Abstract
The interaction of cadherin–catenin complex with the actin-based cytoskeleton through α-catenin is indispensable for cadherin-based cell adhesion activity. We reported previously that E-cadherin–α-catenin fusion molecules showed cell adhesion and cytoskeleton binding activities when expressed in nonepithelial L cells. Here, we constructed deletion mutants of E-cadherin–α-catenin fusion molecules lacking various domains of α-catenin and introduced them into L cells. Detailed analysis identified three distinct functional domains of α-catenin: a vinculin/α-actinin-binding domain, a ZO-1-binding domain, and an adhesion-modulation domain. Furthermore, cell dissociation assay revealed that the fusion molecules containing the ZO-1-binding domain in addition to the adhesion-modulation domain conferred the strong state of cell adhesion activity on transfectants, although those lacking the ZO-1-binding domain conferred only the weak state. The disorganization of actin-based cytoskeleton by cytochalasin D treatment shifted the cadherin-based cell adhesion from the strong to the weak state. In the epithelial cells, where α-catenin was not precisely colocalized with ZO-1, the ZO-1-binding domain did not completely support the strong state of cell adhesion activity. Our studies showed that the interaction of α-catenin with the actin-based cytoskeleton through the ZO-1-binding domain is required for the strong state of E-cadherin–based cell adhesion activity.
Keywords: α-catenin, ZO-1, vinculin, E-cadherin, adhesion
The cadherins are a family of transmembrane proteins responsible for Ca2+-dependent cell–cell adhesion (Takeichi, 1991). Intracellularly, they interact with a group of proteins collectively termed catenins (Ozawa et al., 1989; Nagafuchi et al., 1993). Association with catenins is necessary for cadherins to express their full function as cell adhesion molecules, and this complex is now regarded as a functional unit for cell adhesion. A unique property of this cadherin–catenin complex is its intimate interaction with the actin-based cytoskeleton. At cell–cell contact sites, this complex is colocalized with actin filaments and resists nonionic detergent extraction (Hirano et al., 1987). This complex is a major constituent of intercellular adherens junctions (AJ),1 where actin filaments are densely associated with the plasma membrane through its well-developed plaque structure (Tsukita et al., 1993). Although the cytoskeletal interaction of cadherin– catenin complex is thought to be essential for adhesion, the detailed molecular mechanism of this interaction remains elusive.
α-Catenin associates with the COOH-terminal end of the cadherin cytoplasmic domain (catenin-binding site) via β-catenin. Sequence analysis showed that α-catenin has similarity to vinculin, another constituent of AJ (Herrenknecht et al., 1991; Nagafuchi et al., 1991), which interacts with various actin-based cytoskeletal components including actin itself (Jockusch et al., 1995). This suggested that α-catenin may interact with the actin-based cytoskeleton. α-Actinin, ZO-1, vinculin, and actin itself were reported to interact directly with α-catenin (Rimm et al., 1995; Itoh et al., 1997; Nieset et al., 1997; Watabe-Uchida et al., 1998; Weiss et al., 1998). ZO-1 was identified originally as a tight junction (TJ)-associated peripheral membrane protein (Stevenson et al., 1986; Anderson et al., 1988), but it is also concentrated at cadherin-based cell–cell contact sites together with vinculin in nonepithelial cells lacking TJ such as fibroblasts and cardiac muscle cells (Itoh et al., 1993).
The functions of α-catenin have been analyzed mainly using two cell lines, the human lung carcinoma cell line PC9, and L cells, a mouse fibroblast cell line. PC9 cells express E-cadherin and β-catenin but not α-catenin. They do not form tight cell aggregates after rotation culture and resultant aggregates are easily dissociated into single cells (Shimoyama et al., 1992). The exogenous expression of α-catenin in these cells restored not only the full cell adhesion activity of cadherins but also the cadherin–cytoskeleton interaction (Hirano et al., 1992; Watabe et al., 1994). Mouse L fibroblasts do not express cadherin molecules and lack the cadherin-based cell adhesion activity. When E-cadherin was introduced into L cells, stable transfectants showed the full cell adhesion activity through the interaction of introduced E-cadherin with endogenous α- and β-catenins, but the mutant E-cadherin lacking the catenin-binding site did not confer such cell adhesion activity on L cells (Nagafuchi et al., 1987; Nagafuchi and Takeichi, 1988; Ozawa et al., 1989). When the full-length or COOH-terminal half of α-catenin was covalently connected to this nonfunctional mutant E-cadherin, these fusion molecules showed the full cell adhesion activity in L cells without interacting with endogenous catenins. Moreover, the introduced fusion molecules were associated with the cytoskeleton (Nagafuchi et al., 1994). These findings indicated that α-catenin, especially its COOH-terminal half, is crucial for the full cadherin-based cell adhesion activity as well as cadherin–cytoskeleton interaction.
The L cell transfection system is advantageous for examination of the function of the cadherin–catenin complex in cell adhesion and cytoskeleton interaction. Since L cells show little cell–cell adhesion activity, these functions of introduced cadherin or its fusion molecules can be selectively analyzed. Using this system, we examined the domains of α-catenin responsible for the cadherin-based cell adhesion activity and the interaction with cytoskeletal components such as ZO-1, vinculin, and α-actinin. We also evaluated the functions of these α-catenin functional domains in α-catenin–deficient epithelial cell lines.
Materials and Methods
Cells and Antibodies
Mouse L cells were grown in DME supplemented with 10% FCS. Transfectants expressing E-cadherin (ELβ1; Nose et al., 1988), nEα(1-906), nEαN(1-508), and nEαC(509-906) (nEαL2, nEαNL28, and nEαCL1, respectively; Nagafuchi et al., 1994), and other E-cadherin–α-catenin fusion molecules (see Fig. 1) were grown in the same medium containing 150 μg/ ml of G418. nEα(1-906), nEαN(1-508), and nEαC(509-906) were originally called nEα, nEαN, and nEαC, respectively (Nagafuchi et al., 1994). We named transfectants by combining the name of the fusion protein with L; for example, L cells expressing nEα(1-906) were designated as nEα(1-906)L cells. Mouse PC9 cells were grown in a 1:1 mixture of DME and Ham's F12 supplemented with 10% FCS (DH10). PC9 cells expressing α(1-184/509-643)-HA (see below) were grown in the same medium containing 150 μg/ml of G418. Human colon carcinoma DLD-1/R2/7, abbreviated to R2/7, and its transfectants expressing αE(1-890), αE(1-325/510-890), αE(1-509), and αE(1-325) (Watabe-Uchida et al., 1998) were also cultured in DH10 medium.
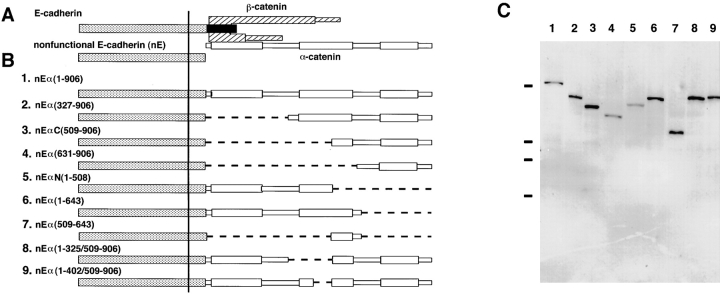
Figure 1.
Schematic representation of E-cadherin–α-catenin fusion proteins. (A) E-cadherin–catenin complex and nonfunctional E-cadherin. Direct binding of β-catenin (hatched boxes) to E-cadherin (dotted and closed boxes) and α-catenin (open boxes) links the NH2-terminal domain of α-catenin to the COOH-terminal domain of E-cadherin. The COOH-terminal 70 amino acids of E-cadherin (closed box) are responsible for catenin binding. E-cadherin lacking this catenin-binding domain did not interact with catenins and was nonfunctional. (B) Fusion molecules between the nonfunctional E-cadherin and mutant α-catenins with various deletions (1–9). The amino acid residues of α-catenin contained in fusion molecules are shown in parentheses. nEα(1-906), nEαN(1-508), and nEαC(509-906) correspond to the previously reported fusion molecules nEα, nEαN, and nEαC, respectively (Nagafuchi et al., 1994). (C) Expression of fusion proteins in stable transfectants. Total lysates derived from 105 cells expressing nEα(1-906) (lane 1), nEα(327-906) (lane 2), nEαC(509-906) (lane 3), nEα(631-906) (lane 4), nEαN(1-508) (lane 5), nEα(1-643) (lane 6), nEα(509-643) (lane 7), nEα(1-325/509-906) (lane 8), and nEα(1-402/509-906) (lane 9) were separated by SDS-PAGE (7.5%) and immunoblotted with anti–E-cadherin mAb, ECCD-2. The numbers marking the lanes in C correspond to the numbers of constructs in B. Several minor bands with lower molecular mass, which may have been degradation products of fusion molecules, were detected. Size markers of 200, 116, 97, and 66 kD are indicated on the left.
Mouse anti–ZO-1 mAb (T8-754) was obtained and characterized as described (Itoh et al., 1991). Anti–E-cadherin mAb (ECCD-2), which was concentrated by ammonium sulfate precipitation, was a generous gift from Dr. M. Takeichi (Kyoto University, Kyoto, Japan; Shirayoshi et al., 1986). Mouse anti-vinculin mAb (hVIN-1) and mouse anti–α-actinin mAb (BM-75.2) were purchased from Sigma Chemical Co. Mouse anti–HA-tag mAb (12CA5) was purchased from Boehringer Mannheim Biochemicals. Cy2-labeled anti–rat IgG and Cy3-labeled anti–mouse IgG antibodies were purchased from Amersham. DTAF (dichlorotriazinyl amino fluorescence)-labeled anti–rat IgG was purchased from Chemicon International, Inc.
Constructs
We constructed pBATEα(327-906), pBATEα(631-906), pBATEα(1-643), pBATEα(509-643), pBATEα(1-325/509-906), and pBATEα(1-402/509-906), expression vectors for nEα(327-906), nEα(631-906), nEα(1-643), nEα(509-643), nEα(1-325/509-906), and nEα(1-402/509-906), respectively (see Fig. 1). For construction of these vectors, we used three plasmids: (a) pBATEM2, mouse E-cadherin expression vector (Nose et al., 1988), the ClaI-XbaI fragment of which corresponds to the catenin-binding site and was replaced with the α-catenin cDNA fragments in constructs; (b) pSK102B, which contains the α-catenin cDNA with a PstI-BglII adaptor inserted into the PstI site just before the initiation methionine codon; and (c) pBATEα, the ClaI-XbaI fragment of pBATEM2 was replaced with the BglII-XbaI fragment of pSK102B including the whole ORF of the α-catenin cDNA (Nagafuchi et al., 1994). In the open reading frame of the α-catenin cDNA sequence, we used four restriction sites, PmaCI, ScaI, ClaI, and SmaI, corresponding to amino acid residues 326, 403, 508, and 670, respectively. The XbaI site at the 3′ terminal of α-catenin cDNA in pSK102B was also used. For the production of pBATEα(327-906), the ClaI-XbaI fragment of pBATEM2 was replaced with the PmaCI-XbaI fragment of pSK102B. For construction of pBATEα(631-906), a ClaI site (630ClaI) was introduced at the position corresponding to amino acid residue 630 of α-catenin cDNA in pSK102B by PCR, then the ClaI-XbaI fragment of pBATEM2 was replaced with the 630ClaI-XbaI fragment. For production of pBATEα(1-643), an XbaI site (644XbaI) was introduced at a position corresponding to amino acid residue 644 of α-catenin cDNA in pSK102B by PCR, then the ClaI-XbaI fragment of pBATEα was replaced with the ClaI-644XbaI fragment. For production of pBATEα(1-325/509-906) and pBATEα(1-402/509-906), the PmaCI-ClaI and the ScaI-ClaI fragments, respectively, were excised from pBATEα. All junctions newly produced in the α-catenin coding sequence were arranged in frame using adaptors or linkers as necessary.
For construction of pEFα(1-184/509-643)-HA, an EcoRI site (185 EcoRI) was introduced at the position corresponding to amino acid residue 185 of α-catenin cDNA in pSK102B by PCR. The BglII-185EcoRI fragment, ClaI-644XbaI fragment, and a HAα3′ fragment, which contains an HA epitope tag sequence, a stop codon, and 3′ noncoding region of α-catenin, were tandemly ligated and inserted into the pEFMC1-neo expression vector (Visvader et al., 1992).
We also constructed pGEX-αN(1-508), pGEX-αC(509-906), and pGEX-α(671-906), expression vectors for GST–α-catenin fusion molecules, using pSK102B and pGEX vectors (Pharmacia LKB Biotechnology). For production of pGEX-αN(1-508), pGEX-αC(509-906), and pGEX-α(631-906), the BglII-ClaI, ClaI-XbaI, and SmaI-EcoRI fragments of pSK102B were inserted into the BamHI-SmaI sites of pGEX-2T, the SmaI site of pGEX-3X, and the SmaI-XhoI sites of pGEX-4T-3 (Pharmacia LKB Biotechnology), respectively.
Transfection
L cells (5 × 105 per 3-cm plate) were cotransfected with 1 μg of each expression vector and 0.05 μg of pSTneoB (Katoh et al., 1987) by the lipofectamine method (Life Technologies, Inc.). After 48 h of incubation, the cells were replated on pairs of 9-cm dishes and cultured in the presence of 400 μg/ml G418 to select stable transfectants. Colonies of G418-resistant cells were isolated, recloned, and subsequently maintained in complete medium with 150 μg/ml of G418. We isolated several stable clones for each transfection experiment. Since nEα(327-906)L-11, nEα(631-906)L-7, nEα(1-643)L-9, nEα(509-643)L-32, nEα(1-325/509-906)L-2, and nEα(1-402/509-906)L-23 clones expressed relatively large amounts of fusion molecules, we mainly used these in this study.
Trypsinized PC9 cells (105) were suspended in 500 μl of Hepes-buffered (pH 7.4) Ca2+- and Mg2+-free saline and mixed with 10 μg of expression vector and 1 μg of pSTneoB. Electroporation was performed at 960 μF, 250 V. The cells were selected in G418 (0.2 mg/ml)-containing medium.
Immunohistochemistry
All procedures were performed at room temperature. Cells cultured on coverslips were fixed with 3.5% (for hVIN-1 and BM-75.2) or 1.0% (for T8-754) formaldehyde solution in HMF (Hepes-buffered magnesium-free saline) for 15 min. After three washes with PBS, cells were soaked in blocking solution (1% BSA in PBS) for 30 min and subsequently incubated with ECCD-2 diluted with PBS containing 1% BSA for 30–60 min at room temperature. The cells were then washed three times with PBS and soaked in 0.2% Triton X-100 in PBS for 15 min. After rinsing with PBS, the cells were treated with 1% BSA in PBS for 30 min and subsequently incubated with hVIN-1, T8-754, or BM-75.2 for 30–60 min. After extensive washing with PBS, the specimens were incubated with fluorescence-labeled second antibodies (Cy2- or DTAF-labeled goat anti–rat IgG for ECCD-2 and Cy3-labeled donkey anti–mouse IgG [H&L] for hVIN-1, T8-754, and BM-75.2) diluted with PBS containing 1% BSA for 30 min at room temperature. After washing thoroughly with PBS, the preparation was mounted with 90% glycerol-PBS containing 0.1% para-phenylendiamine and 1% n-propylgalate. Samples were observed with a Zeiss Axiophot photomicroscope (Carl Zeiss). Images were recorded with a cooled CCD camera (SenSys 0400, 768 × 512 pixels; Photometrics) controlled by a Power Macintosh 7600/132 and the software package IPLab Spectrum V3.1 (Signal Analytic Corp.).
SDS-PAGE and Immunoblotting
SDS-PAGE (10 or 7.5%) and immunoblotting were performed as described previously (Nagafuchi et al., 1994). Samples were solubilized in SDS sample buffer, separated by SDS-PAGE, and gels were stained with Coomassie brilliant blue R-250. For immunoblotting, proteins were electrophoretically transferred onto nitrocellulose sheets. Nitrocellulose membranes were then incubated with ECCD-2, T8-754, or 12CA5. Antibody detection was performed using an Amersham biotin-streptavidin kit with biotinylated anti–rat or anti–mouse Ig and NBT-BCIP.
In Vitro Binding Assay Using GST Fusion Proteins
In vitro binding assays were performed as previously described (Itoh et al., 1997). In brief, GST–α-catenin fusion proteins were expressed in Escherichia coli and purified using glutathione-Sepharose 4B beads (Pharmacia LKB Biotechnology) as previously described (Itoh et al., 1997). Then, 2 ml of the cell lysate of Sf9 cells expressing N-ZO-1 was added, followed by incubation for 3 h at 4°C. The beads were again washed with PBS containing 0.1% Triton X-100, 2 mM PMSF, and 4 μg/ml of leupeptin, and then bound proteins were eluted with 1 ml of 50 mM Tris-HCl buffer, pH 8.0, containing 10 mM glutathione. The amounts of GST fusion proteins in each eluate were determined by SDS-PAGE.
Detergent Extraction of Cells
Confluent cultured cells (∼4 × 106 cells per 6-cm dish) were extracted with 2.5% NP-40 in HMF, then centrifuged at 100,000 rpm for 30 min as described previously (Nagafuchi and Takeichi, 1988). To the supernatant, 2× SDS sample buffer was added to make the total volume 0.4 ml and used as the detergent-soluble fraction. On the other hand, the pellet fraction was dissolved in 0.4 ml of 1× SDS sample buffer and used as the detergent-insoluble fraction.
Trypsin Treatment, Aggregation, and Dissociation of Cells
Cells were trypsinized by two different methods for the differential removal of E-cadherin or its fusion molecules, as described by Takeichi (1977). In brief, cells were treated with 0.01% trypsin in the presence of 1 mM CaCl2 (TC treatment) or 1 mM EGTA (TE treatment) at 37°C for 30 min. Generally, cadherins are left intact after TC treatment, but are digested by TE treatment.
For the cell aggregation assay, cells were dispersed after TC treatment as described by Takeichi (1977). L and R2/7 transfectants were pretreated with 1 μM cytochalasin D in culture medium for 2 h. Although this treatment is necessary for the dissociation of L cells expressing nEαC(509-906) and R2/7 cells expressing αE(1-890), it did not affect cell aggregation of other transfectants. Aliquots of 5 × 105 dissociated cells were plated in each well of a Falcon 12-well plate with 0.5 ml HMF and allowed to aggregate, as described previously (Nagafuchi et al., 1987). The extent of cell aggregation was represented by the index N t/N 0 where N t is the total particle number after incubation time t and N 0 is the total particle number at the initiation of incubation.
For the cell dissociation assay, confluent cultures were treated with TC and TE and dissociated by pipetting 10 times (Nagafuchi et al., 1994). The extent of cell dissociation was represented by N TC/N TE, where N TC and N TE are the total particle number after TC and TE treatment, respectively. In some experiments, confluent cultures were pretreated with 1 μM cytochalasin D in culture medium for 2 h, then cell dissociation analyses were performed in the presence of 1 μM cytochalasin D.
Results
Involvement of α-Catenin in the Recruitment of Vinculin and ZO-1 to Cadherin-based Cell–Cell Adhesion Sites
In L cell transfectants expressing E-cadherin, two cytoskeletal proteins, vinculin and ZO-1, are precisely colocalized with E-cadherin at cell–cell contact sites (Itoh et al., 1993). In parental L cells, vinculin is concentrated exclusively at cell-substrate AJ, and ZO-1 does not show specialized localization but some condensation at tips of cellular processes (data not shown).
To determine whether α-catenin is involved in the recruitment of vinculin and ZO-1 to E-cadherin–based cell adhesion sites, we used L cell transfectants expressing nEα(1-906), which is a fusion molecule consisting of nonfunctional E-cadherin lacking its catenin-binding domain and full-length α-catenin (Fig. 1 B; Nagafuchi et al., 1994). As previously reported, this molecule showed similar cell adhesion and cytoskeleton interaction activities to the normal E-cadherin–catenin complex. Immunocytochemical analysis clearly revealed that both vinculin and ZO-1 were precisely colocalized with nEα(1-906) at cell–cell contact sites in L cell transfectants (Fig. 2). As reported previously, nonfunctional E-cadherin does not interact with the cytoskeleton and nEα(1-906) is not associated with endogenous β-catenin (Nagafuchi and Takeichi, 1988; Ozawa et al., 1989; Nagafuchi et al., 1994). These observations indicated that α-catenin was crucial for the recruitment of vinculin and ZO-1 to cell–cell contact sites in transfected L cells.
Figure 2.
Subcellular localization of vinculin, ZO-1, and nEα(1-906) in transfectants expressing nEα(1-906). Cells were doubly stained with anti-vinculin mAb (a)/anti–E-cadherin (a′) or anti– ZO-1 (b)/anti–E-cadherin (b′) mixture. nEα(1-906) was colocalized with both vinculin and ZO-1. Bar, 25 μm.
Involvement of Residues 327-402 of α-Catenin in the Recruitment of Vinculin
To determine the domain of α-catenin necessary for the recruitment of vinculin or ZO-1, we constructed several expression vectors encoding various E-cadherin–α-catenin fusion molecules, in which distinct domains of α-catenin were deleted (Fig. 1 B). These expression vectors were introduced into mouse L cells, and stable transfectant clones were isolated for each construct. Each mutant molecule expressed in transfectants had the expected apparent molecular mass (Fig. 1 C).
The subcellular localization of vinculin was compared with those of expressed fusion molecules in various transfectants (Fig. 3). nEα(327-906) in which the NH2-terminal 326 residues had been truncated was precisely colocalized with vinculin (Fig. 3, a and a′). However, nEαC(509-906) with a longer NH2-terminal deletion showed no colocalization with vinculin (Fig. 3, b and b′). In cells expressing nEαN(1-508) with truncation of the COOH-terminal 398 residues, expressed fusion molecule was also colocalized with vinculin at sites where it was heavily condensed (data not shown). These observations suggested that residues 327-508 are important for the recruitment of vinculin. Consistently, vinculin was not colocalized with nEα(1-325/ 509-906) lacking residues 326-508 (Fig. 3, c and c′). Residues 326-508 of α-catenin include the direct α-actinin-binding site (325-394 residues) reported previously (Nieset et al., 1997). When this α-actinin-binding site was added to nEα(1-325/509-906), the resultant fusion molecule nEα(1-402/509-906) retained the ability to colocalize with vinculin (Fig. 3, d and d′). Double-immunostaining for E-cadherin and α-actinin revealed that the constructs which recruited vinculin, such as nEα(327-906) and nEα(1-402/509-906), could recruit α-actinin (Fig. 4, a, a′, c, and c′). In contrast, nEα(1-325/509-906) was not colocalized with either vinculin or α-actinin (Fig. 4, b and b′). These results demonstrated that residues 327-402 of α-catenin are crucial for the recruitment of vinculin as well as α-actinin to cadherin-based cell adhesion sites of L cell transfectants (see Fig. 11 A).
Figure 3.

Subcellular localization of vinculin and E-cadherin– α-catenin fusion proteins in transfectants expressing nEα(327-906) (a and a′), nEαC(509-906) (b and b′), nEα(1-325/509-906) (c and c′), and nEα(1-402/509-906) (d and d′). Cells were doubly stained with anti-vinculin mAb (a–d) and anti–E-cadherin mAb (a′–d′) mixture. Arrows indicate the colocalization of fusion molecules with vinculin. Arrowheads indicate the absence of vinculin at cell–cell boundaries where fusion molecules were condensed. Bar, 25 μm.
Figure 4.
Subcellular localization of α-actinin and E-cadherin– α-catenin fusion proteins in transfectants expressing nEα(327-906) (a and a′), nEα(1-325/509-906) (b and b′), and nEα(1-402/ 509-906) (c and c′). Cells were doubly stained with anti–α-actinin mAb (a–c) and anti–E-cadherin mAb (a′–c′). Arrows indicate the colocalization of fusion molecules with α-actinin. Arrowheads indicate the absence of α-actinin at cell–cell boundaries where fusion molecules were condensed. Bar, 25 μm.
Figure 11.
Functional domains of α-catenin in L cell transfectants. (A) Summary of each fusion molecule phenotype with regard to cytoskeleton interaction and cell adhesion activity. The table on the right indicates whether a given fusion protein was colocalized with vinculin and α-actinin (vinculin/α-actinin) or ZO-1 (ZO-1) in transfectants (indicated as + or −). Whether a given fusion protein functioned as a cell adhesion molecule (adhesion activity) is also indicated (+ or −). The states of adhesion activity are indicated as S (strong state) or W (weak state). Heavily, lightly hatched, and cross-hatched boxes were required for vinculin/a-actinin-binding, ZO-1-binding, and cell adhesion activity, respectively. (B) The functional domains identified in this study, i.e., the adhesion-modulation domain (509-643 residues; cross-hatched), ZO-1-binding domain (631-906 residues; lightly hatched), and vinculin/α-actinin-binding domain (327-402 residues; heavily hatched), are indicated. These domains performed their functions independently. Both the adhesion-modulation domain and ZO-1-binding domain were required for fusion molecules to show the strong state of cell adhesion activity.
Involvement of the COOH-terminal Domain of α-Catenin (Residues 631-906) in the Recruitment of ZO-1
To determine the domain(s) of α-catenin involved in the recruitment of ZO-1, we compared the subcellular localization of ZO-1 with that of expressed fusion molecules in transfectants. In contrast with vinculin, ZO-1 was not precisely colocalized with nEαN(1-508) but with nEαC(509-906) (Fig. 5, a, a′, b, and b′). nEα(631-906), which had the longest NH2-terminal deletion and showed no cell adhesion activity (see below), was also colocalized with ZO-1 at cell–cell boundaries (Fig. 5, c and c′), although their condensation at cell–cell boundaries was not as exclusive compared with those in other transfectants. These observations strongly suggested that the COOH-terminal domain of α-catenin (631-906 residues) is crucial for the recruitment of ZO-1 (see Fig. 11 A).
Figure 5.

Subcellular localization of ZO-1 and E-cadherin– α-catenin fusion proteins in transfectants expressing nEαN(1-508) (a and a′), nEαC(509-906) (b and b′), and nEα(631-906) (c and c′). Cells were doubly stained with anti–ZO-1 mAb (a–c) and anti–E-cadherin mAbs (a′–c′). Arrows indicate the colocalization of fusion molecules with ZO-1. Arrowheads indicate the absence of ZO-1 at cell–cell boundaries where fusion molecules were condensed. Bar, 25 μm.
We have reported previously that the NH2-terminal half of ZO-1 (N-ZO-1) directly interacts with α-catenin (Itoh et al., 1997). Therefore, it was expected that N-ZO-1 would directly interact with the COOH-terminal domain of α-catenin. To test this possibility, we produced four GST fusion proteins, GST-α(1-906), GST-αN(1-508), GST- αC(509-906), and GST-α(671-906), which contained the full-length α-catenin, its NH2-terminal half, its COOH-terminal half, or the COOH-terminal 236 residues, respectively (Fig. 6 A). Then, we analyzed in vitro binding abilities of these fusion molecules with recombinant N-ZO-1 produced in Sf9 cells by baculovirus infection. As shown in Fig. 6 B, N-ZO-1 bound to not only GST-α(1-906) but also GST-αC(509-906) and GST-α(671-906), although the binding affinities to the latter two were lower than that to the former. N-ZO-1 did not bind to GST-αN(1-508). These results strongly suggested that the COOH-terminal 276 amino acids (residues 631-906) of α-catenin recruited ZO-1 to cadherin-based cell–cell contact sites through its direct binding to ZO-1 in transfected L cells.
Figure 6.
Association of α-catenin with N-ZO-1 in vitro. (A) Schematic representation of fusion molecules between GST and various domains of α-catenin. The amino acid residues of α-catenin remaining in fusion molecules are shown in parentheses. (B) Detection of proteins bound to GST-α(1-906), GST-αN(1-508), GST-αC(509-906), and GST-α(671-906). GST fusion proteins were bound to glutathione-Sepharose beads, and incubated with the lysate of Sf9 cells expressing N-ZO-1. After washing, GST fusion proteins were eluted together with their binding proteins from the beads with a buffer containing glutathione. Proteins in the eluates were separated by SDS-PAGE followed by Coomassie brilliant blue staining to estimate the amounts of GST fusion protein in each eluate (GST fusion), or followed by immunoblotting with anti–ZO-1 mAb, T8-754, to detect N-ZO-1 bound to GST fusion proteins (Bound N-ZO-1).
Involvement of Residues 509-643 of α-Catenin in Cell Adhesion Activity of Fusion Molecules
We have reported previously that nEαC(509-906) shows similar cell adhesion activity to the normal E-cadherin– catenin complex (Nagafuchi et al., 1994). To determine the domain which is required for this function, we compared the reaggregative properties of L cell transfectants expressing nEα(509-643) and nEα(631-906); the former was an nEαC(509-906) derivative lacking the ZO-1-binding domain, and the latter contained only the ZO-1-binding domain (see Fig. 1 B). Cells expressing nEα(509-643) aggregated as rapidly as those expressing nEαC(509-906). In contrast, the reaggregation of cells expressing nEα(631-906) was indistinguishable from that of parent L cells (Fig. 7 A). These observations suggested that residues 509-643 of α-catenin are required for cell adhesion activity of fusion molecules. Indeed, when residues 509-643 were added to nEαN(1-508), cells expressing the resultant fusion molecule nEα(1-643) aggregated rapidly, although nEαN(1-508) did not (Fig. 7 A). The levels of expression of nEα(631-906) or nEαN(1-508) in the transfectants examined were relatively low among the fusion molecules examined (Fig. 1 C, lanes 4 and 5). However, the reduced level of fusion molecule expression did not seem to be the cause of loss of their cell adhesion activity since L cells expressing similar or lesser amounts of nEα(1-643) still aggregated rapidly (data not shown). These results demonstrated that residues 509-643 of α-catenin are required for cell adhesion activity of E-cadherin–α-catenin fusion molecules in transfected L cells (see Fig. 11 A). We tentatively called this an adhesion-modulation domain (see Fig. 11 B).
Figure 7.

Aggregation and dissociation assay of L cell transfectants. (A) Aggregation of L cells (open circles) and their transfectants expressing nEαC(509-906) (closed circles), nEα(509-643) (open squares), nEα(631-906) (closed squares), nEα(1-643) (open triangles), and nEαN(1-508) (closed triangles). Cells dissociated by TC treatment in the presence of cytochalasin D were allowed to aggregate in the presence of 1 mM Ca2+. The lower value on the ordinate represents the higher degree of aggregation. (B and C) Dissociation of L cell transfectants expressing nEαC(509-906) (black bar), nEα(509-643) (hatched bar), and nEα(1-643) (white bar) in the absence (B) or in the presence (C) of cytochalasin D. The lower value on the ordinate represents the lower degree of dissociation.
The cell adhesion activities of E-cadherin and its α-catenin fusion molecules were reported to be associated with their interactions with the cytoskeleton (Nagafuchi et al., 1994; Sako et al., 1998). For example, about half of the nEαC(509-906) was resistant to extraction with NP-40 (Fig. 8) Interestingly, most of the nEα(509-643) carrying only the adhesion-modulation domain was extracted with NP-40, suggesting that this fusion molecule did not interact with the cytoskeleton (Fig. 8). In contrast, nEαN(1-508) (data not shown; see Nagafuchi et al., 1994) and nEα(631-906) (Fig. 8) did not show cell adhesion activity but interacted with the cytoskeleton as judged from their resistance to NP-40 extraction. In the cell aggregation assay, the disorganization of actin-based cytoskeleton by cytochalasin D treatment essentially did not affect the cell aggregating activity of any of the transfectants (data not shown). These results suggested that cadherin-based cell adhesion activity and cadherin–cytoskeleton interaction are regulated independently by distinct domains of α-catenin.
Figure 8.
Detergent extraction of E-cadherin–α-catenin fusion proteins. Soluble (S) and insoluble (I) fractions derived from 2 × 105 cells expressing nEαC(509-906), nEα(509-643), nEα(631-906), and nEα(1-643) were separated by SDS-PAGE (10%) and immunoblotted with anti–E-cadherin mAb. Several minor bands with lower molecular mass, which may have been degradation products of fusion molecules, were detected. Cell adhesion activity for each transfectants is represented by + or −. Size markers of 200, 116, and 97 kD are indicated on the left.
Requirement of ZO-1-Binding Domain for the Strong State of Cell Adhesion
We demonstrated previously that cells assumed two states of cadherin-based cell adhesion, strong and weak (Takeda et al., 1995). The cell–cell adhesion in the strong state could hardly be dissociated by pipetting, although that in the weak state was easily dissociated. Using the cell dissociation assay, we examined the states of cell adhesion of various L cell transfectants. As reported previously, L cell transfectants expressing nEαC(509-906), which contained both the adhesion-modulation and ZO-1-binding domains of α-catenin, were hardly dissociated in the cell dissociation assay, indicating that these cells showed the strong state of cell adhesion activity (Fig. 7 B; Nagafuchi et al., 1994). Intact E-cadherin or other fusion molecules containing both of these domains also showed the strong state of cell adhesion activity (data not shown; see Fig. 11 A). In contrast, cells expressing nEα(509-643), which contained only the adhesion-modulation domain of α-catenin, were easily dissociated into single cells under the conditions of the cell dissociation assay (Fig. 7 B), indicating that these cells showed the weak state of cell adhesion activity. Interestingly, cells expressing nEα(1-643) lacking only the ZO-1-binding domain also showed the weak state of cell adhesion activity (Fig. 7 B). It should be noted that nEα(1-643) was hardly extracted with NP-40 (Fig. 8), suggesting its interaction with the cytoskeleton probably through the vinculin/α-actinin-binding domain. In the presence of cytochalasin D, nEαC(509-906)L cells were also easily dissociated under the same condition (Fig. 7 C), although the amount of expressed nEαC(509-906) was not altered in the presence or absence of cytochalasin D (data not shown). These results indicated that the ZO-1-binding domain, but not the vinculin/α-actinin-binding domain, in addition to the adhesion-modulation domain was required for the strong state of cell adhesion activity. It was also suggested that the intact actin-based cytoskeleton is required for this type of adhesion activity.
Functions of α-Catenin in Epithelial Cells
We examined the role of α-catenin in epithelial cell adhesion using α-catenin–deficient epithelial cell lines and their transfectants expressing α-catenin deletion mutants. Since cadherin–catenin complex is not colocalized with ZO-1 in epithelial cells, the roles of α-catenin in epithelial cells are expected to be different, at least in some aspects, from those in nonepithelial cells such as L cells. DLD-1/R2/7, abbreviated to R2/7, is an α-catenin–deficient colon carcinoma line. R2/7 transfectants expressing α-catenin deletion mutants were previously reported (Watabe-Uchida et al., 1998). We used four of these transfectants in this study. α-Catenin deletion mutants expressed in these transfectants are shown in Fig. 9 A. Using the cell aggregation assay, we compared the reaggregative properties of R2/7 and its transfectants (Fig. 9 B). R2/7 itself showed aggregation activity which was blocked in the presence of E-cadherin blocking antibodies (data not shown, see Watabe-Uchida et al., 1998). R2/7 transfectants expressing αE(1-890), which is indistinguishable from cells expressing intact α-catenin, aggregated more rapidly than parental R2/7 cells. Not only cells expressing αE(1-325/510-890) but also those expressing αE(1-509), which lacks the adhesion-modulation domain, also aggregated more rapidly than parental R2/7 cells. R2/7 cells expressing αE(1-325) showed similar aggregation activity to the parental cell line R2/7. These results demonstrated that the residues 325-509 including the vinculin/α-actinin-binding domain are also involved in cadherin-dependent cell aggregation activity in R2/7. In the cell dissociation assay, R2/7 was readily dissociated but R2/7 transfectants expressing αE(1-890) were hardly dissociated (Fig. 9 C). Although cells expressing αE(1-325/ 510-890) showed some degree of resistance to pipetting, the strong state of cell adhesion activity was not fully restored (Fig. 9 C). This partial restoration of the strong state of cell adhesion activity was also observed in cells expressing not only αE(1-509) but also αE(1-325) (Fig. 9 C). These results suggested that multiple domains of α-catenin were required for the strong state of cell adhesion activity in R2/7.
Figure 9.
Aggregation and dissociation assay of R2/7 transfectants. (A) Schematic drawing of the mutant α-catenin constructs. The amino acid residues of α-catenin contained in mutant molecules are shown in parentheses. T7 tag is connected at the COOH-terminal end of each mutant. (B) Aggregation of R2/7 cells (open squares) and their transfectants expressing αE(1-890) (open circles), αE(1-325/ 510-890) (closed circles), αE(1-509) (open triangles), and αE(1-325) (closed triangles). Cells dissociated by TC treatment in the presence of cytochalasin D were allowed to aggregate in the presence of 1 mM Ca2+. The lower value on the ordinate represents the higher degree of aggregation. (C) Dissociation of R2/7 (black bar) and its transfectants expressing αE(1-890) (hatched bar), αE(1-325/510-890) (lightly hatched bar), αE(1-509) (dotted bar), and αE(1-325) (white bar). The lower value on the ordinate represents the lower degree of dissociation.
To confirm the importance of adhesion-modulation domain in epithelial cells, we used PC9 cells, a human lung carcinoma cell line lacking α-catenin expression. It was reported that PC9 showed aggregation activity to some extent and that this activity was dependent on E-cadherin– β-catenin complex without α-catenin (Shimoyama et al., 1992). We constructed an expression vector encoding α(1-184/509-643), in which only an adhesion-modulation domain was covalently connected to the NH2-terminal β-catenin-binding domain of α-catenin (Fig. 10 A). This vector was introduced into PC9 cells, and several transfectant clones were isolated. α(1-184/509-643) with the expected size was expressed in the transfectants (Fig. 10 B) and colocalized with E-cadherin–β-catenin complex (data not shown). Cell aggregation assay revealed that cells expressing α(1-184/509-643) aggregated more rapidly and more extensively than parental PC9 cells (Fig. 10 C). These aggregates were readily dissociated into single cells under the dissociation assay conditions (data not shown). These observations indicated that an adhesion-modulation domain is involved in the weak state of cell adhesion activity even in epithelial cell lines.
Figure 10.
Aggregation assay of PC9 transfectants. (A) Schematic drawing of α(1-184/509-643). (B) Expression of α(1-184/ 509-643) molecule. Total lysates derived from 2 × 105 PC9 cells (lane 1) and PC9 cells expressing α(1-184/509-643) (lane 2) were separated by SDS-PAGE and immunoblotted with anti–HA-tag mAb, 12CA5. Size markers of 45 and 36 kD are indicated on the right. (C) Aggregation of PC9 cells (open squares) and PC9 cells expressing α(1-184/509-643) (closed circles). Cells dissociated by TC treatment were allowed to aggregate in the presence of 1 mM Ca2+. The lower value on the ordinate represents the higher degree of aggregation.
Discussion
It is generally accepted that the cadherin–catenin cell adhesion complex plays fundamental roles not only in the formation of cell–cell junctions but also in the morphogenesis of tissue and organs, dependent on its strong state of cell–cell adhesion activity and its interaction with the actin-based cytoskeleton (Takeichi, 1991). We identified three distinct functional domains of α-catenin required for the interaction with vinculin, for direct binding to ZO-1, and for the adhesion activity of E-cadherin–α-catenin fusion molecules (Fig. 11 B). Here, we will discuss possible functions of each domain of α-catenin and the relationship between the cell adhesion activity and the interaction of cadherin–catenin complex with the cytoskeleton. We will also discuss the difference of α-catenin function in nonepithelial and in epithelial cells.
Functional Domains of α-Catenin
ZO-1-Binding Domain.
α-Catenin was reported to be directly associated with β-catenin, α-actinin, and actin filaments, and the domains responsible for their binding have been narrowed down on the α-catenin molecule (Rimm et al., 1995; Nieset et al., 1997; Obama and Ozawa, 1997). It was reported recently that ZO-1 bound to α-catenin directly (Itoh et al., 1997), but the domain responsible remained elusive. In this study, using deletion constructs, we showed that the COOH-terminal domain (residues 631-906) of α-catenin recruited ZO-1 to the cell adhesion sites and directly bound to NH2-terminal half of ZO-1 in vitro. We also demonstrated that this ZO-1-binding domain interacted with cytoskeletons judging from the resistance of fusion molecules to NP-40 extraction. It was reported previously that the COOH-terminal halves of ZO-1 are directly associated with actin filaments (Itoh et al., 1997). Based on these properties, we can imagine that the ZO-1-binding domain interact with the actin-based cytoskeleton through ZO-1. We demonstrated that this domain is essential for the strong state of cadherin-based cell adhesion in L cell transfectants, which was dependent on the intact actin-based cytoskeleton. The functional importance of the ZO-1-binding domain was also reported using mouse embryos expressing mutant α-catenin (Torres et al., 1997). Taken together, we concluded that the ZO-1-binding domain (COOH-terminal 276 residues) of α-catenin plays a fundamental role in the cadherin–catenin cell adhesion system probably through its interaction with ZO-1 and/or the actin-based cytoskeleton.
We cannot exclude the possibility that other cytoskeletal proteins interact with the ZO-1-binding domain. This domain is known to interact with actin in vitro (Rimm et al., 1995). The physiological role of this interaction remains to be elucidated. The ZO-1-binding domain was also shown to directly interact with vinculin in vitro (Weiss et al., 1998). However, we found that the ZO-1-binding domain in fusion molecules did not recruit vinculin to the cell–cell boundaries in L cell transfectants. Since the reported binding constant of the ZO-1-binding domain to vinculin was lower than that to ZO-1 (Itoh et al., 1997; Weiss et al., 1998), the binding of vinculin to the ZO-1-binding domain may be prevented by ZO-1 in L cell transfectants. ZO-2 and ZO-3, homologues of ZO-1, are other candidates as binding proteins to the ZO-1-binding domain. In fact, it was reported recently that ZO-2 showed very similar properties with ZO-1 and directly bound to α-catenin (Itoh et al., 1999). However, these proteins are not involved in the cadherin-based cell adhesion in L cell transfectants, since their expression was not detected in L cell transfectants (our unpublished observation).
Domain Required for the Recruitment of Vinculin.
We found that residues 327-402 of α-catenin were required for fusion molecules to recruit not only α-actinin but also vinculin in transfected L cells. This is consistent with previous data that this domain directly binds to both vinculin (Watabe-Uchida et al., 1998) and α-actinin (Nieset et al., 1997), and these two molecules interact with each other (Wachsstock et al., 1987). It is not clear whether vinculin and α-actinin interact with this short domain with 76 residues simultaneously or competitively in vivo. E-cadherin– α-catenin fusion molecules conferred full adhesion activity in L cell transfectants even if they lacked the vinculin/ α-actinin-binding domain. This raised the question of what is the function of the vinculin/α-actinin-binding domain. We reported previously that intact E-cadherin conferred a flexible adhesive phenotype upon L cells, but E-cadherin– α-catenin fusion molecules conferred inflexible phenotypes (Nagafuchi et al., 1994). If the vinculin/α-actinin-binding domain is involved in this flexible adhesion activity, its function would not be observed using E-cadherin– α-catenin molecules expressed in L cells. On the other hand, it was reported recently that this domain is involved in the organization of apical junctional complex and the activation of cadherin-based cell adhesion in epithelial cells (Watabe-Uchida et al., 1998). It has been reported also that vinculin is colocalized with the cadherin–catenin complex in epithelial cells but not in some fibroblastic cell lines (Knudsen et al., 1995), and that vinculin is one of the major components of cell–cell AJ in epithelial cells (Geiger et al., 1980). These observations suggest that the vinculin/α-actinin-binding domain is required for the function of cadherin–catenin complex, especially for junctional complex formation, only in epithelial cells but not in fibroblastic cells.
Functional Domain Involved in Cell Adhesion Activity.
Deletion constructs showed that residues 509-643 of α-catenin are required for fusion molecules to function as cell adhesion molecules. We tentatively called this domain an adhesion-modulation domain. When mutant α-catenin containing the β-catenin-binding and the adhesion-modulation domains was expressed in α-catenin–deficient PC9 cells, such cells aggregated more rapidly than parental PC9 cells. These results suggested that the adhesion-modulation domain is involved in cell adhesion in the “natural” cadherin/catenin complex.
Although several sites of α-catenin were reported to be required for the interaction with the cytoskeleton, the adhesion-modulation domain does not correspond to these cytoskeletal interaction sites. Moreover, the fusion molecule carrying only this domain was easily extracted with NP-40, suggesting that this molecule did not interact with the cytoskeleton. These findings indicated that the adhesion-modulation domain might function without the interaction with the cytoskeleton. It has been accepted that the insoluble fraction of E-cadherin was active in cell adhesion and the soluble one was not, since E-cadherin molecules which could not be extracted with NP-40 were strictly localized at cell–cell contact sites (Nagafuchi and Takeichi, 1988). However, our present results suggested that some fraction of soluble E-cadherin–catenin complex was also active in cell adhesion.
The molecular mechanism of the activation of E-cadherin extracellular domain remains unclear. One simple interpretation is that the adhesion-modulation domain supports the lateral aggregation of E-cadherin molecules, which may mediate the weak state of cell adhesion. Alternatively, this domain may trigger off the other adhesion-modulation system. Fusion molecules used contained the membrane proximal, p120-binding domain of E-cadherin (Yap et al., 1998) and are colocalized with endogenous p120 protein in transfected L cells (our unpublished observation). It was reported that this membrane proximal domain might positively or negatively regulate cadherin-based cell adhesion (Ozawa and Kemler, 1998; Yap et al., 1998). It is possible that the adhesion-modulation domain of α-catenin affects the potential activity of the membrane proximal domain of E-cadherin.
Some fusion molecules lacking the adhesion-modulation domain were detected at cell–cell boundaries in transfectants, although nonfunctional E-cadherin itself was not (Nagafuchi and Takeichi, 1988). These observations raised the question of how these fusion molecules were condensed at cell–cell boundaries, although they did not function as cell adhesion molecules. The main difference between these fusion molecules and nonfunctional E-cadherin is that the former interacted with cytoskeletal proteins such as ZO-1 or vinculin/α-actinin but the latter did not. As discussed below, ZO-1 is likely to facilitate the lateral aggregation of its membrane binding partners. Vinculin is also expected to form clusters through its interaction with various cytoskeletal components (Otto, 1990). So, the interaction with cytoskeletal components may cause the clustering of fusion molecules in the plasma membrane, which then induces the association of cadherin complexes on apposed cell membranes (Shapiro et al., 1995).
Relationship between Interaction with the Cytoskeleton and Adhesion Activity of Cadherin–Catenin Complex
As previously reported, cadherin-based cell adhesion can be classified into the strong state and the weak state, using cell dissociation and aggregation assays (Takeda et al., 1995). In the cell aggregation assay, cells form aggregates in both adhesive states. In the cell dissociation assay, however, cells in the strong state were hardly dissociated into single cells but those in the weak state were dissociated readily. It is known that the adhesive state is regulated by the phosphorylation level of the cytoplasmic components (Matsuyoshi et al., 1992; Behrens et al., 1993). We also demonstrated that disorganization of the actin-based cytoskeleton shifted the cadherin-based cell adhesion from the strong to the weak state. Since the level of cadherin expression on the cell surface was not affected in either case, the strong state and the weak state may reflect qualitative differences in cell adhesion activity but not quantitative differences in cell adhesion molecules. The present results are consistent with this idea, since cells in both adhesive states aggregated in a similar manner.
We found that all of the fusion molecules that showed the strong state of cell adhesion activity interacted with the cytoskeleton, suggesting that the interaction with the cytoskeleton is required for the strong state of cell adhesion activity. This was supported by the present observation that cytochalasin D treatment shifted cadherin-based cell adhesion to the weak state. Interestingly, the fusion molecule lacking only the ZO-1-binding domain showed the weak state of cell adhesion activity, although it contained a vinculin/α-actinin-binding domain and may interact with the cytoskeletons, judging from the refractoriness to NP-40 extraction. Thus, we concluded that the cytoskeleton interaction through the ZO-1-binding domain, but not through the vinculin/α-actinin binding domain or other domain(s), is required for the strong state of cell adhesion activity in L cell transfectants. As discussed above, ZO-1 and actin are possible binding proteins to this domain in L cell transfectants. ZO-1 is a member of the MAGUK family. Another member of this family, PSD95, is known to facilitate the lateral aggregation of its membrane binding partners such as NMDA receptors and K+ channels (Kim et al., 1995; Niethammer et al., 1996). Thus, it is possible that ZO-1 strengthens the cell–cell adhesion activity not only by cross-linking α-catenin to actin filaments (Itoh et al., 1997) but also by facilitating the lateral aggregation of E-cadherin or its fusion molecules in L cell transfectants. The role of actin binding to this domain remains unclear. Furthermore, we cannot exclude the possibility that unknown factor(s) interacted with the ZO-1-binding domain and supported the strong state of cell adhesion activity.
Function of α-Catenin in Epithelial Cells
At the immunoelectron microscopic level, cadherin–catenin complex is known to be colocalized with ZO-1 in nonepithelial cells such as L cells but not in epithelial cells. In epithelial cells, ZO-1 is highly condensed at the TJ and is thought to directly interact with TJ membrane proteins including occludin (Furuse et al., 1994). These results suggested that the ZO-1-binding domain of α-catenin may have different functions in nonepithelial cells and in epithelial cells. In fact, it was demonstrated that this domain could not cause redistribution of ZO-1 in R2/7, an epithelial colon carcinoma cell line (Watabe-Uchida et al., 1998). It remains unclear why the ZO-1-binding domain does not associate with ZO-1 and how this domain functions in epithelial cells. Further studies to address this question will provide important information regarding the mechanisms of junctional complex formation in epithelial cells.
It was reported recently that the vinculin/α-actinin-binding domain directly binds to vinculin and this interaction functions to organize the apical junctional complex, including TJ, in R2/7 cells (Watabe-Uchida et al., 1998). This domain, instead of the ZO-1-binding domain, functions to recruit ZO-1 at cell–cell boundaries in R2/7 cells. Since we demonstrated that this domain did not interact with ZO-1 directly, the redistribution of ZO-1 may be dependent on the reorganization of TJ caused by α-catenin–vinculin interaction in R2/7 cells. This is consistent with the previous observation that, when the vinculin/α-actinin binding domain was replaced with the vinculin tail domain, the resultant α-catenin–vinculin fusion molecule recruited ZO-1 to cell–cell boundaries (Watabe-Uchida et al., 1998).
The role of α-catenin in epithelial cell adhesion also seems to be different from that in nonepithelial cells, although the adhesion-modulation domain seems to be generally used in nonepithelial and epithelial cells. We found that the ZO-1-binding domain, which cannot cause redistribution of ZO-1, also failed to fully restore the strong state of cell adhesion activity in epithelial cells, supporting our speculation that ZO-1 is required for the strong state of cell adhesion activity. In epithelial cells, both the NH2-terminal half and COOH-terminal half domains are partially involved in the strong state of cell adhesion activity and these two domains cooperatively support this activity. Thus, α-catenin in epithelial cells supports the strong state of cell adhesion activity in a different manner from that in nonepithelial cells. In fact, it was reported recently that the COOH-terminal 236 residues, the in vitro binding domain to ZO-1, were not involved in the cadherin-based cell adhesion activity in epithelial cells, although the COOH- terminal 273 residues of α-catenin were involved in this activity (Ozawa, 1998). The 37 residues between 634 and 670 of α-catenin were not involved in the adhesion activity or binding to ZO-1 in L cell transfectants (our unpublished observation). The mechanism by which α-catenin is involved in these processes in epithelial cells remains to be elucidated.
Of course, here, we cannot completely exclude the possibility that differences of α-catenin functions observed in L cells and R2/7 cells are not due to differences of the cell type (nonepithelium versus epithelium) but that of the expressed molecule (fusion versus nonfusion). However, our recent observations using α-catenin–deficient F9 cells did not favor this possibility (Maeno, Y., and A. Nagafuchi, unpublished observations).
In this study, we identified three functional domains of α-catenin, i.e., the ZO-1-binding domain, vinculin/α-actinin-binding domain and adhesion-modulation domain. We also clarified the effects of the cytoskeleton interaction on the different states of cadherin-based cell adhesion activity. Furthermore, we demonstrated that α-catenin may have different functions in nonepithelial and epithelial cells. Further studies of the regulatory mechanism of cadherin-based cell adhesion by α-catenin will lead to a better understanding of the physiological functions of the cadherin–catenin complex which plays a pivotal role in morphogenesis in multicellular organisms.
Acknowledgments
We would like to thank all the members of our laboratory for their helpful discussions. Our thanks are also due to Drs. M. Takeichi, M. Watabe-Uchida, and S. Aono for their generous gift of anti–E-cadherin mAb, ECCD-2, R2/7 transfectants, and helpful discussions.
This work was supported in part by a Grant-in-Aid for Cancer Research and a Grant-in-Aid for Scientific Research (to A. Nagafuchi, M. Itoh, and S. Tsukita) from the Ministry of Education, Science, Sports and Culture of Japan, and by Special Coordination Funds for promoting Science and Technology (to A. Nagafuchi) from the Science and Technology Agency of Japan.
Abbreviations used in this paper
- AJ
adherens junctions
- TJ
tight junction
References
- Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988;106:1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Tokuyasu KT, Dutton AH, Singer SJ. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci USA. 1980;77:4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R. The uvomorulin-anchorage protein α catenin is a vinculin homologue. Proc Natl Acad Sci USA. 1991;88:9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol. 1987;105:2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992;70:293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Itoh M, Yonemura S, Nagafuchi A, Tsukita S, Tsukita S. A 220-kD undercoat-constitutive protein: its specific localization at cadherin-based cell–cell adhesion sites. J Cell Biol. 1991;115:1449–1462. doi: 10.1083/jcb.115.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani YT, Tsukita S, Tsukita S. The 220-kD protein colocalizing with cadherins in nonepithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to α catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and α catenin. J Biol Chem. 1999;274:5981–5986. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- Jockusch BM, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rudiger M, Schluter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- Katoh K, Takahashi Y, Hayashi S, Kondoh H. Improved mammalian vectors for high expression of G418 resistance. Cell Struct Funct. 1987;12:575–580. doi: 10.1247/csf.12.575. [DOI] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell–cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell–cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO (Eur Mol Biol Organ) J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Tsukita S, Takeichi M. Transmembrane control of cadherin-mediated cell-cell adhesion. Semin Cell Biol. 1993;4:175–181. doi: 10.1006/scel.1993.1021. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin–α-catenin fusion molecules. J Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ. Characterization of the interactions of α-catenin with α-actinin and β-catenin/plakoglobin. J Cell Sci. 1997;110:1013–1022. doi: 10.1242/jcs.110.8.1013. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Obama H, Ozawa M. Identification of the domain of α-catenin involved in its association with β-catenin and plakoglobin (γ-catenin) J Biol Chem. 1997;272:11017–11020. doi: 10.1074/jbc.272.17.11017. [DOI] [PubMed] [Google Scholar]
- Otto JJ. Vinculin. Cell Motil Cytoskeleton. 1990;16:1–6. doi: 10.1002/cm.970160102. [DOI] [PubMed] [Google Scholar]
- Ozawa M. Identification of the region of α-catenin that plays an essential role in cadherin-mediated cell adhesion. J Biol Chem. 1998;273:29524–29529. doi: 10.1074/jbc.273.45.29524. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Kemler R. The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity. J Cell Biol. 1998;142:1605–1613. doi: 10.1083/jcb.142.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO (Eur Mol Biol Organ) J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako Y, Nagafuchi A, Tsukita S, Takeichi M, Kusumi A. Cytoplasmic regulation of the movement of E-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: corralling and tethering by the membrane skeleton. J Cell Biol. 1998;140:1227–1240. doi: 10.1083/jcb.140.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Nagafuchi A, Fujita S, Gotoh M, Takeichi M, Tsukita S, Hirohashi S. Cadherin dysfunction in a human cancer cell line: possible involvement of loss of α-catenin expression in reduced cell-cell adhesiveness. Cancer Res. 1992;52:5770–5774. [PubMed] [Google Scholar]
- Shirayoshi Y, Nose A, Iwasaki K, Takeichi M. N-linked oligosaccharides are not involved in the function of a cell-cell binding glycoprotein E-cadherin. Cell Struct Funct. 1986;11:245–252. doi: 10.1247/csf.11.245. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrens J, Birchmeier W, Tsukita S. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and β catenin is not required for the shift. J Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977;75:464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Torres M, Stoykova A, Huber O, Chowdhury K, Bonaldo P, Mansouri A, Butz S, Kemler R, Gruss P. An α-E-catenin gene trap mutation defines its function in preimplantation development. Proc Natl Acad Sci USA. 1997;94:901–906. doi: 10.1073/pnas.94.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Submembranous junctional plaque proteins include potential tumor suppressor molecules. J Cell Biol. 1993;123:1049–1053. doi: 10.1083/jcb.123.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, Elefanty AG, Strasser A, Adams JM. GATA-1 but not SCL induces megakaryocytic differentiation in an early myeloid line. EMBO (Eur Mol Biol Organ) J. 1992;11:4557–4564. doi: 10.1002/j.1460-2075.1992.tb05557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsstock DH, Wilkins JA, Lin S. Specific interaction of vinculin with α-actinin. Biochem Biophys Res Commun. 1987;146:554–560. doi: 10.1016/0006-291x(87)90564-x. [DOI] [PubMed] [Google Scholar]
- Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell–cell association and retardation of growth by activation of the E-cadherin–catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, Takeichi M. α-catenin–vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EE, Kroemker M, Rudiger AH, Jockusch BM, Rudiger M. Vinculin is part of the cadherin–catenin junctional complex: complex formation between α-catenin and vinculin. J Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]