Abstract
Myoblasts, the precursors of skeletal muscle fibers, can be induced to withdraw from the cell cycle and differentiate in vitro. Recent studies have also identified undifferentiated subpopulations that can self-renew and generate myogenic cells (Baroffio, A., M. Hamann, L. Bernheim, M.-L. Bochaton-Pillat, G. Gabbiani, and C.R. Bader. 1996. Differentiation. 60:47–57; Yoshida, N., S. Yoshida, K. Koishi, K. Masuda, and Y. Nabeshima. 1998. J. Cell Sci. 111:769–779). Cultured myoblasts can also differentiate and contribute to repair and new muscle formation in vivo, a capacity exploited in attempts to develop myoblast transplantation (MT) for genetic modification of adult muscle. Our studies of the dynamics of MT demonstrate that cultures of myoblasts contain distinct subpopulations defined by their behavior in vitro and divergent responses to grafting. By comparing a genomic and a semiconserved marker, we have followed the fate of myoblasts transplanted into muscles of dystrophic mice, finding that the majority of the grafted cells quickly die and only a minority are responsible for new muscle formation. This minority is behaviorally distinct, slowly dividing in tissue culture, but rapidly proliferative after grafting, suggesting a subpopulation with stem cell–like characteristics.
Keywords: skeletal muscle, myoblast transplantation, cell heterogeneity, stem cells, mdx mouse
Adult skeletal muscle exhibits a remarkable capacity for regeneration following damage, an ability which resides in a population of precursor cells, termed satellite cells, sequestered between the basement membrane and sarcolemma of individual muscle fibers. Such cells are normally quiescent, but following tissue damage, become activated to migrate, proliferate, and differentiate, thereby effecting repair and regeneration (reviewed in Pastoret and Partridge, 1998). In certain respects, regeneration in the adult recapitulates the embryogenesis of skeletal muscle during which muscle precursor cells (MPCs)1 migrate from the somitic mesoderm, proliferate, and finally differentiate to form muscle fibers (Buckingham, 1992). However, although the precise origins and lineage of satellite cells are ill-defined, in vitro studies have demonstrated clear differences between MPCs prepared from prenatal and adult muscle (Cossu et al., 1992; Smith et al., 1993). This distinction has also been suggested by in vivo studies which show that myonuclei derived from MPCs involved in muscle development and growth migrate from the center to the periphery of the maturing muscle fiber, whereas MPCs derived from satellite cells involved in postnatal regeneration in mice give rise to permanently centrally nucleated fibers (Grounds et al., 1980; Pastoret and Sebille, 1995). The presence of two populations of MPC has also been demonstrated in postnatal, growing rat muscle, with a majority of rapidly dividing cells, available for incorporation with little or no proliferation during growth, and a slowly dividing, but potentially highly proliferative reserve population involved in the generation of the former population and self-renewal (Rantanen et al., 1995; Schultz, 1996). Clonal analysis of satellite cells isolated from growing and regenerating rat muscles further suggests that these populations may continue to be distinguishable in vitro (Schultz and Jaryszak, 1985). Recent studies have also revealed heterogeneity within myogenic cultures derived from single cells (Baroffio et al., 1995, 1996; Yoshida et al., 1998), a minority of cells remaining undifferentiated when cultured in conditions designed to induce terminal differentiation, while retaining the ability to proliferate and apparently self-renew.
The ability of myoblasts to incorporate into postnatal skeletal muscle during regeneration is exploited by myoblast transplantation (MT; reviewed in Blau and Springer, 1995; Partridge and Davies, 1995), a potential therapeutic approach to the treatment of primary inherited myopathies (Partridge et al., 1989; Vilquin et al., 1996) and diseases that could be ameliorated by the systemic delivery of nonmuscle proteins from host muscle (Barr and Leiden, 1991; Dhawan et al., 1991; Yao et al., 1994; Hamamori et al., 1995). MT involves delivery of exogenous myoblasts which contribute to the formation of new muscle fibers during repair and regeneration, and therefore result in genetic modification of the host muscle. Thus far, the relevance of myoblast heterogeneity to MT has not been explored, specifically, whether all MPCs are equivalent in terms of their behavior following transplantation into regenerating adult muscle.
We have followed the behavior of donor myoblasts during the first few days after murine MT using a dual marker system designed to measure the short-term persistence and proliferation of donor cells (Beauchamp et al., 1997). The system involves transplantation of male MPCs labeled with radioactive thymidine into female mouse muscles, thereby providing two donor cell markers, the Y chromosome and incorporated radiolabel, which can be measured in the same muscle extract. These markers have different modes of inheritance. Following division, each daughter contains the same amount of Y chromosome and half of the radiolabel present in the parent cell. The total number of donor-derived nuclei in a host muscle can be determined from the amount of Y chromosome. The radiolabel, by contrast, is passed semiconservatively to the progeny of the originally labeled cell and is released from this population only upon cell death. Thus, the total radioactivity within the host muscle provides an estimate of the surviving proportion of the initial dose of donor DNA. Any in vivo replication within the donor cell population will manifest as a change in the ratio of the two markers, with a relative increase in the amount of Y chromosome.
Using this system, we show that even in an immunodeficient, irradiated mdx host, an optimized environment for MT (Morgan et al., 1990, 1993), all the donor-derived muscle is produced from a minority of donor cells that survive and proliferate rapidly in vivo under conditions in which the majority of cells undergo rapid death. This minority is also refractory to a period of culture in the presence of concentrations of [3H]thymidine which are cytotoxic to rapidly dividing cells, suggesting that myogenic cultures contain a discrete subpopulation which divide slowly in vitro, but are activated to undertake rapid and extensive proliferation upon grafting into regenerating host muscle. These cells may represent a permanent, distinct subpopulation of stem cells capable of generating more rapidly dividing progeny and self-renewal, or may be comprised of cells in transit through a specific part of the cell cycle at the time of transplantation. Whatever their origin, these cells are distinct in their ability to survive transplantation (unlike the vast majority) and contribute to regeneration in the postnatal host muscle. The presence of behaviorally distinct populations of MPCs in mature muscle has implications for our understanding of growth, repair, and regeneration in this tissue.
Materials and Methods
Cell Culture and Radiolabeling
H-2Kb clone 18, a cloned, conditionally immortal myogenic line, was derived from a 4-wk-old male H-2Kb-tsA58 heterozygote, carrying a single copy of the thermolabile tsA58 mutant SV-40 large T antigen (TAg) under the control of the H-2Kb promoter (Morgan et al., 1994). The line was maintained at low density in DME (high glucose, with sodium pyruvate; Life Technologies Inc.) supplemented with 20% FCS, 2% chick embryo extract, 4 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma Chemical Co.). Murine recombinant IFN-γ was added to the medium (20 U/ml; Life Technologies Inc.) to increase transgene expression through the H-2Kb promoter, and cultures were grown at 33°C in 10% CO2, a temperature at which the thermolabile TAg protein is active.
Primary MPCs were prepared by enzymatic disaggregation of leg muscles from 1-d-old male C57Bl/10 mice. Muscles were minced and digested in HBSS (25 ml/mouse) containing 1 U/ml collagenase type III (Sigma Chemical Co.) for 10 min at 37°C before repeated cycles of aspiration and expulsion through the tip of a Pasteur pipette. Released cells were discarded and fragments were subjected to two rounds of digestion in HBSS containing 0.25% trypsin (Sigma Chemical Co.) as above. The trypsin was inactivated by addition of an equal volume of HBSS containing 20% FCS, and cells were collected by centrifugation at 350 g for 10 min at 4°C. Cell pellets were resuspended in growth medium consisting of DME (high glucose, with sodium pyruvate) supplemented with 20% FCS, 2% chick embryo extract, 4 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Undigested tissue fragments were removed by filtration through 45 μM-pore-diameter nylon mesh, and cells were plated and cultured at 37°C in 10% CO2.
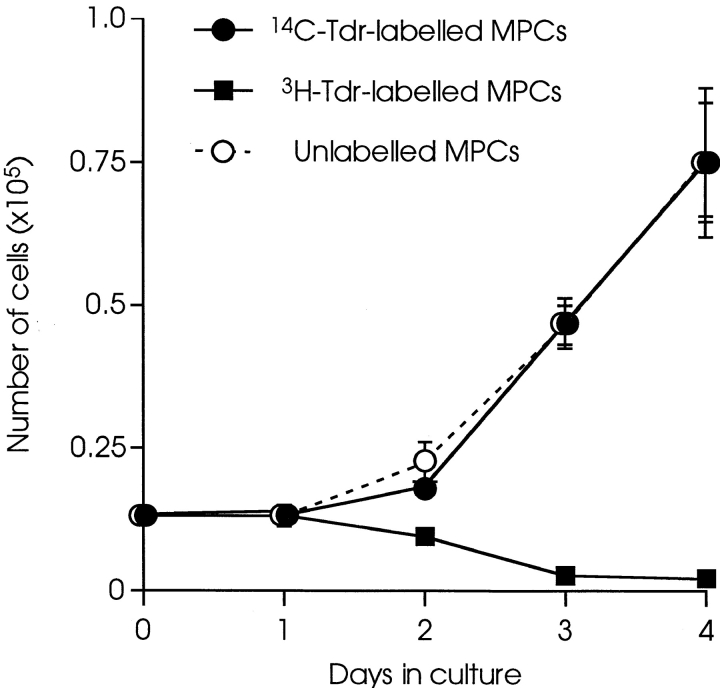
MPCs were radiolabeled by culturing for 16 h in growth medium containing 0.25 μCi/ml [methyl-3H]thymidine (5 Ci/mmol) or [methyl-14C] thymidine (54 mCi/mmol; Nycomed Amersham). Using this labeling regime, >95% of the radiolabel could be TCA-precipitated (data not shown). To investigate the effects of radiolabeling on MPCs, [14C]thymidine–labeled, [3H]thymidine–labeled, and unlabeled H-2Kb clone 18, MPCs were plated at 500 cells/cm2. At each time point, cultures were examined by phase-contrast microscopy and total cell number was calculated from mean number of cells in 12 random fields of known area.
5-Bromo-2′-deoxyuridine (BrdU) Incorporation
H-2Kb clone 18 MPCs were plated at 500 cells/cm2, cultured for 16 h in growth medium containing 4 μM BrdU, and then immunostained with mouse anti-BrdU antibody as described (Kaufman and Foster, 1988). In brief, cultures were fixed at −20°C in 95% ethanol, rinsed in PBS, and then incubated for 30 min at room temperature in 2 M HCl. After three 20-min washes in 50 mM NaCl, 100 mM Tris-HCl, pH 7.4, the cells were incubated for 1 h with mouse anti-BrdU antibody (diluted 1:20 in PBS), for 30 min with a biotinylated rabbit polyclonal anti–mouse immunoglobulins antibody (diluted 1:250 in PBS), and finally for 30 min with streptavidin-peroxidase (diluted 1:250). All antibodies were obtained from DAKO Corp. Peroxidase activity was visualized with 3,3′-diaminobenzidine tetrahydrochloride and after counterstaining with hematoxylin, cultures were examined by light microscopy. Percentages of BrdU-positive cells were counted in six random fields, each containing ≥200 cells.
Myoblast Transplantation
MPCs were detached from the culture vessel by incubation in 0.05% trypsin/0.02% EDTA. An equal volume of growth medium was added to inactivate the trypsin, and cells were collected by centrifugation at 350 g for 10 min at 4°C. Cell pellets were resuspended in HBSS such that 5 × 105 cells could be transplanted in a total volume of 5–10 μl. 3–4-wk-old female mdx nude mice were anaesthetized, the skin overlaying the tibialis anterior (TA) muscle was opened, and 5 × 105 MPCs were injected into the TA muscle using a Hamilton 7005 syringe. The needle was inserted longitudinally into the muscle and withdrawn slowly as the plunger was depressed to deliver the cells along the length of the muscle. Irradiated host muscles had been exposed to 18 Gray of X-irradiation 3 d before transplantation, a pretreatment previously shown to enhance MT in mice (Morgan et al., 1990, 1993).
Measurement of Y Chromosome and Radiolabel in Host Muscles
At the indicated time points, host muscles were removed, snap-frozen in liquid nitrogen, and stored at −80°C. Amounts of radiolabel and Y chromosome were measured as described previously (Beauchamp et al., 1997). In brief, muscles were thawed on ice, minced, and then digested to homogeneity for 16 h at 50°C in 50 mM Tris-HCl, 100 mM EDTA, 100 mM NaCl, pH 8.0, containing 500 μg/ml proteinase K (Sigma Chemical Co.), and 0.5% (wt/vol) SDS, with regular vortexing. To determine the amount of radiolabel present, an aliquot was mixed with Hionic-Fluor liquid scintillation counting fluid (Canberra Packard Ltd.) and the amount of isotope measured using a Beckman LS6000SC counter system. DNA was extracted from the remaining digestion mixture and slot blotted onto uncharged nylon membrane (Hybond-N; Nycomed Amersham), together with appropriate control dilution series of male and female DNA. Before slot blotting, the amount of isotope present in an aliquot of each DNA preparation was measured as above to determine the efficiency of extraction. Slot blots were hybridized with a Y chromosome–specific DNA probe, 145SC5 (Nishioka, 1988), and labeled with [α-32P]dCTP (Nycomed Amersham) by random priming using Ready-To-Go DNA labeling beads (Pharmacia Biotech Co). Hybridized membranes were exposed to a phosphor screen which was then scanned using a PhosphoImager 445 SI (Molecular Dynamics, Inc.). Quantitative analyses were carried out using ImageQuant software (Molecular Dynamics, Inc.) and the amount of male DNA present in each sample was determined by reference to a standard curve obtained from control male DNA dilution series.
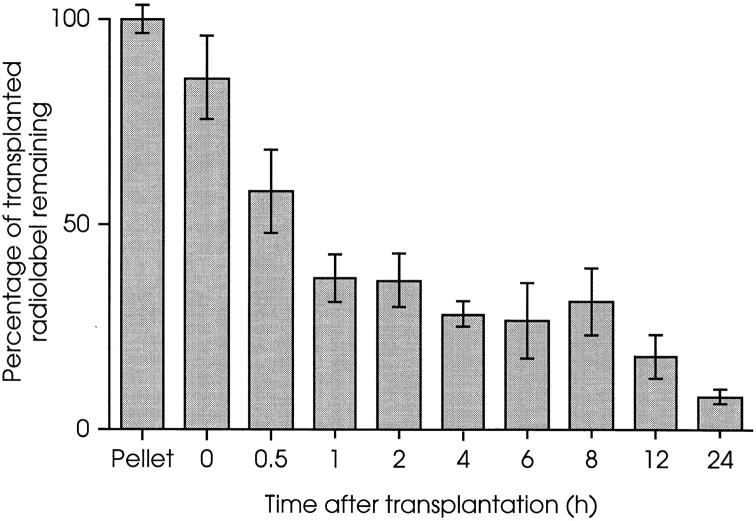
In the experiment presented in Fig. 2, host muscles were removed, solubilized in Soluene®-350 (Canberra Packard Ltd.), and the amount of 14C present was measured by scintillation counting after the addition of scintillation counting fluid, as above.
Figure 2.
Biphasic loss of donor MPCs from irradiated host muscles during the initial 24 h after transplantation. [14C]Thymidine– labeled H-2Kbclone 18 MPCs were injected into irradiated left TA muscles of nude mdx mice. Each muscle received 5 × 105 cells and at the times indicated, amount of 14C present in host muscles was measured. Less than 10% of the injected radiolabel remained 24 h after MT. 60% was lost during the first 60 min and a second event, initiated ≥8 h after MT, resulted in the subsequent disappearance of most of the remaining label. Results are presented as percentages of 14C present in 5 × 105 labeled MPCs, and each time point is the mean value from four muscles ± 1 SD. Muscles taken at 0 h were removed as quickly as possible after MT (i.e., <5 min) and contained ∼85% of the injected label.
Immunofluorescent Staining
8-μm-thick cryosections were cut and immunostained for dystrophin using a sheep polyclonal antibody to the 60-kD dystrophin peptide (provided by Professor E.P. Hoffman, University of Pittsburgh School of Medicine, Pittsburgh, PA) as described (Partridge et al., 1989). In brief, unfixed sections were blocked in 5% horse serum in PBS, and then incubated for 1 h with the primary antibody (1:1,000 dilution), for 1 h with an affinity-purified, biotinylated donkey antibody raised against sheep immunoglobulins (diluted 1:400; Nycomed Amersham), and for 30 min with streptavidin– Texas red (diluted 1:100; Nycomed Amersham). All dilutions were in 5% horse serum in PBS. The slides were examined and photographed using a Zeiss Axiophot fluorescence microscope.
Results
Most Transplanted MPCs Are Lost Rapidly from the Host Muscle
Initial studies were carried out using a cloned, conditionally immortal myogenic cell line, H-2Kb clone 18, derived from a male mouse transgenic for the tsA58 mutant TAg under the control of the inducible H-2Kb promoter (Morgan et al., 1994). Donor MPCs were labeled with either [3H]thymidine or [14C]thymidine, and then injected into preirradiated TA muscles of 3–4-wk-old female nude mdx mice, an optimized host environment for MT (Morgan et al., 1990, 1993).
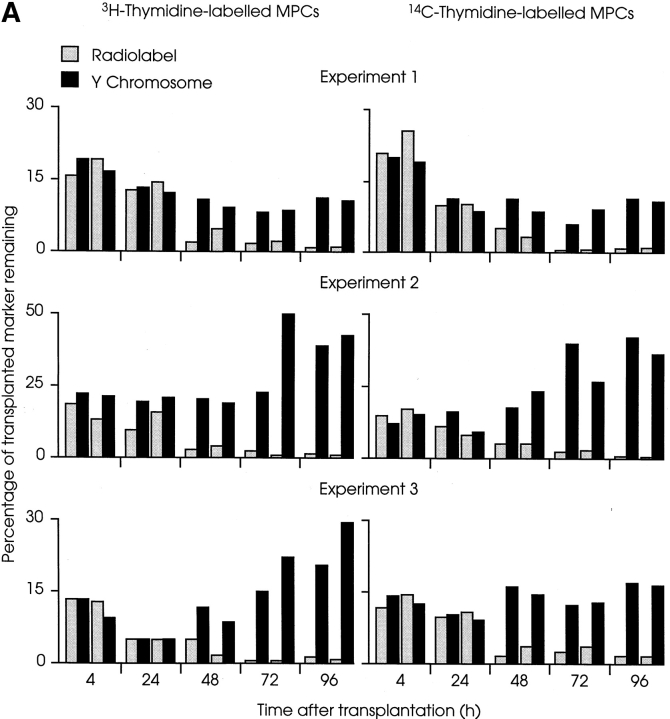
Identical results were obtained with [3H] and [14C]thymidine–labeled MPCs (Fig. 1). In the experiments shown, each of which was carried out using a different preparation of H-2Kb clone 18 MPCs, there was a rapid loss of 80–95% of radiolabel and Y chromosome during the first day after transplantation (at 24 h, mean percentage of radiolabel remaining from all experiments = 10.2 ± 4.2; mean percentage of male DNA remaining = 11.2 ± 1.6 [± SEM, n = 12]). During this period, the loss of the two markers was concordant. The mean ratio of percentage of radiolabel to percentage of male DNA present at 24 h remained at 0.96 ± 0.04 (± SEM, n = 12) compared with the initial ratio of 1.0. Equivalent loss of Y chromosome demonstrates that the disappearance of radiolabels was not due to leakage of unincorporated thymidine. Furthermore, in some experiments, entire legs of host mice were solubilized after removal of the injected TA muscles, and were found to contain <10% of the injected isotope 4 h after MT (data not shown). This, together with the finding that MPCs incorporated within a fibrin clot are lost to the same extent as when delivered in suspension (Beauchamp et al., 1997), demonstrates that the disappearance of donor cells is not simply due to leakage from the injection site into surrounding compartments.
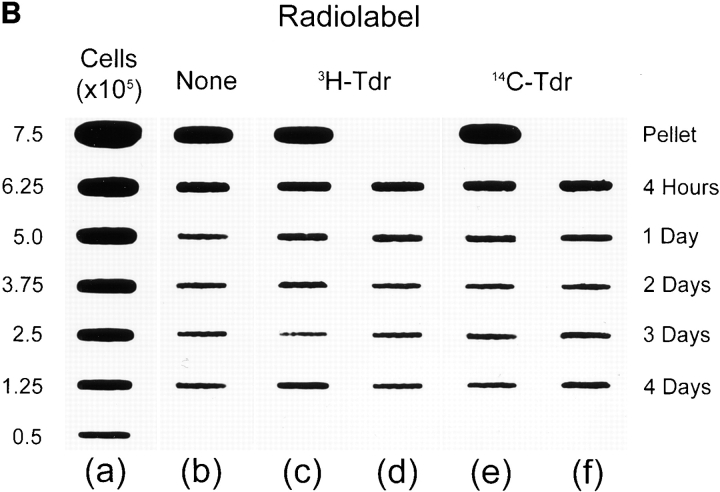
Figure 1.
Loss and proliferation of MPCs following myoblast transplantation into irradiated host muscle. (A) Irradiated left TA muscles of female nude mdx mice were transplanted with 5 × 105 radiolabeled male H-2Kbclone 18 MPCs. At the times indicated, mice were killed and amounts of Y chromosome (filled columns) and radiolabel (open columns) were measured and are presented as percentages. In each experiment, most donor MPCs disappeared from host muscles during the initial 24 h after MT, as shown by a coincident loss of the majority of donor cell markers. During the subsequent 3 d, amounts of Y chromosome revealed marked proliferation of surviving cells, despite the continued, progressive disappearance of the radiolabeled population. In each experiment shown, four mice were killed at each time point, two had received [3H]thymidine–labeled MPCs (left), and two had been transplanted with [14C]thymidine–labeled MPCs (right). Each pair of columns represents percentages of radiolabel and Y chromosome remaining in an individual muscle. (B) Representative slot blot of DNA extracted from irradiated female nude mdx mouse muscles injected with unlabeled or radiolabeled male MPCs, probed for Y chromosome. (a) Series of control samples prepared from noninjected female muscles combined with 7.5–0.5 × 105 male MPCs; (b) DNA extracted from muscles injected with 5 × 105 unlabeled cells; (c and d) DNA extracted from muscles injected with the same number of [3H]thymidine–labeled MPCs; and (e and f) DNA from muscles injected with [14C]thymidine–labeled cells. The time at which each muscle was harvested after MT is shown at the right of the figure. The top slots in b, c, and e contain DNA extracted from representative cell pellets containing 5 × 105 unlabeled or labeled MPCs. The signal from each slot was measured using a PhosphoImager and values obtained from a were used to construct a linear standard curve of signal against number of donor cells (data not shown). Signals obtained from injected muscles were within the linear range of this system, which is maintained with as little as 1 ng of target DNA (equivalent to ∼200 genome copies; Beauchamp et al., 1997). Values obtained from this blot are presented in A, experiment 1.
Rapid Cell Death Following MT Is Biphasic
The rapid disappearance of donor MPCs from host muscles was confirmed and further defined by a detailed investigation of the first 24 h after MT (Fig. 2). Muscles harvested immediately after injection contained 85% of the amount of label present in the transplanted cell population, confirming that most of the cells were successfully deposited within the muscle. However, only 30% of the injected label (∼35% present immediately after injection) remained after 1 h. Following the initial fall, amounts of radiolabel present in muscles taken between 1 and 8 h after injection were relatively constant, at ∼25% of that transplanted. However, a second loss of label was observed beginning 8–12 h after transplantation, suggesting a biphasic loss of donor MPCs following MT: α rapid loss of ∼75% of the injected cell population, followed by a second event beginning 8–12 h later, result in the loss of most of the remaining cells, such that only ∼1% of the injected radiolabel remained 4 d after grafting (Fig. 1).
Surviving Transplanted MPCs Proliferate in the Host Muscle
After the dramatic disappearance of donor cells during the initial 24 h after injection, there was a progressive loss of radiolabel such that ∼1% of the injected radiolabeled-thymidine remained 4 d after transplantation (mean percentage of radiolabel remaining after 4 d = 1.1 ± 0.1 [± SEM, n = 12]; Fig. 1 A). This value may actually overestimate the persistence of the transplanted MPCs as some label may have been released by dying cells and reused by host cells. However, continued disappearance of donor cells was accompanied by division of the remaining cells, as revealed by a marked divergence of the labels. Thus, although only 1% of the injected radiolabel persisted, the mean percentage of Y chromosome present after 4 d from all experiments was equivalent to 23.5% of the initial population (SEM ± 3.8, n = 12). It is notable that, although the percentages of Y chromosome present in individual muscles after 4 d varied from 8 to 43%, the ranges within each experiment were small (experiment 1, 8–11%; 2, 36– 43%; 3, 16–29%; Fig. 1 A), demonstrating that within an equivalent group of host mice, each particular cell preparation gave rise to equal numbers of donor cells irrespective of the radiolabel used. Furthermore, when Y chromosome was measured in muscles transplanted with unlabeled MPCs, the same results were obtained as with equivalent labeled MPCs (Fig. 1 B, column b).
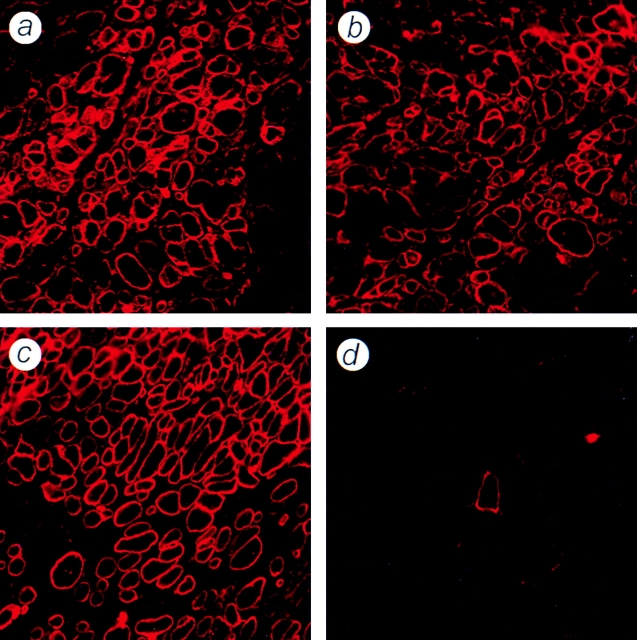
Extensive proliferation of the surviving fraction of donor cells and their subsequent contribution to the formation or repair of muscle fibers was also evident from the large numbers of dystrophin-positive profiles present in muscles taken 3 wk after MT (Fig. 3). Again, no differences in the numbers or distributions of dystrophin-positive fibers were observed between muscles that had received [3H]thymidine–labeled, [14C]thymidine–labeled, or unlabeled MPCs. Together, these results demonstrate what would appear to be a catastrophic loss of donor cells in the immediate aftermath of MT, cited as an explanation for failure of this technique (Huard et al., 1994; Fan et al., 1996; Guérette et al., 1997), is in fact a standard feature of successful transplantations, as in the above experiments, and that success is due to extensive proliferation of a small proportion of the cells rather than survival of the majority of the transplanted population.
Figure 3.
Dystrophin expression in sections of irradiated mdx muscles, 3 wk after transplantation of unlabeled or radiolabeled normal MPCs. Cryosections of irradiated, nude mdx TA muscles were immunostained for the presence of dystrophin 3 wk after transplantation of 5 × 105 H-2Kbclone 18 MPCs. No differences in numbers or distribution of dystrophin-positive fibers were observed between muscles injected with [3H]thymidine–labeled (a), [14C]thymidine–labeled (b), or unlabeled (c) donor cells. A control muscle injected with 5 μl of medium is shown, containing a single dystrophin-positive revertant fiber (d).
MPCs That Contribute to Muscle Formation In Vivo Divide Slowly In Vitro
It was surprising that identical results were obtained in vivo with MPCs labeled with [3H]thymidine and [14C]thymidine (Fig. 1). In vitro, [3H]thymidine was cytotoxic for the majority of the population, whereas [14C]thymidine had no significant effect on survival or proliferation (Fig. 4). Previous studies, published >30 yr ago, reported that [3H]thymidine of high specific activity, such as used in the present studies, was cytotoxic to HeLa cells, and only cells which had not entered S phase during the period of labeling (and therefore remained unlabeled) retained the potential to form colonies (Drew and Painter, 1962). Since intranuclear [3H]thymidine is cytotoxic to MPCs in vitro, this suggests that cells that survive MT and proliferate within the host muscle environment had not undergone DNA synthesis during the 16 h of radiolabeling, and may therefore represent a subpopulation of MPCs defined by a relatively slow generation time in vitro. When H-2Kb clone 18 MPCs were cultured under the same conditions used for radiolabeling, but in the presence of BrdU, immunostaining for BrdU incorporation revealed that 3 ± 0.3% (± SEM, n = 8) of the population had not entered S phase during that time, suggesting an upper limit to size of the subpopulation responsible for muscle formation in vivo.
Figure 4.
Differential effects of labeling with [14C]thymidine and [3H]thymidine on MPCs in vitro. H-2Kbclone 18 MPCs were cultured for 16 h in the presence of 0.25 μCi/ml of either [14C]thymidine or [3H]thymidine, and then replated at 500 cells/cm2. The numbers of cells in cultures of [14C]thymidine–labeled (filled circles), [3H]thymidine–labeled (filled squares), and control unlabeled (open circles) cells were counted over 4 d. Each point is the mean of three cultures ± 1 SD. The growth of MPCs precultured in presence of equivalent concentrations of unlabeled thymidine (4.6 μM for [14C]thymidine and 50 pM for [3H]thymidine) was indistinguishable from the control, unlabeled cells at thymidine concentrations of standard medium (data not shown).
Preirradiation of the Host Muscle Enhances Donor MPC Proliferation, but Not Survival
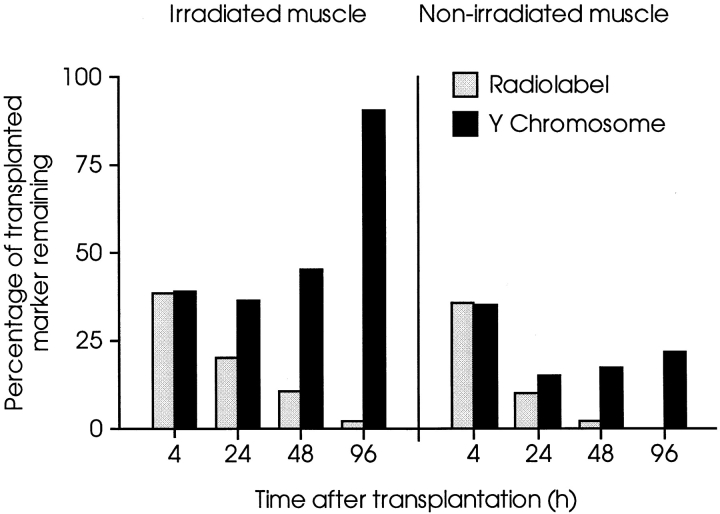
The preceding data are from experiments in which MPCs were injected into preirradiated host muscles, a treatment previously shown to increase the efficacy of MT (Morgan et al., 1990, 1993). To investigate the effects of preirradiation of host muscles on the short-term behavior of transplanted MPCs, the right legs of a series of female nude mdx mice were irradiated before bilateral MT with radiolabeled primary cells derived from male neonatal muscle. In the irradiated muscles and the contralateral, nonirradiated controls, there was a similar loss, both in timing and extent, of virtually all the transplanted radiolabel over the experimental period (Fig. 5). As with the conditionally immortal cell line, the amount of Y chromosome showed a progressive relative increase from 24 h after MT onwards compared with the amount of radiolabel, in both the irradiated and control muscles. However, the amount of Y chromosome increased to a greater extent in the irradiated muscles, suggesting that there was greater proliferation of surviving MPCs than in nonirradiated muscles. Thus, the increased efficacy of MT in preirradiated host muscles is not the result of increased cell survival, but may be attributed to increased proliferation of the surviving subpopulation. Furthermore, data presented in Fig. 5 also show that successful MT through the rapid expansion of a small number of surviving MPCs is not peculiar to conditionally immortal myogenic lines. In fact, when injected into irradiated host muscles, more donor-derived nuclei were generated from primary cells than from the same numbers of cloned, conditionally immortal MPCs, although a similarly large proportion of cells was lost in the immediate aftermath of transplantation.
Figure 5.
Comparative behavior of primary MPCs in irradiated and nonirradiated host muscles. TA muscles of female nude mdx mice were injected with 5 × 105 [3H]thymidine–labeled male primary MPCs. The right legs of host mice had been irradiated 3 d before transplantation. Amounts of Y chromosome (filled columns) and 3H (open columns) were measured in muscles of host mice killed at times indicated after MT. In both irradiated and contralateral, nonirradiated muscles, there was similar progressive loss of radiolabel over 4 d. However, after an initial fall at 4 h equal to that of radiolabel, amounts of Y chromosome increased in both series, although increase in irradiated muscles was considerably greater than in nonirradiated, contralateral muscles. Each column is the mean of two muscles, presented as percentages of marker injected, determined by analysis of cell pellets prepared as for transplantation.
Discussion
Successful genetic modification of skeletal muscle by MT requires the survival of donor myoblast nuclei and their stable incorporation into muscle fibers within the host tissue. Attempts to develop MT into a viable approach to gene therapy have focused on modification of the host tissue and control of inflammatory and immunological responses to the graft (Tremblay and Guerette, 1997). In contrast, scant attention has been paid to donor cells until recently (Gussoni et al., 1997; Qu et al., 1998). In the context of MT, the term myoblast is simply used to describe a mononucleated, undifferentiated cell with the potential to initiate the myogenic program, withdraw from the cell cycle, and differentiate to form skeletal muscle. However, our studies of early events following MT reveal markedly divergent behavior within myoblast populations, even in X-irradiated muscles of nude mdx mice, a highly permissive environment for MT (Morgan et al., 1990, 1993). The vast majority of donor MPCs die in the aftermath of MT, and muscle of donor-origin is derived from only a tiny proportion of the injected population which undergo rapid and extensive proliferation in vivo. This phenomenon was observed with both primary MPCs and a myogenic cell line derived from the H-2Kb-tsA58 transgenic mouse, suggesting that expression of TAg maintains the population capable of repopulating muscle in vivo.
To follow the fate of donor myoblasts during the first few days after transplantation into dystrophic host muscle, we have developed a method based upon quantitative comparisons between a genetic marker (Y chromosome) and a semiconserved label (radiolabeled thymidine) of donor DNA (Beauchamp et al., 1997). Since both labels are measured directly, this combination has an advantage over other commonly used reporter systems such as β-galactosidase and green fluorescent protein, the use of which may be confounded by variation in the level of gene expression or accumulation of product, particularly when labeled cells may be at different stages of differentiation. Loss of either label will occur only on cell death (loss of radiolabeled thymidine due to DNA repair would be trivial in the time course of this experiment). Thus, in our system, the amount of Y chromosome provides an absolute measure of the number of copies of donor cell–derived genome, while the amount of radiolabel directly reflects the proportion of originally grafted donor cell DNA which has survived in the recipient muscle. Our intention to use the change in ratio between the genetic and semiconserved labels to assess cell proliferation is predicated on uptake of radiolabeled thymidine by a single homogeneous population. However, the almost complete loss of radiolabel in conjunction with increasing amounts of Y chromosome revealed that labeling was not homogenous and suggested the presence of two donor cell populations of diverse behavior. One, proliferative in culture and therefore readily poisoned by [3H]thymidine, is sensitive to transplantation into muscles. The second, a minor subpopulation, slowly dividing in culture and thus refractory to [3H]thymidine, survives transplantation and proliferates rapidly in vivo. This unexpectedly clear-cut distinction, to some extent, vitiates the use of the ratio of labels as a quantitative measure of cell proliferation. Thus, with progressive decline of radiolabel towards background levels, the best indicator of the behavior of the surviving subpopulation is the change in the absolute measures of Y chromosome content which, for example, illustrates a clear difference between the rapid proliferation of cells in preirradiated muscles and slower rates in nonirradiated muscles.
Our findings raise two fundamental questions. Why should a consistently small, but highly prolific, subpopulation of donor MPCs survive the apparent rigors of MT, and what causes the death of the vast majority of transplanted MPCs?
The identity of cells which proliferate and contribute to muscle formation is crucial to the first of these questions. Our data suggest that the surviving cells constitute a distinct minority which, for unknown reason(s), are resistant to the early events after MT, and are not merely chance survivors of a purely stochastic process of cell death. We observed the same dynamics of rapid cell death and eventual outcome of MT in terms of new muscle formation with unlabeled, [14C]thymidine–labeled, or [3H]thymidine–labeled MPCs, despite the fact that the latter label is cytotoxic to dividing cells. Cells which proliferate after transplantation and give rise to the donor muscle therefore must have been those that are refractory to [3H]labeling, presumably because they had not passed through S phase during the 16-h period of in vitro labeling. That the loss of injected MPCs was identical irrespective of labeling suggests that cells in rapid cycle in vitro (which are readily labeled) are those which die following MT, and are irrelevant to the final outcome. Further, this demise is an innate feature of this category of cell since its occurrence is unaffected by the toxicity of the label. In contrast, the cells which do not incorporate radiolabel in vitro further demonstrate their inherent behavioral individuality by undertaking rapid proliferation soon after transplantation into irradiated muscle, in conditions which the previously proliferative majority are dying. These findings parallel those in heterogeneous populations of hematopoietic cells, where exposure to high specific activity [3H]thymidine has been shown to selectively kill cycling cells while preserving long-term culture-initiating cells (Ponchio et al., 1995), and argue in favor of the idea that cells which give rise to muscle on transplantation into muscle constitute a distinct subpopulation, apparently with some stem cell–like properties.
The existence of a stem cell–like subpopulation of MPC has also been suggested by recent studies of myoblasts in tissue culture. Yoshida et al. (1998) identified a population of reserve cells in the myogenic cell line, C2, which, when exposed to differentiation conditions, persisted as slowly cycling, undifferentiated, mononuclear cells with the capacity to self-renew and give rise to differentiating cells. Phenotypically distinct, nonfusing cells that can divide asymmetrically to self-renew and generate committed cells have also been identified within individual clones of human MPCs (Baroffio et al., 1996). These presumptive stem cells comprised ∼2% of each clone (very similar to our 3% upper limit of murine muscle-forming MPCs which did not enter S phase during labeling) and decreased in number with successive passages, which may account for the finding that culture of avian myoblasts results in a progressive decrease in the amount of muscle formed following transplantation (DiMario and Stockdale, 1995). Recently, Dominov et al. (1998) reported that Bcl-2, an apoptosis-inhibiting protein, is expressed by myoblasts at an early stage of differentiation and may promote clonal expansion of myogenic cells. Intriguingly, only 1–4% of cells in primary myogenic cultures were found to be positive for Bcl-2 (Dominov et al., 1998). It is possible that the subpopulation with some stem cell–like properties, previously identified in vitro (Baroffio et al., 1995, 1996; Dominov et al., 1998; Yoshida et al., 1998), may correspond to those we define by their behavior in vivo, i.e., relative robustness when transplanted.
Why most donor MPCs die following MT remains unclear. Our results show that ∼99% of radiolabeled donor cells had died and were cleared from the host muscles 4 d after grafting. The remaining 1% defines an upper limit to the survival of radiolabeled cells, as some reutilization of label released from dead donor MPCs may have occurred, although the extent of any reutilization was clearly insignificant compared with the total amount of label transplanted. We found no evidence of donor cell apoptosis during the first 6 h after MT when sections of host muscle were analyzed by terminal deoxynucleotidyl transferase– mediated deoxyuridine triphosphate endlabeling, a technique which reveals DNA strand breaks characteristic of apoptotic nuclei (data not shown). The mechanism is also unlikely to be a specific immunological response to donor MPCs as death occurs within 2–3 d following either transplantation into isogenic (Huard et al., 1994), congenic (Fan et al., 1996), immunodeficient, or immunosuppressed hosts (Beauchamp, J.R., J.E. Mogan, C.N. Pagel, and T.A. Partridge. 1994. Muscle Nerve. 18:S261; Guérette et al., 1997). A recent study has implicated a nonspecific inflammatory reaction, possibly initiated in response to tissue damage, which may be inhibited by antibody-mediated blockade of leukocyte function associated molecule-1 (Guérette et al., 1997). Our analysis of the first 24 h after MT suggests that the rapid loss of cells may involve two events, the second of which, beginning 8–12 h after injection, may indeed be attributable to such an inflammatory reaction. However, this is unlikely to account for the initial loss of ≤70% of donor cells within 1 h of transplantation. Rather, this rapid necrotic demise of the most proliferative portion of the population of grafted MPCs suggests the cells are maladapted to some aspect of the environment within the recipient muscle, perhaps anoxia, although the rapid removal of released radiolabel from the graft site seems to imply that it is efficiently perfused. In the study of Guérette et al. (1997), the percentage of donor cells surviving 3 d after grafting was calculated relative to that measured 1 h after MT. Our results show that significant cell death occurs during the first hour after grafting, so the initial phase of cell death following grafting would have been missed. However, recent findings of Qu et al. (1998) also imply a biphasic loss of donor MPCs following MT. When either an mdx cell line or myoblasts isolated from isolated muscle fibers were grafted, substantial losses of donor cells were measured 12 h after injection. In marked contrast, primary myogenic cells selected by a sequential preplating regime survived transplantation, apparently without loss. However, although the selected primary cells persisted for 48 h, there were marked losses during the subsequent 3 d. Furthermore, when the mdx cell line was engineered to produce IL-1 receptor antagonist protein intended to block any early inflammatory reaction, the initial loss of donor cells was largely unaffected, although the subsequent disappearance observed 2–5 d after transplantation was prevented (Qu et al., 1998). These results support the idea that loss of donor MPCs following MT involves two events, the first as yet undefined and to which different preparations of donor MPC may be differentially susceptible, followed by a second, inflammation-mediated event.
The identification of a relatively undifferentiated subpopulation, slowly dividing (and probably selected against) in culture, but driven into rapid proliferation upon being grafted into preirradiated muscle, and in consequence responsible and therefore required for successful MT in vivo, has significant implications for human MT. Failure to reproduce the promising results obtained with mdx mice in clinical trials of MT on boys with Duchenne muscular dystrophy has been variously attributed to lack of host muscle regeneration, the presence of significant connective tissue barriers (both consequences of recipient age), and immune rejection (Gussoni et al., 1992; Karpati et al., 1993; Roy et al., 1993; Tremblay et al., 1993a,b; Mendell et al., 1995; Morandi et al., 1995; Miller et al., 1997). The ability of human MPCs to survive transplantation was also questioned (Gussoni et al., 1992; Karpati et al., 1993) and subsequent reports of rapid donor cell death during the aftermath of MT in mice (Beauchamp, J.R., J.E. Mogan, C.N. Pagel, and T.A. Partridge. 1994. Muscle Nerve. 18: S261; Huard et al., 1994; Fan et al., 1996; Guérette et al., 1997) have suggested that this may have contributed to the failure of clinical trials. Our results also demonstrate that the vast majority of transplanted MPCs die within hours of delivery. However, we have shown that this phenomenon does not lead to inevitable failure, as it occurs during successful MT. Recent reevaluation of biopsies of recipient muscles from an earlier clinical trial of human MT on boys with Duchenne muscular dystrophy (Gussoni et al., 1992) revealed the persistence of unexpectedly large numbers of donor-derived nuclei, many of which had become incorporated into mature myofibers, but did not express dystrophin (Gussoni et al., 1997). Therefore, at least in human MT, the ability of donor cells to survive grafting does not inevitably lead to biochemical modification of host tissue, possibly due to environmental influences encountered in diseased, dystrophic muscle. Nevertheless, the ability to isolate and maintain a population of human MPCs equivalent to those responsible for successful MT in mice could greatly enhance the therapeutic potential of a given dose of donor cells, and might obviate the extensive cloning and expansion in vitro (Law et al., 1993; Tremblay et al., 1993a,b; Mendell et al., 1995), or selection on the basis of neural cell adhesion molecule expression (Gussoni et al., 1992; Karpati et al., 1993; Morandi et al., 1995; Miller et al., 1997), which may have helped to confound the potential success of MT in human trials. Considerable interest in the possible use of stem cells for genetic conversion of adult skeletal muscle has resulted from the demonstration that bone marrow–derived cells can contribute to skeletal muscle regeneration, following either direct grafting into host muscle or bone marrow transplantation (Ferrari et al., 1998). Whether derived from bone marrow or isolated from heterogeneous myogenic cultures (Baroffio et al., 1995, 1996; Dominov et al., 1998; Yoshida et al., 1998), precursor cells with the potential to contribute to new muscle formation, particularly after the initial transplantation, would be invaluable for the development of MT.
Acknowledgments
This work was supported by The Medical Research Council, The Muscular Dystrophy Group of Great Britain and Northern Ireland, The Leopold Muller Foundation, and The David and Frederick Barclay Foundation.
Abbreviations used in this paper
- BrdU
5-Bromo-2′-deoxyuridine
- MT
myoblast transplantation
- MPCs
muscle precursor cells
- TA
tibialis anterior
- TAg
SV-40 large T antigen
References
- Baroffio A, Bochaton-Piallet M-L, Gabbiani G, Bader CR. Heterogeneity in the progeny of single human muscle satellite cells. Differentiation. 1995;59:259–268. doi: 10.1046/j.1432-0436.1995.5940259.x. [DOI] [PubMed] [Google Scholar]
- Baroffio A, Hamann M, Bernheim L, Bochaton-Piallat M-L, Gabbiani G, Bader CR. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 1996;60:47–57. doi: 10.1046/j.1432-0436.1996.6010047.x. [DOI] [PubMed] [Google Scholar]
- Barr E, Leiden JM. Systemic delivery of recombinant proteins by genetically modified myoblasts. Science. 1991;254:1507–1509. doi: 10.1126/science.1962212. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Pagel CN, Partridge TA. A dual-marker system for quantitative studies of myoblast transplantation in the mouse. Transplantation. 1997;63:1794–1797. doi: 10.1097/00007890-199706270-00015. [DOI] [PubMed] [Google Scholar]
- Blau HM, Springer ML. Muscle-mediated gene therapy. N Engl J Med. 1995;333:1554–1556. doi: 10.1056/NEJM199512073332308. [DOI] [PubMed] [Google Scholar]
- Buckingham M. Making muscle in mammals. Trends Genet. 1992;8:144–149. doi: 10.1016/0168-9525(92)90373-C. [DOI] [PubMed] [Google Scholar]
- Cossu, G., M.G. Cusella-De Angelis, L. De Angelis, A. Mezzogiorno, P. Murphy, M. Coletta, E. Vivarelli, M. Bouché, and M. Molinaro. 1992. Multiple myogenic cell precursors and their possible role in muscle histogenesis. In Neuromuscular Development and Disease. A.M. Kelly and H.M. Blau, editors. Raven Press, Ltd., New York. 183–194.
- Dhawan J, Pan LC, Pavlath GK, Travis MA, Lanctot AM, Blau HM. Systemic delivery of human growth hormone by injection of genetically engineered myoblasts. Science. 1991;254:1509–1512. doi: 10.1126/science.1962213. [DOI] [PubMed] [Google Scholar]
- DiMario JX, Stockdale FE. Differences in the developmental fate of cultured and noncultured myoblasts when transplanted into embryonic limbs. Exp Cell Res. 1995;216:431–442. doi: 10.1006/excr.1995.1054. [DOI] [PubMed] [Google Scholar]
- Dominov JA, Dunn JJ, Miller JB. Bcl-2 expression identifies an early stage of myogenesis and promotes clonal expansion of muscle cells. J Cell Biol. 1998;142:537–544. doi: 10.1083/jcb.142.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew RM, Painter RB. Further studies on the clonal growth of HeLa S3 cells treated with tritiated thymidine. Radiat Res. 1962;16:303–311. [PubMed] [Google Scholar]
- Fan Y, Maley M, Beilharz M, Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19:853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stronaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic precursors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Grounds M, Partridge TA, Sloper JC. The contribution of exogenous cells to regenerating skeletal muscle: an isoenzyme study of muscle allografts in mice. J Pathol. 1980;132:325–341. doi: 10.1002/path.1711320404. [DOI] [PubMed] [Google Scholar]
- Guérette B, Asselin I, Skuk D, Entman M, Tremblay JP. Control of inflammatory damage by anti–LFA-1: increase success of myoblast transplantation. Cell Transplant. 1997;6:101–107. doi: 10.1177/096368979700600203. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L, Blau HM. Normal dystrophin transcripts in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992;356:435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nature Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- Hamamori Y, Samal B, Tian J, Kedes L. Myoblast transfer of human erythropoietin gene in a mouse model of renal failure. J Clin Invest. 1995;95:1808–1813. doi: 10.1172/JCI117859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J, Acsadi G, Jani A, Massie B, Karpati G. Gene transfer into skeletal muscles by isogenic myoblasts. Hum Gene Ther. 1994;5:949–958. doi: 10.1089/hum.1994.5.8-949. [DOI] [PubMed] [Google Scholar]
- Karpati G, Ajdukovic D, Arnold D, Gledhill RB, Guttmann R, Holland P, Koch PA, Shoubridge E, Spence D, Vanasse M, et al. Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol. 1993;34:8–17. doi: 10.1002/ana.410340105. [DOI] [PubMed] [Google Scholar]
- Kaufman SJ, Foster RF. Replicating myoblasts express a muscle-specific phenotype. Proc Natl Acad Sci USA. 1988;85:9606–9610. doi: 10.1073/pnas.85.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PK, Goodwin TG, Fang Q, Deering MB, Duggirala V, Larkin C, Florendo JA, Kirby DS, Li HJ, Chen M, et al. Cell transplantation as an experimental treatment for Duchenne muscular dystrophy. Cell Transplant. 1993;2:485–505. doi: 10.1177/096368979300200607. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, et al. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Miller RG, Sharma KR, Pavlath GK, Gussoni E, Mynhier M, Yu P, Lanctot BS, Greco CM, Steinman L, Blau HM. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 1997;20:469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Morandi L, Bernasconi P, Gebbia M, Mora M, Crosti F, Mantegazza R, Cornelio F. Lack of mRNA and dystrophin expression in DMD patients three months after myoblast transfer. Neuromuscul Disord. 1995;5:291–295. doi: 10.1016/0960-8966(94)00070-p. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Hoffman EP, Partridge TA. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J Cell Biol. 1990;111:2437–2449. doi: 10.1083/jcb.111.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JE, Pagel CN, Sherratt T, Partridge TA. Long-term persistence and migration of myogenic cells injected into pre-irradiated muscles of mdxmice. J Neurol Sci. 1993;115:191–200. doi: 10.1016/0022-510x(93)90224-m. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Beauchamp JR, Pagel CN, Peckham M, Ataliotis P, Jat PS, Noble MD, Farmer K, Partridge TA. Myogenic cell lines derived from transgenic mice carrying a thermolabile T antigen: a model system for the derivation of tissue-specific and mutation-specific cell lines. Dev Biol. 1994;162:486–498. doi: 10.1006/dbio.1994.1103. [DOI] [PubMed] [Google Scholar]
- Nishioka Y. Application of Y chromosomal repetitive sequences to sexing mouse embryos. Teratology. 1988;38:181–185. doi: 10.1002/tera.1420380211. [DOI] [PubMed] [Google Scholar]
- Partridge TA, Davies KE. Myoblast-based gene therapies. Br Med Bull. 1995;51:123–137. doi: 10.1093/oxfordjournals.bmb.a072942. [DOI] [PubMed] [Google Scholar]
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Pastoret C, Sebille A. Age-related differences in regeneration of dystrophic (mdx) and normal muscle in the mouse. Muscle Nerve. 1995;18:1147–1154. doi: 10.1002/mus.880181011. [DOI] [PubMed] [Google Scholar]
- Pastoret, C., and T.A. Partridge. 1998. Muscle regeneration. In Cellular and Molecular Basis of Regeneration: From Invertebrates to Humans. P. Ferretti and J. Géraudie, editors. John Wiley and Sons Ltd., Chichester, UK. 309–333.
- Ponchio L, Conneally E, Eaves C. Quantitation of the quiescent fraction of long-term culture-initiating cells in normal human blood and marrow and the kinetics of their growth factor-stimulated entry into S-phase in vitro. Blood. 1995;86:3314–3321. [PubMed] [Google Scholar]
- Qu Z, Balir L, van Deutekom JCT, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transplantation therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen J, Hurme T, Lukka R, Heino J, Kalimo H. Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest. 1995;72:341–347. [PubMed] [Google Scholar]
- Roy R, Tremblay J-P, Huard J, Richards C, Malouin F, Bouchard J-P. Antibody formation after myoblast transplantation in Duchenne-dystrophic patients, donor HLA compatible. Transplant Proc. 1993;25:995–997. [PubMed] [Google Scholar]
- Schultz E. Satellite cell proliferative compartments in growing skeletal muscles. Dev Biol. 1996;175:84–94. doi: 10.1006/dbio.1996.0097. [DOI] [PubMed] [Google Scholar]
- Schultz E, Jaryszak DL. Effects of skeletal muscle regeneration on the proliferation potential of satellite cells. Mech Ageing Dev. 1985;30:63–72. doi: 10.1016/0047-6374(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Smith TH, Block NE, Rhodes SJ, Konieczny SF, Miller JB. A unique pattern of expression of the four muscle regulatory factor proteins distinguishes somitic from embryonic, fetal and newborn mouse myogenic cells. Development. 1993;117:1125–1133. doi: 10.1242/dev.117.3.1125. [DOI] [PubMed] [Google Scholar]
- Tremblay JP, Guerette B. Myoblast transplantation: a brief review of the problems and some solutions. Basic Appl Myology. 1997;7:221–230. [Google Scholar]
- Tremblay JP, Bouchard JP, Malouin F, Théau D, Cottrell F, Collin H, Rouche A, Gilgenkrantz S, Abbadi N, Tremblay M, et al. Myoblast transplantation between monozygotic twin girl carriers of Duchenne muscular dystrophy. Neuromuscul Disord. 1993a;3:583–592. doi: 10.1016/0960-8966(93)90121-y. [DOI] [PubMed] [Google Scholar]
- Tremblay JP, Malouin F, Roy R, Huard J, Bouchard JP, Satoh A, Richards CL. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993b;2:99–112. doi: 10.1177/096368979300200203. [DOI] [PubMed] [Google Scholar]
- Vilquin J-T, Kinoshita I, Roy B, Goulet M, Engvall E, Tomé F, Fardeau M, Tremblay JP. Partial laminin α2 chain restoration in α2 chain-deficient dy/dymouse by primary muscle cell culture transplantation. J Cell Biol. 1996;133:185–197. doi: 10.1083/jcb.133.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao SN, Smith KJ, Kurachi K. Primary myoblast-mediated gene transfer: persistent expression of human factor-IX in mice. Gene Ther. 1994;1:99–107. [PubMed] [Google Scholar]
- Yoshida N, Yoshida S, Koishi K, Masuda K, Nabeshima Y. Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates “reserve cells.” . J Cell Sci. 1998;111:769–779. doi: 10.1242/jcs.111.6.769. [DOI] [PubMed] [Google Scholar]