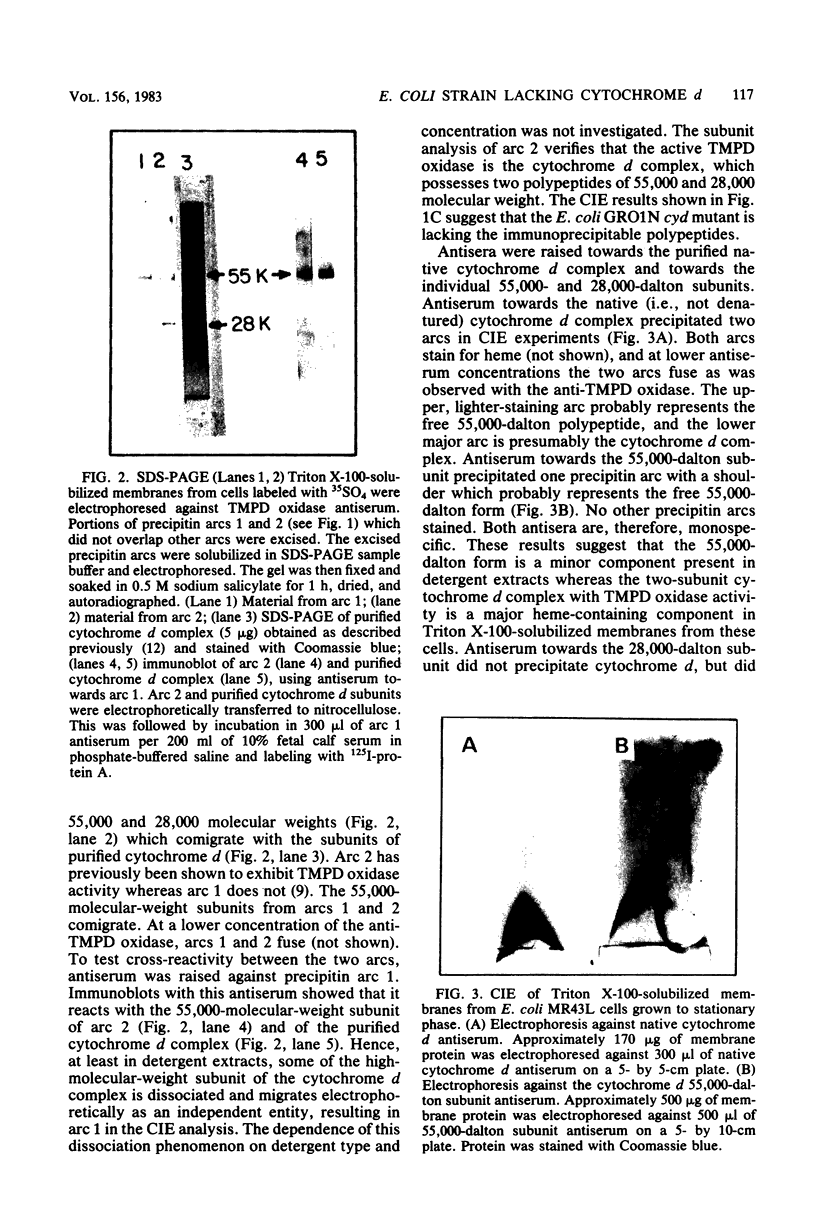
Abstract
The isolated membranes from an Escherichia coli mutant strain which lacks spectroscopically detectable levels of cytochromes d, a1, and b558 also have abnormally low levels of N,N,N',N'-tetramethyl-p-phenylenediamine oxidase activity. In this paper, it is shown that the material previously identified as the N,N,N',N'-tetramethyl-p-phenylenediamine oxidase is, in fact, the two-subunit cytochrome d complex. Antisera directed against the native cytochrome d complex as well as against each of two subunits apparent on sodium dodecyl sulfate-polyacrylamide gels were used to show that the mutant strain lacks both subunits of the cytochrome d complex. Introduction of F-prime F152 into the mutant strain restored the two subunits along with the spectroscopic and enzymatic activity associated with the cytochrome d complex.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Downie J. A., Cox G. B. Sequence of b cytochromes relative to ubiquinone in the electron transport chain of Escherichia coli. J Bacteriol. 1978 Feb;133(2):477–484. doi: 10.1128/jb.133.2.477-484.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. N., Gennis R. B. Isolation and characterization of an Escherichia coli mutant lacking cytochrome d terminal oxidase. J Bacteriol. 1983 Jun;154(3):1269–1275. doi: 10.1128/jb.154.3.1269-1275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Shrager R. I. Potentiometric analysis of Escherichia coli cytochromes in the optical absorbance range of 500 nm to 700 nm. J Biol Chem. 1979 Nov 25;254(22):11288–11299. [PubMed] [Google Scholar]
- Hendler R. W., Towne D. W., Shrager R. I. Redox properties of beta-type cytochromes in Escherichia coli and rat liver mitochondria and techniques for their analysis. Biochim Biophys Acta. 1975 Jan 31;376(1):42–62. doi: 10.1016/0005-2728(75)90203-0. [DOI] [PubMed] [Google Scholar]
- Kita K., Kasahara M., Anraku Y. Formation of a membrane potential by reconstructed liposomes made with cytochrome b562-o complex, a terminal oxidase of Escherichia coli K12. J Biol Chem. 1982 Jul 25;257(14):7933–7935. [PubMed] [Google Scholar]
- Kita K., Yamato I., Anraku Y. Purification and properties of cytochrome b556 in the respiratory chain of aerobically grown Escherichia coli K12. J Biol Chem. 1978 Dec 25;253(24):8910–8915. [PubMed] [Google Scholar]
- Kranz R. G., Gennis R. B. A quantitative radioimmunological screening method for specific gene products. Anal Biochem. 1982 Dec;127(2):247–257. doi: 10.1016/0003-2697(82)90169-5. [DOI] [PubMed] [Google Scholar]
- Kranz R. G., Gennis R. B. Isoelectric focusing and crossed immunoelectrophoresis of heme proteins in the Escherichia coli cytoplasmic membrane. J Bacteriol. 1982 Apr;150(1):36–45. doi: 10.1128/jb.150.1.36-45.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Gennis R. B. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J Biol Chem. 1983 Aug 10;258(15):9159–9165. [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Inhibition by cyanide of the respiratory chain oxidases of Escherichia coli. Arch Biochem Biophys. 1974 Oct;164(2):682–693. doi: 10.1016/0003-9861(74)90081-2. [DOI] [PubMed] [Google Scholar]
- Scott R. I., Poole R. K. A re-examination of the cytochromes of Escherichia coli using fourth-order finite difference analysis: their characterization under different growth conditions and accumulation during the cell cycle. J Gen Microbiol. 1982 Aug;128(8):1685–1696. doi: 10.1099/00221287-128-8-1685. [DOI] [PubMed] [Google Scholar]
- Shipp W. S. Cytochromes of Escherichia coli. Arch Biochem Biophys. 1972 Jun;150(2):459–472. doi: 10.1016/0003-9861(72)90063-x. [DOI] [PubMed] [Google Scholar]
- Shipp W. S., Piotrowski M., Friedman A. E. Apparent cytochrome gene dose effects in F-lac and F-gal heterogenotes of Escherichia coli. Arch Biochem Biophys. 1972 Jun;150(2):473–481. doi: 10.1016/0003-9861(72)90064-1. [DOI] [PubMed] [Google Scholar]
- Van Wielink J. E., Oltmann L. F., Leeuwerik F. J., De Hollander J. A., Stouthamer A. H. A method for in situ characterization of b- and c-type cytochromes in Escherichia coli and in complex III from beef heart mitochondria by combined spectrum deconvolution and potentiometric analysis. Biochim Biophys Acta. 1982 Aug 20;681(2):177–190. doi: 10.1016/0005-2728(82)90021-4. [DOI] [PubMed] [Google Scholar]
- Yamato I., Anraku Y., Hirosawa K. Cytoplasmic membrane vesicles of Escherichia coli. A simple method for preparing the cytoplasmic and outer membranes. J Biochem. 1975 Apr;77(4):705–718. doi: 10.1093/oxfordjournals.jbchem.a130774. [DOI] [PubMed] [Google Scholar]