Abstract
The intermediates in the biosynthetic pathway of murein were examined in two strains of Escherichia coli to determine whether they synthesized oligomeric precursors in vivo. No oligomeric precursors could be detected; the only intermediates found were the previously described UDP-N-acetylmuramyl peptides, and the two lipid-linked compounds, N-acetylglucosamyl-N-acetylmuramyl-(pentapeptide)-pyrophosphoryl-undecaprenol and N-acetylmuramyl-(pentapeptide)-pyrophosphoryl-undecaprenol. It was concluded that lipid-linked monomers are directly incorporated into the murein sacculus in vivo and do not pass through an oligomeric stage.
Full text
PDF


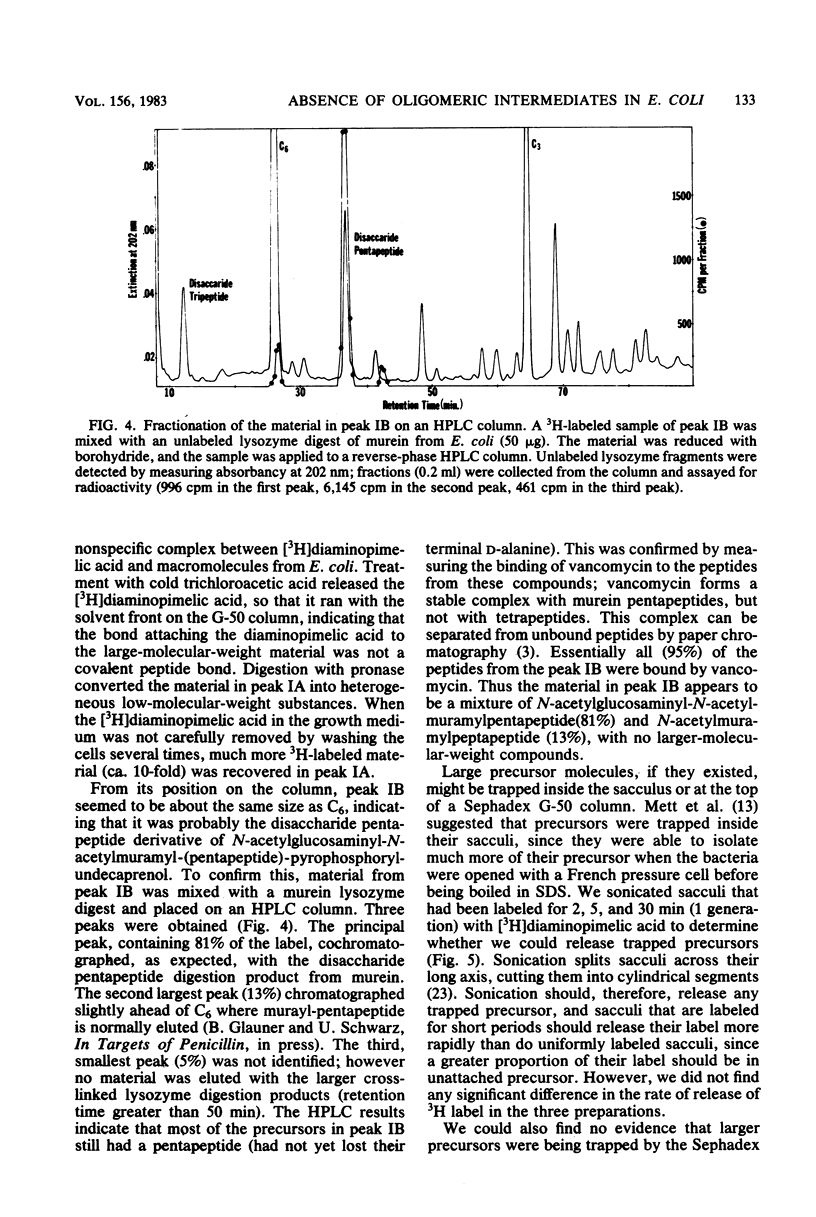
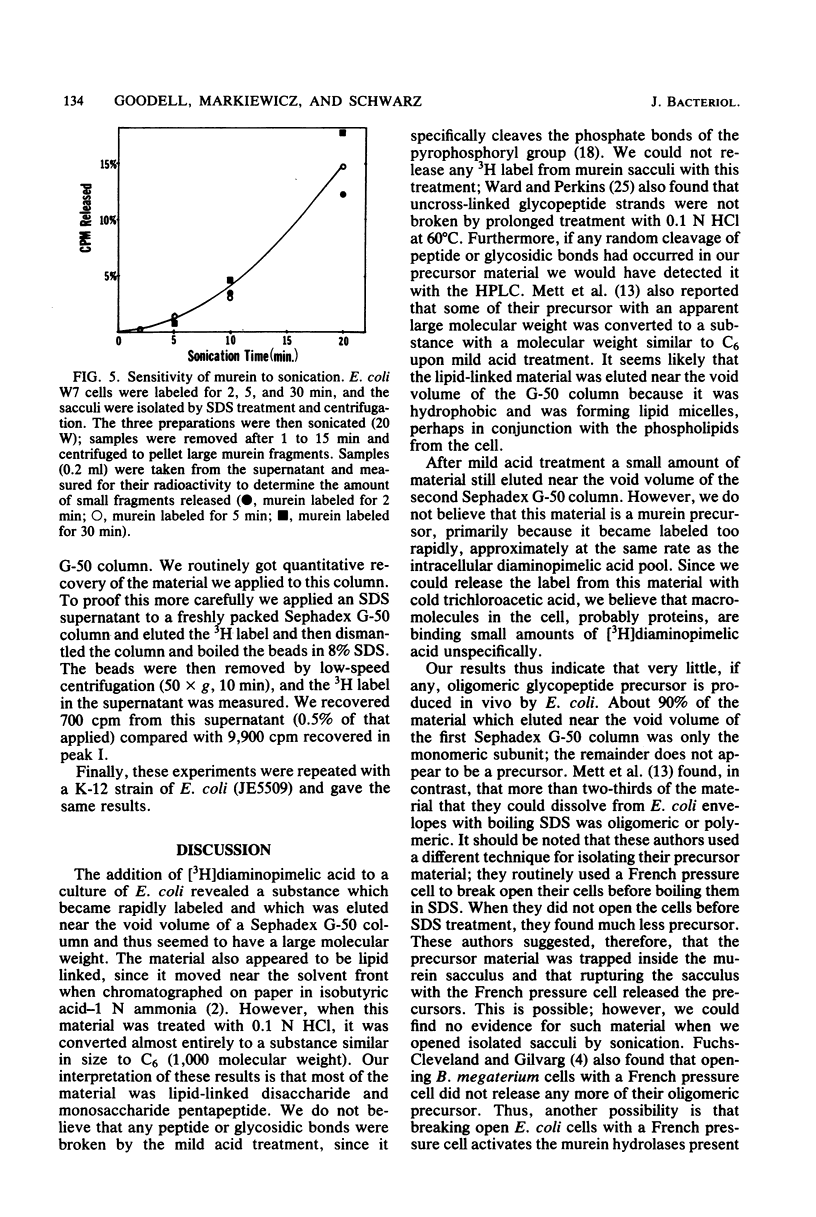

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. S., Matsuhashi M., Haskin M. A., Strominger J. L. Biosythesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J Biol Chem. 1967 Jul 10;242(13):3180–3190. [PubMed] [Google Scholar]
- Fuchs-Cleveland E., Gilvarg C. Oligomeric intermediate in peptidoglycan biosynthesis in Bacillus megaterium. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4200–4204. doi: 10.1073/pnas.73.11.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Fazio M., Tomasz A. Effect of benzylpenicillin on the synthesis and structure of the cell envelope of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1978 Mar;13(3):514–526. doi: 10.1128/aac.13.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Goodell E. W., Schwarz U. Compartmentalization of murein hydrolases in the envelope of Escherichia coli. FEBS Lett. 1974 Apr 1;40(2):261–264. doi: 10.1016/0014-5793(74)80240-1. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Bock-Hennig S. B., Schwarz U. Murein hydrolases in the envelope of Escherichia coli. Properties in situ and solubilization from the envelope. Eur J Biochem. 1974 Jan 3;41(1):203–208. doi: 10.1111/j.1432-1033.1974.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Site of initiation of cellular autolysis in Streptococcus faecalis as seen by electron microscopy. J Bacteriol. 1970 Aug;103(2):504–512. doi: 10.1128/jb.103.2.504-512.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Mett H., Bracha R., Mirelman D. Soluble nascent peptidoglycan in growing Escherichia coli cells. J Biol Chem. 1980 Oct 25;255(20):9884–9890. [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Role of the penicillin-sensitive transpeptidation reaction in attachment of newly synthesized peptidoglycan to cell walls of Micrococcus luteus. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3355–3359. doi: 10.1073/pnas.69.11.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Studies on the elongation of bacterial cell wall peptidoglycan and its inhibition by penicillin. Ann N Y Acad Sci. 1974 May 10;235(0):326–347. doi: 10.1111/j.1749-6632.1974.tb43275.x. [DOI] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XI. Formation of the isoglutamine amide group in the cell walls of Staphylococcus aureus. J Biol Chem. 1968 Feb 25;243(4):783–790. [PubMed] [Google Scholar]
- Strominger J. L., Izaki K., Matsuhashi M., Tipper D. J. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Fed Proc. 1967 Jan-Feb;26(1):9–22. [PubMed] [Google Scholar]
- Strominger J. L. Penicillin-sensitive enzymatic reactions in bacterial cell wall synthesis. Harvey Lect. 1968 1969;64:179–213. [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Yasuda S., Nishimura A., Yamada M., Hirota Y. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol Gen Genet. 1978 Nov 16;167(1):1–9. doi: 10.1007/BF00270315. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Verwer R. W., Beachey E. H., Keck W., Stoub A. M., Poldermans J. E. Oriented fragmentation of Escherichia coli sacculi by sonication. J Bacteriol. 1980 Jan;141(1):327–332. doi: 10.1128/jb.141.1.327-332.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B., Perkins H. R. The direction of glycan synthesis in a bacterial peptidoglycan. Biochem J. 1973 Dec;135(4):721–728. doi: 10.1042/bj1350721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B. The synthesis of peptidoglycan in an autolysin-deficient mutant of Bacillus licheniformis N.C.T.C. 6346 and the effect of beta-lactam antibiotics, bacitracin and vancomycin. Biochem J. 1974 Jul;141(1):227–241. doi: 10.1042/bj1410227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pedro M. A., Schwarz U. Heterogeneity of newly inserted and preexisting murein in the sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5856–5860. doi: 10.1073/pnas.78.9.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]


