Abstract
Nonsymbiotic hemoglobins are broadly present across the plant kingdom; however, the function of these proteins is unknown. Cultured maize cells have been transformed to constitutively express a barley hemoglobin gene in either the sense (HB+) or antisense (HB−) orientation. Hemoglobin protein in the transformed cell lines correspondingly was higher or lower than in wild-type cells under normal atmospheric conditions. Limiting oxygen availability, by placing the cells in a nitrogen atmosphere for 12 hr, had little effect on the energy status of cells constitutively expressing hemoglobin, but had a pronounced effect on both wild-type and HB− cells, where ATP levels declined by 27% and 61%, respectively. Total adenylates in these cells were approximately 35% lower than in HB+ cells. Energy charge was relatively unaffected by the treatment in HB+ and wild-type cells, but was reduced from 0.91 to 0.73 in HB− cells, suggesting that the latter were incapable of maintaining their energy status under the low oxygen regime. Treatment of the cells grown in an air atmosphere with antimycin A gave essentially the same results. It is suggested that nonsymbiotic hemoglobins act in plants to maintain the energy status of cells in low oxygen environments and that they accomplish this effect by promoting glycolytic flux through NADH oxidation, resulting in increased substrate-level phosphorylation. Hypoxic acclimation of plants is an example of this effect in nature. Nonsymbiotic hemoglobins are likely ancestors of an early form of hemoglobin that sequestered oxygen in low oxygen environments, providing a source of oxygen to oxidize NADH to provide ATP for cell growth and development.
Hemoglobins are widespread throughout the biosphere (1). They are found in a broad range of organisms from bacteria, through unicellular eukaryotes, to plants and animals, suggesting that they predate divergence of life into plant and animal forms. Plant hemoglobins have been classified into symbiotic and nonsymbiotic types (2). Symbiotic hemoglobins are found in plants that are capable of participating in microbial symbioses, where they function in regulating oxygen supply to nitrogen-fixing bacteria. Nonsymbiotic hemoglobins only recently have been discovered and are thought to be evolutionary predecessors of the more specialized symbiotic leghemoglobins. The ubiquitous nature of nonsymbiotic hemoglobins is evidenced by their broad presence across the plant kingdom (3). The widespread presence and long evolutionary history of plant hemoglobins suggest a major role for them in the life of plants. Very little, however, is known about their function, although it has been proposed that nonsymbiotic hemoglobins may act either as oxygen carriers to facilitate oxygen diffusion or oxygen sensors to regulate expression of anaerobic proteins during periods of low oxygen supply. The proteins from barley (4) and rice (5) and AHB1 from Arabidopsis (6) have been shown to have high oxygen avidity, with dissociation constants for oxyhemoglobin of 2.86 nM, 0.55 nM, and 1.6 nM, respectively, resulting in conditions whereby the free protein will remain oxygenated at oxygen concentrations far below those at which anaerobic processes are activated. It is, thus, unlikely that these hemoglobins would function as either facilitators of oxygen diffusion or sensors of oxygen, unless the oxygen avidity was modified by interaction with another component within the cell. In this paper, we provide evidence to suggest that nonsymbiotic hemoglobins function to maintain the energy status of cells exposed to low oxygen tensions and that this property may be a common feature of plant cells, either during exposure to hypoxia or under high energy demand.
MATERIALS AND METHODS
Cell Cultures.
Black Mexican Sweet (BMS), HB+, and HB− maize cells were cultured in 250-ml flasks as cell suspensions in 50 ml of MS medium (7) macro- and microelements supplemented with 0.5 mg/liter of thiamine, 150 mg/liter of l-asparagine, 2 mg/liter of 2,4-D, and 20 g/liter of sucrose. Cultures were shaken at 150 rpm at 25°C. Cells were subcultured every 7 days. Nitrogen treatment was applied by replacing air in culture flasks with nitrogen and closing the flasks with rubber stoppers; otherwise, culture flasks were closed with caps, allowing for free exchange of air. Antimycin-A was added as a 27-mM stock solution in 2-propanol to give final concentration of 0.2 mM. Cell samples were collected by filtration. Cell samples used for adenylate measurements were frozen immediately in liquid nitrogen and stored at −80°C until used.
Construction of Vectors.
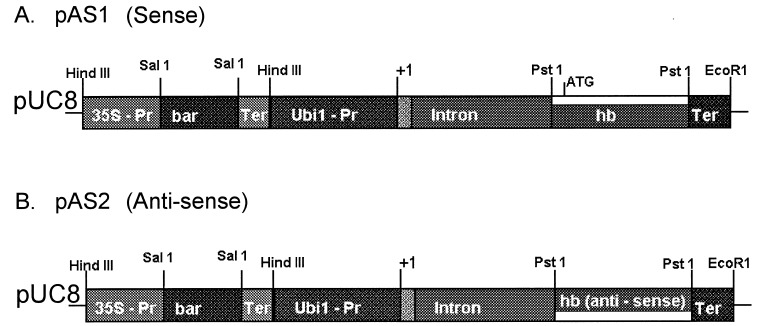
SalI/NotI-digested and end-filled barley hemoglobin cDNA was cloned into BamHI-digested and end-filled pAHC17 plasmid (8) in sense and antisense orientation to generate pAS1 (sense) and pAS2 (antisense) plasmids. An EcoRI-digested, end-filled 1.35-kb fragment from pDB1 (9), possessing a synthetic HindIII linker and containing a 35S promoter-bar gene-35S terminator, HindIII-digested pAS1 and pAS2.
Transformation and Selection.
A silicon carbide fibers-mediated transformation system was used as described (10) to transform BMS maize cells with pAS1 and pAS2 vectors. Resistant colonies were selected on culture medium solidified with 0.2% Phytagel (Sigma) and supplemented with 5 mg/liter of glufosinate ammonium.
Protein Immunoblots.
SDS/PAGE, protein transfer to nitrocellulose membrane, and antibody detection were performed according to standard Bio-Rad protocol (Bio-Rad bulletin 1721). Hemoglobin protein in transformed lines was detected by immunoblots, by using a polyclonal antibody raised against barley-recombinant hemoglobin. Protein concentration was calculated by densitometric comparison of immunoblots (in four repetitions) with a standard curve of known concentrations of recombinant hemoglobin by using a Sharp Diversity 1, PDI-325OE scanner.
Measurements.
Culture growth was measured by sedimentation in 25-ml graduated pipettes. Adenylates were extracted in 1 M perchloric acid from frozen cell samples at −10°C, and ATP, ADP, and AMP were assayed spectrophotometrically by established protocols as described (11).
Alcohol dehydrogenase activity was measured, in ethanol-acetaldehyde direction, in fresh cell extracts. Enzyme extraction and spectrophotometric measurements were performed as described previously (12).
For measurements of CO2 evolution from cell cultures, 1-ml gas samples were collected with an air-tight syringe, from stoppered culture flasks, and analyzed by gas chromatography (Shimadzu GC-8AIT).
Oxygen uptake was measured polarographically with an O2 electrode (Rank Brothers, Cambridge, U.K.) for 5–30 min. The incubation cell contained 2 ml of culture medium and 0.2 ml (sedimented cell volume) of cells. In some measurements 0.2 mM antimycin A was added.
RESULTS
We have transformed cultured maize (var. BMS) cells with a barley hemoglobin gene to observe the effect of increasing or decreasing hemoglobin expression on cell metabolism. Transformation vectors (Fig. 1) were prepared containing the ORF of a barley hemoglobin cDNA in sense and antisense orientations, which were placed under the control of a strong constitutive maize ubiquitin (Ubi1) promoter. A herbicide-resistance gene (Bar), conferring resistance to glufosinate ammonium, was cloned head to tail with the hemoglobin gene constructs to enable selection of transformed cell lines. Twenty-four independently transformed sense (pAS1) and 38 antisense (pAS2) lines were obtained. Transformation was confirmed by Southern blot analysis and PCR. A sense line (HB+) expressing hemoglobin at levels 10-fold higher than wild type (BMS) and an antisense line (HB−) with 10 times lower expression of hemoglobin than BMS (Fig. 2) were selected for further studies.
Figure 1.
Schematic representation of plasmids used in transformation experiments.
Figure 2.
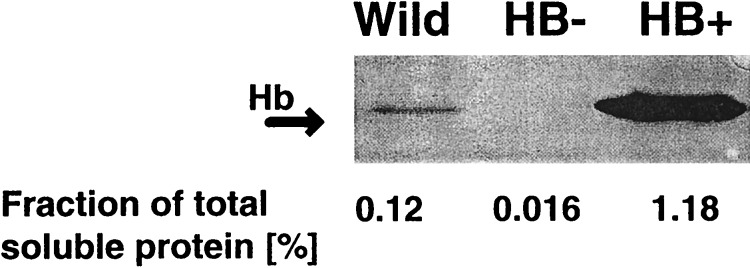
Protein immunoblot analysis of hemoglobin expression in wild-type (BMS), HB+, and HB− maize cells with recombinant barley hemoglobin-specific antibody (17). Protein (100 μg) in crude cell extract was loaded into each lane. Arrow indicates an 18.5-kDa hemoglobin band. Numbers below the lanes represent concentration of hemoglobin as a fraction of total soluble protein.
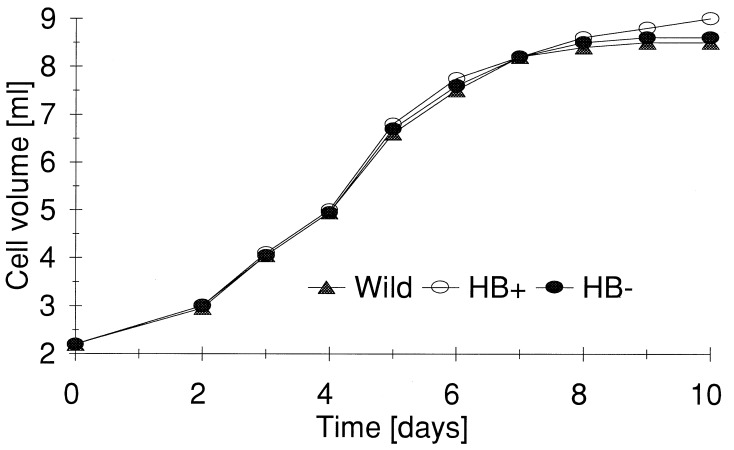
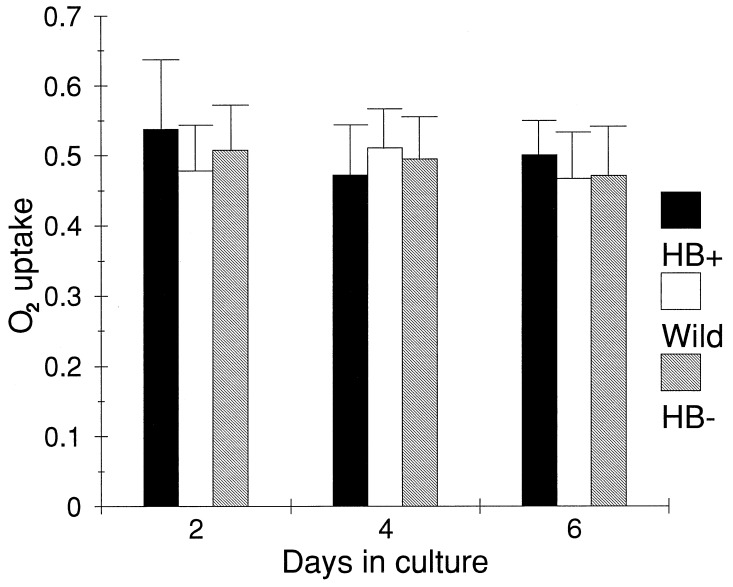
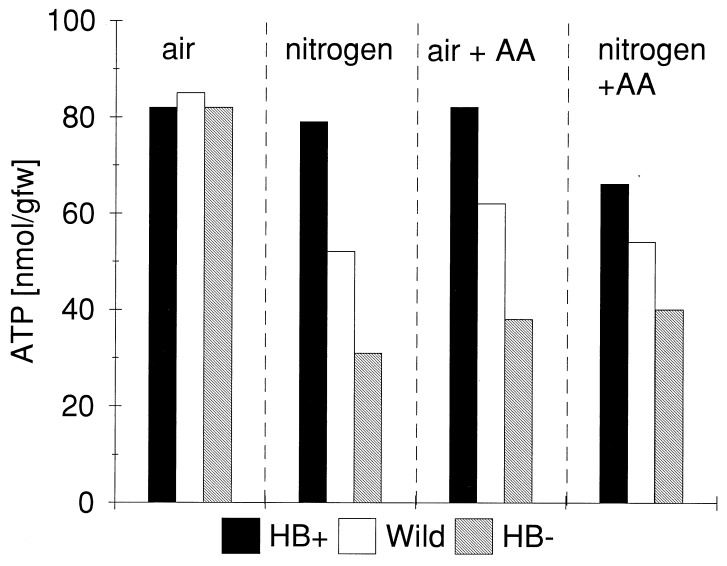
The three cell lines, grown in an air environment, did not differ significantly from one another with respect to culture growth rates (Fig. 3) and consumption of oxygen (Fig. 4). Furthermore, steady-state ATP levels essentially were the same in the three types of cells (Fig. 5). After incubating the cells for a further 12 hr under an atmosphere of nitrogen gas, significant differences were observed in the ATP levels of the cell types. The level of ATP was highest in HB+ cells, being only marginally lower than under normal atmospheric conditions. ATP levels in wild-type (BMS) cells were 27% lower than HB+ cells, whereas those in HB− cells were 61% lower. Differences in energy charge and total adenylates also were observed in cells exposed to nitrogen atmospheres (Table 1). Energy charge was relatively the same in all three cell types under normal atmospheric conditions and in BMS and HB+ cell lines after 12 hr of a nitrogen atmosphere. HB− cells, on the other hand, were unable to maintain energy charge during the 12-hr exposure to a nitrogen atmosphere. Total adenylates remained the same in all three cell lines under atmospheric conditions and in HB+ cells in a nitrogen atmosphere. In BMS and HB− cells, however, the total adenylates declined by about 35%.
Figure 3.
Culture growth of wild-type (BMS), HB+, and HB− maize cell lines under normal air conditions.
Figure 4.
Oxygen uptake by maize wild-type (BMS), HB+, and HB− cells calculated as μmol O2⋅min−1⋅ml−1 cells ± SE (n = 3).
Figure 5.
Levels of ATP in wild-type (BMS), HB+, and HB− maize cells grown under normal atmospheric conditions and after 12 hr of treatment with N2, antimycin-A (0.2 mM), and N2 in combination with antimycin-A. SE (n = 3) in all measurements was less than 4%.
Table 1.
Energy charge and total adenylates in maize cells before and after exposure to a nitrogen atmosphere for 12 hr
| Cell line | Energy charge
|
Total adenylates, nmol/g fresh weight
|
||
|---|---|---|---|---|
| Air | Nitrogen | Air | Nitrogen | |
| HB− | 0.93 | 0.93 | 96 | 92 |
| Wild | 0.94 | 0.93 | 94 | 61 |
| HB− | 0.91 | 0.73 | 99 | 59 |
Results are expressed as nmol/g fresh weight. Maximum SE (n = 3) was 5%.
We have attempted to determine what part of the cell’s metabolism contributes to this increased ability to maintain energy status in the presence of hemoglobin. To examine the possibility that hemoglobin might provide oxygen to generate ATP via cytochrome-mediated respiratory processes, we used antimycin A (0.2 mM), which blocks mitochondrial electron transport in the span from cytochrome b to c and has been shown to induce hemoglobin expression in barley aleurone layers (13). Antimycin A inhibited 80% of the oxygen uptake by maize cells within 30 min of treatment. After 12 hr of exposure to antimycin A in an air environment, ATP levels in the three cell types were similar to those of untreated cells after 12 hr under nitrogen (Fig. 5). Upon placing antimycin A-treated cells in a nitrogen atmosphere for 12 hr, the cell lines all showed decreases in ATP, but, consistent with the previous experiments, the levels of ATP decreased in the order HB+, BMS, and HB−. This provides evidence that the increase in ATP brought about by the presence of hemoglobin was not the result of cytochrome-mediated mitochondrial respiration. It is also unlikely that the increased ATP is the result of oxyhemoglobin supporting mitochondrial alternative-oxidase activity, which would increase substrate phosphorylation through glycolysis.
CO2 evolution from hypoxic HB+ cells was 20–30% lower than CO2 levels evolved from BMS or HB− cells (Fig. 6), which would not be anticipated if the Krebs cycle was being maintained through alternative oxidase activity.
Figure 6.
CO2 evolution by maize cells cultured under an N2 atmosphere. Results were calculated as mg CO2 evolved per g fresh weight of cells ± SE (n = 3).
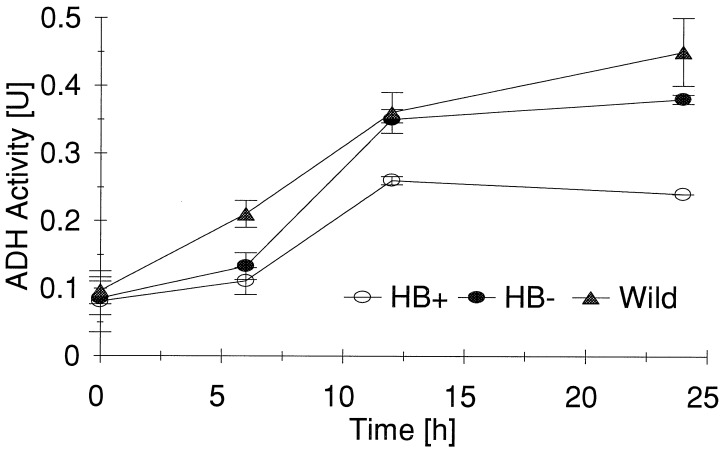
An examination of alcohol dehydrogenase activity (ADH) in the cell lines showed that ADH increased in all three lines over the course of the experiments, but the ADH activity was significantly lower in the sense transformants (HB+) than in antisense transformants (HB−) or wild-type cells (Fig. 7). Fluorescein diacetate staining (14) showed no difference in the viability of the cells lines at the end of the incubation period. The reduced ADH activity along with lower CO2 evolution in HB+ cells likely reflects lower ethanolic fermentation rates, suggesting that a fermentative pathway may be the main source of carbon dioxide production in this system.
Figure 7.
Activity of alcohol dehydrogenase in maize cells cultured under N2 atmosphere. Results are expressed as μmol⋅min−1⋅mg−1 protein [U] ± SE (n = 3).
DISCUSSION
Higher plant hemoglobins are cytoplasmic proteins (1). With this in mind, transformation constructs were designed for cytoplasmic expression of hemoglobin. Barley hemoglobin cDNA hybridizes to only one locus in barley and maize genomes (15), and, therefore, sense and antisense expression of this cDNA would not be expected to affect the expression of any other genes. The polyclonal antihemoglobin that was used in our experiments was raised and titrated against recombinant barley hemoglobin. It is, thus, possible that our assessments underestimate the amount of hemoglobin in all three cell lines. It is clear, however, that there is over- and underexpression of hemoglobin in the transgenic cells.
The lack of effect of hemoglobin on cell growth and oxygen uptake under normal air conditions likely reflects the fact that barley (15) and maize (unpublished data) hemoglobin genes are induced under conditions of limited oxygen availability, resulting in the protein having little effect when oxygen supplies are not impaired. The results, however, show clearly that the energy status of maize cells when oxygen is limiting is affected by the ability of the cells to produce hemoglobin. Total adenylates and ATP levels are maintained during the period of exposure to limiting oxygen when hemoglobin is constitutively expressed in the cells. Alternatively, when hemoglobin expression is suppressed by constitutive expression of antisense barley hemoglobin message, the cells are unable to maintain their energy status during oxygen limitation. In wild-type (BMS) cells, it would appear that the induction of native maize hemoglobin was sufficient to maintain the energy charge, but not the total adenylate pool. A decline in the adenylate pool has been noted during hypoxia in maize root tips (16). Under limiting oxygen, plant cells turn their metabolism toward fermentation to oxidize the NADH that is necessary to maintain glycolytic substrate phosphorylation. Lower alcohol dehydrogenase activity in HB+ cells suggests that hemoglobin provides an alternative to potentially harmful fermentation. Carbon dioxide is produced by the HB+ in lower amounts than by HB− and wild-type maize cells, reflecting lower ADH activity and suggesting that the ethanolic fermentation is the only source of CO2. The dissociation constant of barley oxyhemoglobin is about 3 nM (4), indicating that oxyhemoglobin acting alone would be ineffective in providing oxygen to maintain mitochondrial respiratory processes. This is confirmed by the observation that antimycin A has no effect on the ability of hemoglobin-containing cells in maintaining their energy status under low oxygen tensions. Our results suggest that hemoglobin maintains energy status of the cell by means different from mitochondrial oxidative phosphorylation, probably by facilitating glycolysis to generate ATP through substrate-level phosphorylation.
Hemoglobins of barley (15) and maize (I. R. Silva and R.D.H., unpublished data) as well as Arabidopsis AHB1 (6) are hypoxia-inducible. It has been demonstrated that in barley hemoglobin, this is not a result of a lack of oxygen per se, but a response to insufficient mitochondrial ATP synthesis. Nonsymbiotic hemoglobins are expressed in metabolically active tissues such as roots (5, 6, 15), aleurone (15), vascular tissues of leaves, stems, and seedling cotyledons (17). Taken together, the data support a hypothesis that nonsymbiotic hemoglobins utilize available oxygen in cells exposed to low oxygen tensions or other conditions that reduce cell ATP levels to maintain the cells’ energy status. The very low dissociation constant of barley oxyhemoglobin makes it an ideal candidate for sequestering oxygen in low oxygen environments. Interaction with another compound, perhaps a flavoprotein, could create a complex capable of oxidizing NADH, in a manner analogous to Hmp protein of Escherichia coli (18). This would provide an efficient means of oxidatively regenerating NAD to support glycolysis, bypassing the fermentative route to ethanol.
The effects of expression of sense and antisense hemoglobin on energy charge are reminiscent of hypoxic acclimation of plant tissues. Maize root tips develop a tolerance to short-term anoxia if they have been acclimated by exposure to hypoxic conditions (19, 20). Acclimation is accompanied by increased energy charge (21) resulting from a sustained glycolytic rate compared with nonacclimated root tips (22, 23). Similarly, winter cereals show increased survival to hypoxia caused by ice encasement if they have been acclimated by exposure to hypoxic conditions (24). Acclimated plants maintain higher levels of adenylates and ATP during ice encasement, as a result of accelerated rates of glycolysis, than nonacclimated plants (25). Maximum induction of barley hemoglobin message occurs within 12 hr of exposure to hypoxic conditions (15), which is well within the time interval used for acclimation in the above examples. Furthermore, we have shown that the expression of hemoglobin is not influenced directly by oxygen usage or availability but is influenced by the availability of ATP in the tissue (13). We would, therefore, suggest that the increased survival of plants to anoxia as a result of hypoxic acclimation is a consequence of hemoglobin gene induction resulting from declining ATP levels during acclimation.
From an evolutionary standpoint it has been suggested that nonsymbiotic hemoglobins represent one of the more ancient forms of plant hemoglobins (17). Our results add credence to this idea. Because early life on earth existed in oxygen-poor environments, the presence of a hemoglobin capable of utilizing oxygen at low oxygen tensions would have provided an evolutionary advantage to an organism. Oxygen produced during photosynthesis and retained as oxyhemoglobin would provide a source of oxygen to oxidize NADH, maintaining a high glycolytic flux during darkness to provide ATP for cell growth and development.
Acknowledgments
We thank Professor H. Lörz for the pDB1 plasmid, Dr. P. Quail for the Ubi 1 promoter, and Dr. P. Eckes for the bar gene. Special thanks to D. Durnin and S. Ramsay for technical help.
ABBREVIATIONS
- BMS
Black Mexican Sweet
- ADH
alcohol dehydrogenase activity
References
- 1.Wittenberg J B, Wittenberg B A. Annu Rev Biophys Biophys Chem. 1990;19:217–241. doi: 10.1146/annurev.bb.19.060190.001245. [DOI] [PubMed] [Google Scholar]
- 2.Appleby C A. Sci Progress. 1992;76:365–398. [Google Scholar]
- 3.Appleby C A. In: Nitrogen Fixation and CO2 Metabolism. Ludden P W, Burris J E, editors. New York: Elsevier Science; 1985. pp. 41–51. [Google Scholar]
- 4.Duff S M G, Wittenberg J B, Hill R D. J Biol Chem. 1997;272:16746–16752. doi: 10.1074/jbc.272.27.16746. [DOI] [PubMed] [Google Scholar]
- 5.Arredondo-Peter R, Hargrove M S, Sarath G, Moran J F, Lohrman J, Olson J S, Klucas R V. Plant Physiol. 1997;115:1259–1266. doi: 10.1104/pp.115.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trevaskis B, Watts R A, Andersson C, Llewellyn D, Hargrove M S, Olson J S, Dennis E S, Peacock W J. Proc Natl Acad Sci USA. 1997;94:12230–12234. doi: 10.1073/pnas.94.22.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murashige T, Skooge F. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 8.Christensen A H, Quail P H. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- 9.Becker D, Brettshneider R, Lörz H. Plant J. 1994;5:299–307. doi: 10.1046/j.1365-313x.1994.05020299.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaeppler H F, Somers D A, Rines H W, Cockburn A F. Theor Appl Genet. 1992;84:560–566. doi: 10.1007/BF00224152. [DOI] [PubMed] [Google Scholar]
- 11.Lowry O H, Passonneau J V. A Flexible System of Enzymatic Analysis. New York: Academic; 1972. [Google Scholar]
- 12.Hanson A D, Jacobsen J V. Plant Physiol. 1984;75:566–572. doi: 10.1104/pp.75.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie X Z, Hill R D. Plant Physiol. 1997;114:835–840. doi: 10.1104/pp.114.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heslop-Harrison J, Heslop-Harrison Y, Shivanna K R. Theor Appl Genet. 1984;67:367–375. doi: 10.1007/BF00272876. [DOI] [PubMed] [Google Scholar]
- 15.Taylor E R, Nie X Z, MacGregor A W, Hill R D. Plant Mol Biol. 1994;24:853–862. doi: 10.1007/BF00014440. [DOI] [PubMed] [Google Scholar]
- 16.Saint-Ges V, Roby C, Bligny R, Pradet A, Douce R. Eur J Biochem. 1991;200:477–482. doi: 10.1111/j.1432-1033.1991.tb16207.x. [DOI] [PubMed] [Google Scholar]
- 17.Andersson C R, Jensen E O, Llewellyn D J, Dennis E S, Peacock W J. Proc Natl Acad Sci USA. 1996;93:5682–5687. doi: 10.1073/pnas.93.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole R K, Ioannidis N, Orii Y. Microbiology (Reading) 1996;142:1141–1148. doi: 10.1099/13500872-142-5-1141. [DOI] [PubMed] [Google Scholar]
- 19.Saglio P H, Drew M C, Pradet A. Plant Physiol. 1988;86:61–66. doi: 10.1104/pp.86.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson J, Cobb B G, Drew M C. Plant Physiol. 1989;91:837–841. doi: 10.1104/pp.91.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hole D G, Cobb B G, Hole P S, Drew M C. Plant Physiol. 1992;99:213–218. doi: 10.1104/pp.99.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia J H, Saglio P H. Plant Physiol. 1992;100:40–46. doi: 10.1104/pp.100.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia J-H, Roberts J K M. Plant Physiol. 1996;111:227–233. doi: 10.1104/pp.111.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews C J, Pomeroy M K. Can J Bot. 1983;61:142–147. [Google Scholar]
- 25.Andrews C J, Pomeroy M K. Plant Physiol. 1989;91:1063–1068. doi: 10.1104/pp.91.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]