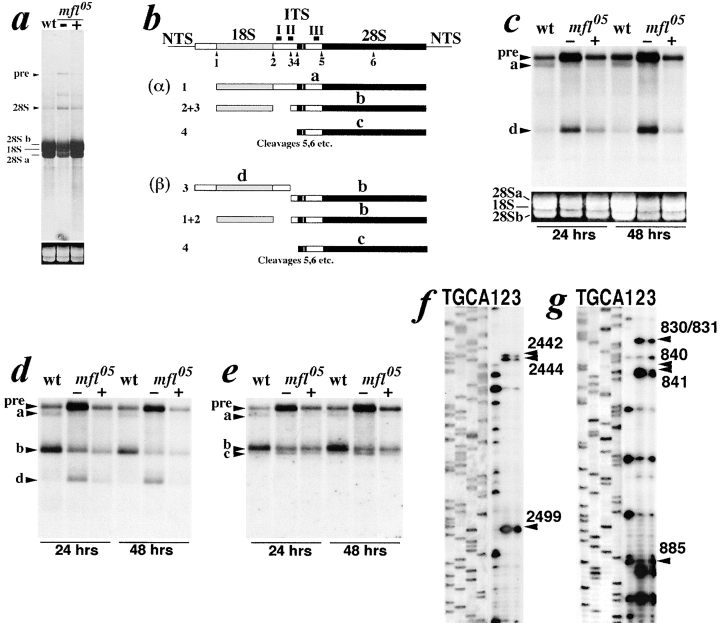
Given the similarity existing between the mfl phenotype and that caused by mutations affecting the synthesis of ribosomal components, we checked the role of the gene on rRNA processing. Electrophoresis of larval rRNA labeled by [3H]uridine incorporation showed that pre-rRNA processing is inefficient in mfl mutants. In fact, with respect to wild-type flies, increased levels of the pre-rRNA and 28S rRNA and reduced amounts of the 18S, 28Sa, and 28Sb mature species were observed (Fig. 5 a; compare the level of the newly synthesized larval rRNA, labeled by [3H]uridine, with the amount of total rRNA composed of both newly synthesized and maternally inherited rRNA, shown by ethidium bromide staining at the bottom). MFL over-expression in mfl transgenic flies is sufficient to reduce rRNA precursor accumulation and to increase the level of the newly synthesized 18S and 28S species (Fig. 5 a).
Figure 5.
(a) [3H]Uridine incorporation in wild-type or mfl05 larvae carrying (+) or not carrying (−) a MFL coding transgene under heat-shock treatment. Below, ethidium bromide staining of the 28Sa, 18S, and 28Sb rRNA species loaded in each lane. In Drosophila, 28S rRNA is cut to generate the 28Sa and 28Sb mature forms. (b) Genomic map of Drosophila rDNA. The two alternative α and β rRNA processing pathways (Long and Dawid, 1980) are depicted. In c–e, Northern analysis of total RNA from wild-type or mfl05 first instar larvae, at 24 or 48 h after egg hatching, with probes derived from the ITS region. The same blot was hybridized in c to probe I, in d to probe II, in e to probe III; probe positions are indicated by solid bars above the rDNA map. (f) Pseudouridylation of Drosophila 28S rRNA at positions U2442, U2444, U2499, and (g) of 18S rRNA, at positions U830/U831, U840, U841, U885. Wild-type untreated (lanes 1) and wild-type (lanes 2) or mfl05 (lanes 3) CMC-alkali treated RNAs were analyzed by primer extension using a 32P-labeled oligonucleotide complementary to the selected Drosophila rRNA sequences (see Materials and Methods). Lanes T, G, C, and A are dideoxy sequence reactions performed by using the same 28S or 18S primers on plasmids carrying the respective rDNA sequences.
Northern blot analysis with three different probes derived from the rDNA internal transcribed spacer (ITS) led us to define in greater detail the abnormal rRNA processing occurring in mfl mutants. In Drosophila the rRNA primary transcript (pre-rRNA) undergoes two alternative types of initial cleavages (Long and Dawid, 1980). The most predominant type occurs in the external transcribed spacer, at site 1, and generates the large type a molecule, from which both 18S and 28S are derived (see pathway α, Fig. 5 b). An alternative cleavage occurs within ITS, at site 3, generating the intermediate d and b forms which are, respectively, 18S and 28S rRNA precursors (see pathway β, Fig. 5 b). Hybridization to a probe derived from the ITS 5′ end (probe I) revealed that the accumulation of the pre-rRNA observed in mfl mutants is accompanied by a reduction of the type a precursor and by an increase of the d form; both effects become more evident with progression of the larval development (Fig. 5 c). Thus, mfl mutations specifically affect site 1 cleavage, inhibiting the formation of type a molecules and the processing of the d intermediate. With pathway blocked, pre-rRNA processing proceeds mainly through pathway β, generating equimolar amounts of d and b intermediate molecules. This is confirmed by hybridization to probe II, which shows that, while in wild-type animals the amount of form b largely exceeds that of d (as expected, being that the b molecule is actively produced by both α and β pathways), in mfl mutants these two forms are detected in similar amounts (Fig. 5 d). However, since the processing of form d is inhibited, this species accumulates progressively along larval development (Fig. 5 d). Conversely, hybridization to probe III indicated that mfl genetic depletion does not impair site 4 cleavage of type b molecule, since the amount of form c observed in the mutants exceeds even that of the control (Fig 5 e). We concluded that form c is generated properly, but its further processing is inhibited by mfl mutations. In mfl transgenic flies, MFL over-expression leads to a reversal of all of the effects observed, although the efficiency of pre-rRNA processing is not fully restored. In heat-shocked transformed animals, in fact, MFL expression causes a decrease of pre-rRNA accumulation and an increase in the production of the type a molecule (Fig. 5 c). Processing of the type a precursor also occurs properly, since, as depicted in Fig. 5 d, these larvae show an excess in form b versus form d, although the amount of the b molecule does not reach that observed in wild-type animals. Finally, the amount of form c appears reduced after the heat-shock (Fig. 5 e), indicating that its processing is at least partially restored.
In yeast, lack of Cbf5 gene activity affects not only rRNA processing, but also rRNA pseudouridylation. Thus, we checked the level of modification in wild-type and mfl mutants at several 28S and 18S Ψ specific sites. With this aim, we used oligonucleotide primers complementary to selected 28S or 18S regions to perform primer extension analyses on CMC-treated Drosophila rRNA. CMC blocks reverse transcription, resulting in a gel band terminating in one residue 3′ of the Ψ site (Bakin and Ofengand, 1993). In planning these experiments, we took advantage of the location of Drosophila 28S rRNA pseudouridines recently reported by Ofengand and Bakin (1997). Instead, none of the 18S Ψ sites checked in our experiments was previously known. In spite of the persistence of maternal rRNA, pseudouridylation appears reduced in mfl05 larvae at several 28S sites, such as the U2442, U2444, and U2499 residues (Fig. 5 f). Similar reduction was observed at various 18S rRNA sites, such as U830/U831, U840, U841, and U885 (Fig. 5 g), indicating that, as Cbf5, mfl is required for efficient rRNA pseudouridylation.
Minifly Hosts an Intron-encoded Box H/ACA snoRNA
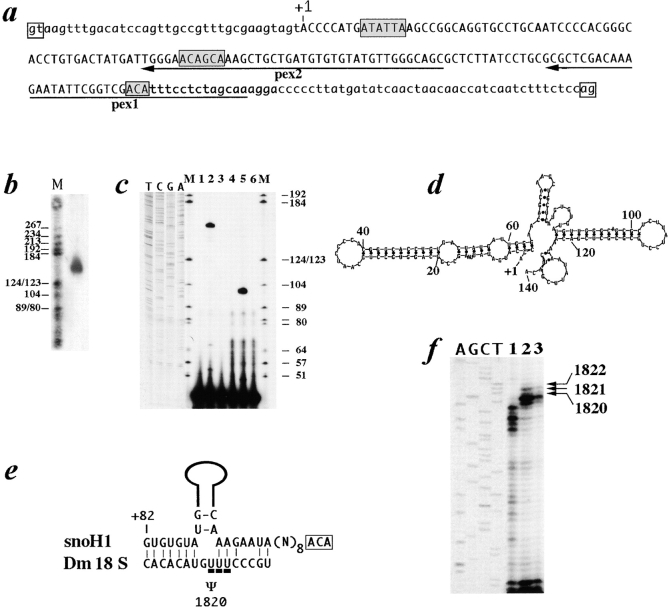
An unexpected feature of the mfl gene structure was revealed by the finding that a small RNA species, ∼0.1 kb in length, hybridized specifically with the genomic sequences of the fourth mfl intron, while it was not detected by any cDNA probe. This small RNA was detected in total RNA preparations from all developmental stages and was specifically enriched in the poly(A)− RNA fraction. The length of the small RNA species was accurately determined on denaturing 6% polyacrylamide gels and its 5′ end precisely mapped by primer extension analysis of total larval RNA using two different oligonucleotides (Fig. 6, b and c). These experiments pointed out that this transcript was ∼140 nt long and derived from position +37 to about +176 of the 235-nt-long fourth mfl intron (Fig. 6 a). Since a large number of small nucleolar RNAs are intron encoded (Smith and Steitz, 1997), we checked for the presence of conserved snoRNA elements within the 0.14-kb RNA sequence. Two H boxes (consensus ANANNA) and a 3′ terminal ACA element were found (Fig. 6 a); in addition, the predicted secondary structure of the mfl intron-encoded RNA (Fig. 6 d) conformed well to the hairpin-hinge-hairpin-tail architecture common to most yeast and vertebrate box H/ACA snoRNAs (Ganot et al., 1997b). Two short regions of complementarity between the mfl intron encoded RNA and Drosophila 18S rRNA were also found (Fig. 6 e). As noticed by Ganot et al. (1997a), short regions of pairing with rRNA flank the site of pseudouridylation, allowing the positioning of the residue to be isomerized at the base of the stem, at the first unpaired position before the 3′ snoRNA helical segment. The pseudouridine selected is found to be separated from the H or ACA box by 14 or, in a few cases, by 15 nucleotides. On the basis of these observations, the rRNA pairing properties of the mfl intron-encoded RNA predicted it may direct pseudouridylation of Drosophila 18S rRNA at position U1820 (Fig. 6 e). Primer extension analysis on CMC-treated Drosophila rRNA shows that the potentially selected residue is actually pseudouridylated (Fig. 6 f). The selected U1820 residue is equivalent to U1698 of human 18S rRNA, whose pseudouridylation has recently been related to the U70 snoRNA (Ganot et al., 1997a). As for U1698 in human rRNA, the Drosophila U1820 residue is the first of three consecutive uridines, all of which are pseudouridylated (Fig. 6 f, lane 2).
Figure 6.
Sequence, structure and properties of the mfl intron encoded snoH1 RNA. (a) Nucleotide sequence of the 235-bp-long fourth mfl intron; open boxes indicate the splicing sites. The estimated 5′ end of the snoH1 RNA, corresponding to position +37 of the mfl fourth intron, is indicated by +1. Shaded boxes indicate the two H and the 3′ terminal ACA element. SnoH1 nucleotide sequence can be obtained from GenBank (accession number AF089836). (b) Northern analysis of total RNA on a denaturing 6% polyacrylamide gel with a probe specific to mfl fourth intron (M, molecular weight marker V; Boehringer Mannheim). (c) 5′ end mapping of the snoH1 RNA by primer extension analysis of total larval RNA. The two oligonucleotides pex1 and pex2, complementary to the sequences underlined by the arrows in a, were used as primers. M, molecular weight marker V (Boehringer Mannheim). In lanes 1 and 4, no RNA was loaded as internal control. In lanes 2 and 3, the RNA was incubated with oligonucleotide pex1, with or without reverse transcriptase. In lanes 5 and 6, the RNA was incubated with oligonucleotide pex2, with or without reverse transcriptase. (d) Predicted secondary structure of the mfl intron encoded RNA. (e) Potential base-pairing interactions between the snoH1 RNA and Drosophila 18S rRNA sequences. The upper strand represents the snoH1 RNA sequence in a 5′ to 3′ orientation. Solid lines schematically represent the hairpin domain of the snoH1 RNA. The 3′ terminal ACA motif is boxed. The ribosomal pseudouridine potentially selected by the snoH1 RNA is indicated by Ψ. (f) Pseudouridylation of Drosophila 18S rRNA, at positions U1820, U1821, and U1822. Untreated (lane 1) or CMC-alkali treated (lanes 2 and 3) RNAs were analyzed by primer extension using an oligonucleotide complementary to the selected Drosophila 18S rRNA sequences (see Materials and Methods). In lanes 1 and 2, RNA was extracted from wild-type larvae; on lane 3, from mfl05 larvae. Lanes A, G, C, and T are dideoxy sequence reactions performed by using the same primer and a plasmid carrying Drosophila 18S rRNA sequences.
In yeast, genetic depletion of most of the box H/ACA snoRNAs has been reported to inhibit pseudouridylation of the specifically selected sites (Ganot et al., 1997a). When we checked modification of the U1820 residue in rRNA preparations obtained from mfl05 first instar larvae, we found that pseudouridylation was reduced not only at U1820, but also at U1821 and U1822 residues (Fig. 6 f, lane 3). This result may be explained by the widespread inhibition of rRNA pseudouridylation observed in mfl mutants. Further experiments are thus required to define the specific functional role, if any, played by the mfl intron-encoded RNA.
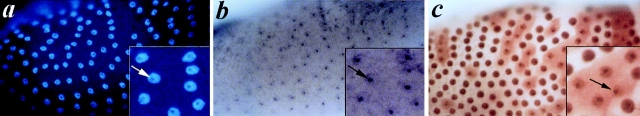
Finally, we checked the localization of the mfl intron encoded RNA by in situ hybridization experiments to whole mount ovary preparations. This analysis showed that a 0.14-kb RNA-specific antisense probe exclusively labeled the nucleoli (Fig. 7, a and b) as it occurs in each tested embryonic or larval tissue (not shown). Specific nucleolar localization may also be observed for MFL (Fig. 7 c), whose ubiquitous expression resulted from both immunolocalization data and histochemical staining of lacZ activity in mfl1 flies (data not shown). In ovarian tissue preparations we noticed that the protein occasionally diffuses into the cytoplasm in several patches of follicle cells. As judged by the presence of well defined, round-shaped nuclei having morphologically well distinguishable nucleoli (Fig. 7 c), these cells should not be in or around mitosis. Moreover, cytoplasmic diffusion can be observed also after stage 10b of oogenesis, when follicular cells endocycles are reported to be terminated (Calvi et al., 1998). It is thus plausible that occasional MFL cytoplasmic localization may be related to ability to carry out nucleolus-cytoplasmic shuttling, as proposed for NAP57 in rat cells (Meier and Blobel, 1994).
Figure 7.
Intracellular distribution of snoH1 RNA and MFL protein in Drosophila ovaries. (a) Nuclei were counterstained with DAPI. (b) In situ hybridization of a snoH1 RNA antisense probe exclusively labeled the nucleoli. (c) Immunohistochemical localization of MFL protein in wild-type ovaries with an affinity-purified rabbit polyclonal anti-MFL antibody (kindly provided by S. Poole). The protein shows a specific nucleolar localization, although occasional intracytoplasmatic diffusion can be observed.
Taken together, the experiments reported indicate that mfl hosts, in its fourth intron, a box H/ACA snoRNA gene, the first member of this class to be identified so far in Drosophila. We have called this gene snoH1 and suggest that it is functionally equivalent to the human U70 snoRNA gene.
Discussion
We reported the cloning of the D. melanogaster mfl gene and established that it encodes an ubiquitous nucleolar protein essential for Drosophila viability and female fertility. Our data also showed that mfl is closely related to the other members of the Cbf5 family so far characterized from higher eukaryotes, the rat Nap57 and the human gene responsible for the X-linked dyskeratosis congenita disease. As cogently predicted (Luzzatto and Karadimitris, 1998), flies carrying mutations in the Drosophila DKC1 orthologue show a pleiotropic phenotype very similar to that caused by mutations that affect the synthesis of ribosomal RNA. In fact, we found that mfl loss-of-function mutations impair rRNA processing and lead to accumulation of rRNA precursors. Although these effects are very similar to those caused by Cbf5 genetic depletion, yeast mutations preferentially affect the production of mature 18S rRNA (Lafontaine et al., 1998), while mfl mutations cause similar reduction of 18S and 28S rRNA species. It would be of interest to know whether this is due to a distinctive feature of Drosophila rRNA processing pathways, or whether it reflects a general property of rRNA processing in higher eukaryotes.
In addition to affecting rRNA maturation, mfl loss-of-function causes reduced levels of pseudouridylation at several 28S and 18S Ψ sites, suggesting that gene activity might be required for fully efficient rRNA pseudouridylation. Again, these results are reminiscent of those obtained in yeast (Lafontaine et al., 1998), and outline the existence of a link between rRNA processing and rRNA pseudouridylation in eukaryotes. By mapping the protein domains conserved among members of the Cbf5p family and investigating the definition of their functional roles, significant information should be generated about the functional role played by rRNA pseudouridylation, which still remains elusive. Although pseudouridylation of eukaryotic rRNAs occurs predominantly on the primary rRNA transcripts before nucleolytic processing, this type of modification is not required for efficient processing of 25S yeast rRNA (Bousquet-Antonelli et al., 1997). It has been suggested that pseudouridylation can contribute to rRNA folding, rRNPs assembly, and ribosomal subunit assembly (Lane et al., 1995; Maden, 1990; Ofengand et al., 1995). Other hypotheses, such as subtle enhancing of ribosomal functions or influencing fidelity of codon recognition, have also been proposed (Ofengand and Bakin, 1997).
An additional role that could be suggested for MFL is based on the observation that it can occasionally diffuse within the cytoplasm. As previously suggested for NAP57 in rat cells, it is tempting to speculate that this may possibly reflect the ability of MFL to structure and export pre-ribosomal RNP particles into the cytoplasm. If confirmed, this would strongly support the view that members of this family are multifunctional proteins involved in different aspects of ribosome biogenesis. It is possible that these proteins may constitute essential components of a single multifunctional complex or, alternatively, they represent common components of structurally and functionally different RNP particles. The definition of the functional interactions required to carry out such a variety of functions will help to clarify this point.
Remarkably, the identification and the characterization of mutations disrupting mfl gene expression has led to establishing the first animal model system for the study of the X-linked dyskeratosis congenita human disease. Some of the results reported here may immediately provide useful information for the comprehension of the molecular basis of the DKC disease. A first relevant point concerns the observation that none of the mfl mutations so far isolated disrupts the gene coding region. Thus, each Drosophila mutant line has certainly quantitative and not qualitative alterations of the gene product which causes the pleiotropic abnormalities observed. The level of MFL protein was found to be critical, and a simple dose-effect rule may be derived: when the protein level is below a crucial threshold, mortality ensues. Instead, while the protein level is lowered but still stands above a critical threshold, the viable, hypomorphic mfl1 phenotype is reached. By analogy, it can be suggested that in man the level of dyskerin activity may be one of the critical parameters able to trigger the DKC disease. The finding that DKC mutations mapped so far all affect the dyskerin coding region (Heiss et al., 1998) is in only apparent contrast with that found in Drosophila. In fact, it is reasonable to suppose that, as observed in Drosophila, total or severe loss-of-function mutations should not be compatible with life. Mutations recovered in patients might be those causing partial loss-of-function, so that the level of dyskerin activity is still compatible with survival. Accordingly, DKC patients might carry hypomorphic mutations, the human counterparts of the viable mfl1 phenotype. Whether these hypomorphic phenotypes are simply a consequence of the inadequate mature rRNA level or are, at least partially, caused by abnormal accumulation of intermediate rRNA species is an important point which deserves further investigation. A further issue concerns the observation that, although MFL and dyskerin are ubiquitous proteins, phenotypic abnormalities are, in Drosophila as in man, restricted to only certain tissues. Since the gene product is presumed to be critically important for protein synthesis in every cell of the body, the finding that abnormalities are developed only by selected cell types is quite surprising. However, if it is accepted that the level of protein activity may be a critical parameter, then it is reasonable to suppose that the amount of properly processed rRNA may be sufficient in cells having a slow growth rate, while in highly proliferating tissues or in cells sustaining a high rate of protein synthesis this would not the case, and degenerative cell defects could progressively be accumulated. Interestingly, inhibition of protein synthesis is known to be one of the stimuli capable of inducing apoptotic cell death, probably by decreasing the levels of essential proteins or by inhibiting the synthesis of proteins that normally suppress the spontaneous activation of apoptosis (reviewed by Wertz and Hanley, 1996). In mfl1 ovary, one of the Drosophila tissues where morphological abnormalities can be observed, we found that degeneration is specifically accompanied by apoptotic cell death. This observation might also suggest a role for apoptosis in the progressive clinical manifestations of the DKC disease.
Finally, we showed that mfl gene organization is intriguing, and leads to the identification of the first member of the box H/ACA class of snoRNAs described so far in Drosophila. As in the case of the snoH1 gene described here, most of the snoRNAs are intron encoded, and snoRNA host genes often encode proteins involved in translation or ribosome biogenesis (reviewed by Smith and Steitz, 1997). These intron-encoded snoRNAs are cotranscribed with their host pre-mRNA and their accumulation is splicing-dependent, since they are released from the excised intron by exonucleolytic processing. Our observation that snoH1 RNA and mfl mRNAs levels are reduced in parallel in each mfl mutant line strongly suggests that snoH1 RNA processing is linked to the splicing of the mfl primary transcript. This feature, which allows coordinated regulation of the host protein and the intron encoded snoRNA, may hinder a precise definition of the specific functional role played by each product. With regard to snoH1, it cannot be excluded, in principle, that it may be required for Drosophila viability and that its depletion might contribute to the generation of mfl phenotype. However, we have observed that snoH1 has little, if any, effect on mfl phenotypic rescue when over-expressed in mfl transgenic flies, either in the presence or in the absence of MFL overexpression (Giordano, E., and M. Furia, unpublished data). This is not surprising, given that all box H/ACA snoRNAs found in yeast, with the exception of snR30 (Morrissey and Tollervey, 1993) and snR10 (Tollervey, 1987; Tollervey and Guthrie, 1985), are dispensable for viability. It will now be interesting to determine whether this type of gene organization is restricted to mfl or is shared by other members of this conserved gene family.
Acknowledgments
We are grateful to S. Poole for the generous gift of the affinity-purified anti-MFL antibody and to D. Bopp and T. Laverty for sending Drosophila stocks. We thank J. Guardiola, L. Luzzatto, S. Banfi, and P. Delli Bovi for encouragement during the course of this work, R. Calogero and G. Iazzetti for friendly and helpful computational assistance, and G. Falcone for excellent photographic assistance.
Abbreviations used in this paper
- AO
acridine orange
- ITS
internal transcribed spacer
- nt
nucleotide
- ORF
open reading frame
- snoRNA
small nucleolar RNA
- Up
uracil-binding pocket
Footnotes
Address correspondence to Maria Furia, Dipartimento di Genetica, Biologia Generale e Molecolare, via Mezzocannone 8, I-80134, Napoli, Italy. Tel.: 39 081 7903413 or 39 081 7903419. Fax: 39 081 5527950. E-mail: furia@biol.dgbm.unina.it
S. Senger was supported by a Ph.D. fellowship from European Union. This work was supported by funds of MURST PRIN Project Molecular regulation of Development to M. Furia.
References
- Abrams JM, White K, Fessler LI, Steller H. Programmed cell death during Drosophilaoogenesis. Development. 1993;117:29–43. doi: 10.1242/dev.117.1.29. [DOI] [PubMed] [Google Scholar]
- Ashburner, M. 1989. Drosophila: A Laboratory Manual. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press. 434 pp.
- Bachellerie JP, Cavaille J. Guiding ribose methylation of rRNA. Trends Biochem Sci. 1997;22:257–261. doi: 10.1016/s0968-0004(97)01057-8. [DOI] [PubMed] [Google Scholar]
- Bakin A, Ofengand J. Four newly located pseudouridylate residues in Escherichia coli23S ribosomal RNA are all at the peptidyltransferase center: analysis by the application of a new sequencing technique. Biochemistry. 1993;32:9754–9762. doi: 10.1021/bi00088a030. [DOI] [PubMed] [Google Scholar]
- Balakin AG, Smith L, Fournier MJ. The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell. 1996;86:823–834. doi: 10.1016/s0092-8674(00)80156-7. [DOI] [PubMed] [Google Scholar]
- Boncinelli E, Graziani F, Polito L, Malva C, Ritossa F. rDNA magnification at the bobbed locus of the Y chromosome in Drosophila melanogaster. . Cell Differ. 1972;1:133–142. doi: 10.1016/0045-6039(72)90036-x. [DOI] [PubMed] [Google Scholar]
- Bousquet-Antonelli C, Henry Y, Gèlugne JP, Caizergues-Ferrer M, Kiss T. A small nucleolar RNP protein is required for pseudouridylation of eukaryotic ribosomal RNAs. EMBO (Eur Mol Biol Organ) J. 1997;16:4770–4776. doi: 10.1093/emboj/16.15.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell C, Yoon HJ, Zebarjadian Y, Carbon J. The yeast nucleolar protein Cbf5p is involved in rRNA biosynthesis and interacts genetically with the RNA polymerase I transcription factor RRN3. Mol Cell Biol. 1997;17:6175–6183. doi: 10.1128/mcb.17.10.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi BR, Lilly MA, Spradling AC. Cell cycle control of chorion gene amplification. Genes Dev. 1998;12:734–744. doi: 10.1101/gad.12.5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JC, Burtis KC, Hogness DS, Thummel CS. The Drosophila E74gene is required for metamorphosis and plays a role in the polytene chromosome puffing response to ecdysone. Development. 1995;121:1455–1465. doi: 10.1242/dev.121.5.1455. [DOI] [PubMed] [Google Scholar]
- Foley K, Cooley L. Apoptosis in late stages Drosophilanurse cells does not require genes within the H99 deficiency. Development. 1998;125:1075–1082. doi: 10.1242/dev.125.6.1075. [DOI] [PubMed] [Google Scholar]
- Ganot P, Bortolin ML, Kiss T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell. 1997a;89:799–809. doi: 10.1016/s0092-8674(00)80263-9. [DOI] [PubMed] [Google Scholar]
- Ganot P, Caizergues-Ferrer M, Kiss T. The family of box ACA small nucleolar RNAs is defined by an evolutionarily conserved secondary structure and ubiquitous sequence elements essential for RNA accumulation. Genes Dev. 1997b;11:941–956. doi: 10.1101/gad.11.7.941. [DOI] [PubMed] [Google Scholar]
- Girard JP, Lehtonen H, Caizergues-Ferrer M, Amalric F, Tollervey D, Lapeyre B. GAR1 is an essential small nucleolar RNP protein required for pre-rRNA processing in yeast. EMBO (Eur Mol Biol Organ) J. 1992;11:673–682. doi: 10.1002/j.1460-2075.1992.tb05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- Jiang W, Middleton K, Yoon HJ, Fouquet C, Carbon J. An essential yeast protein, CBF5p, binds in vitro to centromeres and microtubules. Mol Cell Biol. 1993;13:4884–4893. doi: 10.1128/mcb.13.8.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavli B, Slupphaug G, Mol CD, Arvai AS, Peterson SB, Tainer JA, Krokan HE. Excision of cytosine and thymine from DNA by mutants of human uracil-DNA glycosylase. EMBO (Eur Mol Biol Organ) J. 1996;15:3442–3447. [PMC free article] [PubMed] [Google Scholar]
- Kay MA, Jacobs-Lorena M. Selective translational regulation of ribosomal protein gene expression during early development of Drosophila melanogaster. . Mol Cell Biol. 1985;5:3583–3592. doi: 10.1128/mcb.5.12.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, R.C. 1970. Ovarian Development of Drosophila melanogaster. New York Academic Press.
- Kiss-Laszlo Z, Henry Y, Bachellerie JP, Caizergues-Ferrer M, Kiss T. Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell. 1996;85:1077–1088. doi: 10.1016/s0092-8674(00)81308-2. [DOI] [PubMed] [Google Scholar]
- Lafontaine DLJ, Bousquet-Antonelli C, Henry Y, Caizergues-Ferrer M, Tollervey D. The box H + ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase. Genes Dev. 1998;12:527–537. doi: 10.1101/gad.12.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane BG, Ofengand J, Gray MW. Pseudouridine and O2′-methylated nucleosides. Significance of their selective occurrence in rRNA domains that function in ribosome-catalyzed synthesis of the peptide bonds in proteins. Biochimie. 1995;77:7–15. doi: 10.1016/0300-9084(96)88098-9. [DOI] [PubMed] [Google Scholar]
- Laski FA, Rio DC, Rubin G M. Tissue specificity of DrosophilaP-element transposition is regulated at the level of mRNA splicing. Cell. 1986;44:7–19. doi: 10.1016/0092-8674(86)90480-0. [DOI] [PubMed] [Google Scholar]
- Lindsley, D.L., and G.G. Zimm. 1992. The genome of Drosophila melanogaster. Academic Press Inc., San Diego, California.
- Long EO, Dawid IB. Alternative pathways in the processing of ribosomal RNA precursor in Drosophila melanogaster. . J Mol Biol. 1980;138:873–878. doi: 10.1016/0022-2836(80)90070-4. [DOI] [PubMed] [Google Scholar]
- Luzzatto L, Karadimitris A. Dyskeratosis and ribosomal rebellion. Nat Genet. 1998;19:6–7. doi: 10.1038/ng0598-6. [DOI] [PubMed] [Google Scholar]
- Maden BE. The numerous modified nucleotides in eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1990;39:241–303. doi: 10.1016/s0079-6603(08)60629-7. [DOI] [PubMed] [Google Scholar]
- Meier UT, Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994;127:1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melese T, Xue Z. The nucleolus: an organelle formed by the act of building a ribosome. Curr Opin Cell Biol. 1995;7:319–324. doi: 10.1016/0955-0674(95)80085-9. [DOI] [PubMed] [Google Scholar]
- Morrissey JP, Tollervey D. Yeast snR30is a small nucleolar RNA required for 18S rRNA synthesis. Mol Cell Biol. 1993;13:2469–2477. doi: 10.1128/mcb.13.4.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Tien AL, Fournier MJ. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell. 1997;89:565–573. doi: 10.1016/s0092-8674(00)80238-x. [DOI] [PubMed] [Google Scholar]
- Nicoloso M, Qu LH, Michot B, Bachellerie JP. Intron-encoded, antisense small nucleolar RNAs: the characterization of nine novel species points to their direct role as guides for the 2′-O-ribose methylation of rRNAs. J Mol Biol. 1996;260:178–195. doi: 10.1006/jmbi.1996.0391. [DOI] [PubMed] [Google Scholar]
- O'Connell PO, Rosbash M. Sequence, structure, and codon preference of the Drosophilaribosomal protein 49 gene. Nucleic Acids Res. 1984;12:5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kane CJ, Gehring WJ. Detection in situ of genomic regulatory elements in Drosophila. . Proc Natl Acad Sci USA. 1987;84:9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengand J, Bakin A. Mapping to nucleotide resolution of pseudouridine residues in large subunit ribosomal RNAs from representative eukaryotes, prokaryotes, archaebacteria, mitochondria and chloroplasts. J Mol Biol. 1997;266:246–268. doi: 10.1006/jmbi.1996.0737. [DOI] [PubMed] [Google Scholar]
- Ofengand J, Bakin A, Wrzesinski J, Nurse K, Lane BG. The pseudouridine residues of ribosomal RNA. Biochem Cell Biol. 1995;73:915–924. doi: 10.1139/o95-099. [DOI] [PubMed] [Google Scholar]
- Procunier JD, Tartof KD. Genetic analysis of the 5S RNA genes in Drosophila melanogaster. . Genetics. 1975;81:515–523. doi: 10.1093/genetics/81.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Preston CR, Phillis RW, Johnson-Schlitz DM, Benz WK, Engels WR. A stable genomic source of P element transposase in Drosophila melanogaster. . Genetics. 1988;118:461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophilawith transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. 2nd edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 545 pp.
- Slupphaug G, Mol CD, Kavli B, Arvai AS, Krokan HE, Tainer JA. A nucleotide-flipping mechanism from the structure of human uracil-DNA glycosylase bound to DNA. Nature. 1996;384:87–92. doi: 10.1038/384087a0. [DOI] [PubMed] [Google Scholar]
- Smith CM, Steitz JA. Sno storm in the nucleolus: new roles for myriad small RNPs. Cell. 1997;89:669–672. doi: 10.1016/s0092-8674(00)80247-0. [DOI] [PubMed] [Google Scholar]
- Spradling, A. 1993. Developmental genetics of oogenesis. In Drosophila Development. M. Bate, and A. Martines-Arias, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 1–70.
- Spreji TE. Cell death during the development of imaginal discs of Calliphora erytrocephala. . Netherlands J Zool. 1971;21:221–264. [Google Scholar]
- Thummel CS, Boulet AM, Lipshitz HD. Vectors for DrosophilaP-element-mediated transformation and tissue culture transfection. Gene. 1988;74:445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Tollervey D. A yeast small nuclear RNA is required for normal processing of pre-ribosomal RNA. EMBO (Eur Mol Biol Organ) J. 1987;6:4169–4175. doi: 10.1002/j.1460-2075.1987.tb02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey D, Guthrie C. Deletion of a yeast small nuclear RNA gene impairs growth. EMBO (Eur Mol Biol Organ) J. 1985;4:3873–3878. doi: 10.1002/j.1460-2075.1985.tb04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok T, Tick G, Alvarado M, Kiss I. P-lacW insertional mutagenesis on the second chromosome of Drosophila melanogaster: isolation of lethals with different overgrowth phenotypes. Genetics. 1993;135:71–80. doi: 10.1093/genetics/135.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J, Karpen GH, Craig N, Spradling AC. Preferential transposition of DrosophilaP-elements to nearby chromosomal sites. Genetics. 1993;133:347–359. doi: 10.1093/genetics/133.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz IE, Hanley MR. Diverse molecular provocation of programmed cell death. Trends Biochem Sci. 1996;21:359–364. [PubMed] [Google Scholar]
- Winkles JA, Phillips WH, Grainger RM. Drosophilaribosomal RNA stability increases during slow growth conditions. J Biol Chem. 1985;260:7716–7720. [PubMed] [Google Scholar]