Abstract
Opthopaedic management of femoral head osteonecrosis remains problematic, partly because of inability to systematically compare treatments in an animal model whose natural history parallels the human in terms of progression to femoral head collapse. recently, it was determined that collapse could be consistently achieved for cryogenically induced osteonecrosis in the emu. toward delineating the comparative hip joint biomechanics of emus versus humans, for purposes of establishing the emu as a model for human femoral head osteonecrosis, habitual hip joint activity level was quantified for a group of seven healthy adult emus housed in an outdoor research pen typical of those used in emu farming operations. the daily number of steps taken, and the time spent with the hips loaded (standing, or squatting/sitting) versus unloaded (recumbent), were quantified from 24-hour videotape recordings, analyzed by four independent observers. The average number of steps taken per day was 9,563, which extrapolates to 1.8 million hip loadings per year, a value that falls in the same general range as seen in normal adult humans. On average, the emus spent 4:05 hours per day idly standing, 2:12 hours squatting/sitting, and 10:44 hours recumbent; they underwent an average of 37 transitions per day between the respective posture/activity states.
Introduction
Osteonecrosis of the hip remains an important unsolved problem in the field of orthopaedic surgery. This disorder is caused by a disruption in blood perfusion to the cancellous bone of the femoral head (occurring for any of several reasons), and results in the death of often large regions of weight-bearing bone. Left untreated, osteonecrosis generally progresses to femoral head collapse and subsequent secondary osteoarthritis.1 Due to the mechanical nature of the collapse phenomenon, interventions to hopefully preclude its occurrence ideally should be systematically evaluated at the whole-joint level, under a loading regime as close as practicable to that in humans.
Many animals models have been developed to study pharmacologically- or surgically-induced osteonecrosis. Species utilized have included dogs, rabbits, pigs, horses, goats, rats, and mice.2–9 However, while (usually) successful in reproducing early tissue-level pathology, none of these models has managed to replicate the clinically all-important phenomenon of progression to femoral head collapse. Plausibly, the reason for non-progression has been that all of these species have been quadrupeds, which has allowed spontaneous load-protection of the affected limb, thereby presumably involving lesser hip joint mechanical demand that that typical of the human.
Recently, Conzemius et al.10 demonstrated that cryogenically-induced osteonecrosis progresses to femoral head collapse in the emu, a large flightless (bipedal) bird native to Australia. Emus (Figure 1) are the second-largest member of the ratite family, which along with their bigger cousin the ostrich also includes rheas, cassowaries, and kiwis. Adult emus typically stand about 5 feet (150 cm) tall, and weigh about 100 pounds (440 N). Although not a species previously utilized in biomedical research, emus are potentially attractive for studying various musculoskeletal disorders where bipedal gait is an important consideration, and where human-size-relevant surgical interventions are of interest. Emus nowadays are farmed commercially in many areas of the US as a source of meat and (especially) of cosmetic oils, so they are inexpensive to procure. They also are relatively easy to maintain, requiring only a penned outdoor enclosure/run with sufficient space to freely ambulate year-round, and with shelter available for times of inclement weather.
Figure 1.

Adult emus in an enclosure pen. the animals are about 5 feet in height, and weigh typically 100 pounds. in the presence of human observers, they tend to continually pace, particularly along the edges of the enclosure. note that the ground within the pen is worn down to bare dirt, due to the animals' activity.
Although osteonecrosis in the Conzemius et al. model10 was histologically and morphologically similar to that in the human, the time needed to achieve collapse in that series averaged twelve weeks. This is substantially faster than the average time of twenty-three months11 needed for untreated human femoral head osteonecrosis to progress to collapse. Understanding the reason(s) for the faster-than-human natural history of osteonecrosis progression in the emu is obviously an important consideration for interpreting results from studies potentially using that species as a disease model.
Activity level difference stands out as one obvious possibility. To the casual observer, penned emus appear to relentlessly pace in their enclosure, spending very little time with their hips unloaded. Although most of the lesions induced in that earlier series by Conzemius et al. were more severe than those in many human cases, the emu's apparent high activity level therefore might have contributed appreciably to pathogenesis acceleration. Moreover, in the Conzemius et al. study, histology for two of the three animals that did not develop lameness showed end-stage osteonecrosis, suggesting that emus either experience less pain with structural collapse, or that they are better able to tolerate hip pain, than humans. Besides osteonecrosis, the emu's seeming stoic behavior may also prove useful for modeling other musculoskeletal disorders (e.g., osteoarthritis) whose pathophysiology is influenced by unprotected loading of the involved extremity. Therefore, in addition to helping understand the potential role of the number of loading cycles and the duration of static hip loading as contributors to the rapidity of emu necrotic femoral head collapse per se, an appreciation of the daily activity levels of emus would be informative in the broader context of this species being considered for modeling other musculoskeletal disorders.
For these reasons, a study was undertaken to formally catalog the daily activity levels of emus. An important caveat to the high activity level apparent on casual observation is that emus are at best semidomesticated animals, that react apprehensively to interactions with humans. An observer being visible to the emus would potentially constitute a disturbance that could result in a substantial artifactual increase of their habitual activity level. The study design therefore relied upon a data capture protocol that involved minimal human presence.
Methods
Seven normal adult emus were sedated and marked with spray paint for individual identification. The animals were housed within a 28.2 x 2.8 m outdoor enclosure (Figure 2) that contained a 5.3 x 2.8 m covered shed at one end. This overall enclosure area corresponded to 11.25 m2 per animal. They were allowed food and water ad libitum. These conditions were similar to those typically used in emu farming operations, and were approved by the Iowa State University Institutional Care and Use Committee. Three video cameras were placed along the fence of the enclosure: one in the covered shed, one next to the feeder, and one at the opposite end of the pen, looking towards the shed.
Figure 2.
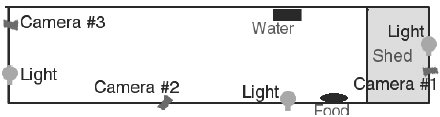
Layout of the outdoor enclosure (not to scale) showing locations of lights and cameras.
A week before the videotaping, one observer (HJL) spent the night inconspicuously observing the emus in their enclosure, to gain an appreciation of their nocturnal activity level. It was determined that around-the-clock videotaping was appropriate, since the animals occasionally got up and walked around at night, although in general such activity was appreciably less than in daylight hours. To facilitate nocturnal videotaping, three small incandescent lights were placed along the enclosure fence, and one light was placed inside the shed, providing just enough illumination to distinguish between emus, while not substantially disturbing their nocturnal behavior.
Videotaping commenced at 3:45 PM. At approximately 9:00 PM the lights were turned on and the cameras were loaded with new tapes. Tapes were exchanged again at approximately 4:30 AM, which caused the emus to awaken and stand for several minutes before returning to sleep. At approximately 10:30 AM the tapes were exchanged a third time. Videotaping was stopped at 3:50 PM. The experiment was conducted during a period of clear summer weather.
Quantifying the video recordings proved to be a substantial undertaking. Due to the animals' frequent intermingling, only one individual could be tracked at any given time. Thus, a total of 504 hours of videotape (3 cameras x 7 emus x 24 hours) needed to be analyzed. Moreover, owing to the seemingly random motions of the animals from site to site within the pen, it was frequently necessary to switch from tape to tape, with corresponding tape rewindings and/or fast forwards to preserve time synchrony. Because of the length and tediousness of videotape analysis, the task was split up among four observers. Each observer processed data (three cameras' tapes) from one of the four (approximately 6-hour) time segments recorded. For each emu the number of steps taken (both walking and running), and the time spent sitting/squatting and lying (Figure 3) were quantified. Occasionally, animals mingled together into tight clusters, such that the particular animal that was being observed was obscured. During the intervals that a particular animal was not distinctly visible on any of the three tapes, its time spend standing, sitting/squat-ting, and lying, and its numbers of steps were estimated in context by the observer, based on all of the animals' activity patterns. Cumulative results of all four observers were then merged to produce a 24-hour compilation of activity levels for each of the seven emus. To verify that observers were counting steps and measuring the standing, sitting/squatting, and lying periods in a consistent manner, three emus in three 30minute representative segments of video were analyzed by all four observers. The number of steps taken, and the time spent standing, sitting/squatting, and lying, were compared using Kendall's W coefficient of concordance, a measure of observer agreement.12 In this statistic, an extremum of 1.0 indicates perfect agreement, and 0.0 indicates absolute disagreement.
Figure 3.

(a) sitting/squatting posture versus (b) lying posture for the emu. for sitting squatting, the proximal-most joint making ground contact is the tibio-tarsus (analog of the human ankle). the hip joint is weight-bearing, in an orientation slightly more flexed that that for standing. the tail makes only marginal contact with the ground, and therefore provides negligible load bypass of the hip. for the lying posture, by contrast, the hips are minimally weight-bearing. most of the hours of darkness were spent in the lying posture.
As an additional point of reference, two emus that had undergone osteonecrosis-inducing cryo-surgery four weeks previously were videotaped for a two-hour period. These two animals were housed indoors in 3 x 3 m pens, the same management procedure as used in the earlier series.10 These two surgery animals were videotaped during daytime hours. Their numbers of steps, and their times spent sitting/squatting and lying down, were quantified by one of the four observers (KLT). These data were then extrapolated to estimate an average of these quantities per hour, for comparison to the non-operated emu cohort.
Results
The Kendall's W statistic for the 30-minute comparisons between all four observers was 0.845 (p=0.0004), which indicates very strong inter-observer agreement. This agreement was judged sufficient to compile the results of the entire 24-hour period as if a single observer had analyzed all the data.
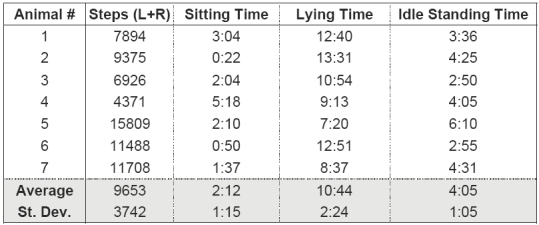
On average, the non-operated emus took 9,653 steps per day (Table 1). This figure extrapolates to 1.8 million loading cycles per hip per annum. An average of 10 hours and 45 minutes per day was spent in a recumbent (hips unloaded) position. For this lying posture, since interest was in the hip joint, no distinction was made between the alert (head held up) versus sleeping (head tucked under the wing or on the ground) states. Both idle standing time and sitting/squatting time represent conditions of static hip loading, and plausibly contribute to osteonecrotic femoral head collapse. The emus spent an average of four hours, five minutes each day standing (without walking), and two hours, thirteen minutes sitting/squatting with their hips loaded. Idle standing time was estimated by assuming that a single step took one second to complete. (This one-second average step duration was estimated by measuring the time and step count for several typical walking episodes). On average, emus had 37 transitions per day between sitting/squatting, standing, and/or lying.
Table 1.
summary of activity data compiled for all seven animals over a 24-hour period
 |
Not surprisingly, activity level varied appreciably over the course of the day (Figures 4 and 5). The animals were most active in the afternoon to mid-evening period (recording period 1, and the first part of period 2). For the most part, they slept through the night (most of recording period 2), occasionally rising for short periods of time. Some animals were substantially more active than others, with one taking as many steps during recording period 1 as another did during the entire 24-hour period. The early morning (recording period 3) had the most variability in how much time the animals spent lying down, presumably because some awoke earlier than others.
Figure 4.
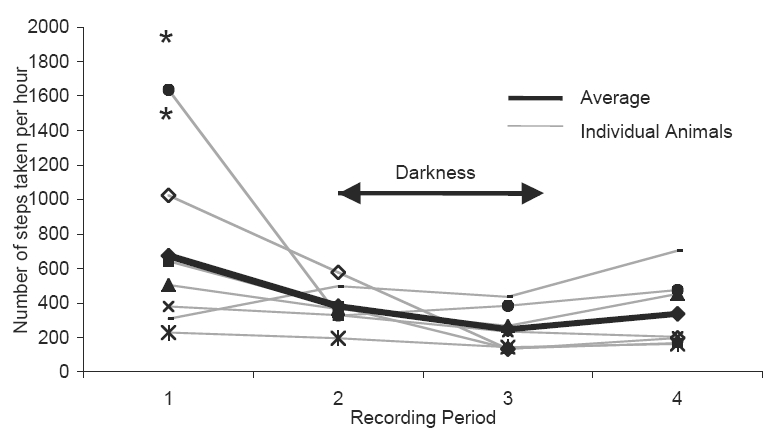
Average number of steps taken by each animal per hour, for the four intervals during the 24-hour taping period. recording periods ran for approximately 6 hours each, commencing at 3:45 PM (period 1). The *s indicate the average number of steps taken per hour for the two-post operative animals.
Figure 5.
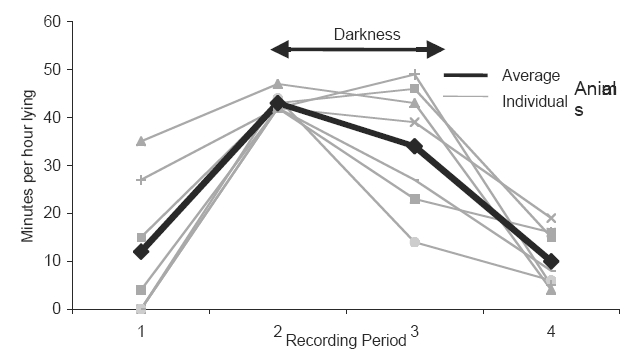
Average number of minutes per hour spent lying down by each animal, for the four recording periods.
In the two-hour videotape of the two animals that had undergone cryo-insult surgery, one took 2,955 steps, and the other took 3,981. The animal that took fewer steps spent 28 minutes lying down, but the other one remained standing for the entirety of the two-hour videotape. The corresponding 1,478 and 1,991 steps per hour for these two animals are labeled with by asterisk in Figure 4, for comparison to the non-operated animals.
Over the course of cataloguing the 24-hour video-tapes, it was noted that emus often arbitrarily paused during walking, while midway through weight shift from one leg to the next. Often they stood frozen in this posture for multiple seconds, and even tens of seconds, before either continuing on with the next step, or shifting the weight back to the trailing leg to adopt a two-legged stance.
Discussion
Historically, formal quantification of activity level has usually been lacking in animal studies where functional loading is important (such as in the case of physical loading stimuli to bones and joints). In the past several years, however, much interest has arisen in cataloging the habitual loading activities both in humans and in experimental animals such as turkeys, dogs, and sheep. For example, Adams et al.13 used 24hour videotaping to document the daily activity patterns responsible for mechanical homeostasis of turkey ulnae. That work was expanded by Fritton et al.,14 who placed strain gauges on the tibiae and ulnae of turkeys, dogs, and sheep, to record 12-24 hour loading histories. Both of those studies were conducted to quantify the numbers and magnitudes of strain-producing activities that contribute to bone maintenance versus remodeling. Habitual activity levels in humans have been documented in a number of studies, primarily in the context of assessing polyethylene wear rates in total hip arthroplasty.15–17
The manual cataloguing methodology used in the present study is similar to that used by Adams et al.13 In each tape, only one emu could be followed at a time, due to their intermingling. Because of the sheer volume of data generated by the videotapes (3 cameras x 7 emus x 24 hours) and the tediousness of the cataloguing measurements, it was necessary to divide the work among several observers, whose consistency it was necessary to verify with Kendall's W statistic. Adams et al. resorted to similar usage of multiple observers, linked via statistical documentation of inter-observer reproducibility, when categorizing wing activity events of turkeys.
Because of the fast progression to femoral head collapse seen in the pilot study by Conzemius et al.,10 it was initially supposed that emus would be documented to have substantially higher habitual activity levels than humans. However, the measured average number of steps per day taken by healthy emus (9,653) turns out to be strikingly consistent with the average number of steps that a healthy human takes each day15,18 (10,400 for men and 8,900 for women, and 8804 for men and 8913 for women, respectively). The present emu data fall near the middle of the range reported by Goldsmith et al.15 for healthy adult humans (395 to 17,718 steps per day). A Student's t-test showed that emus do not take significantly more steps than humans (p=0.55), when compared to data obtained by Sequeira et al.18 Although humans are highly variable, the ∼11 hours per day that emus spend with their hips bearing weight empirically seems appreciably greater than the corresponding period for most humans (and may have contributed to the quick progression to femoral head collapse in the pilot study). While the initial supposition that emus are more active (in terms of number of daily loading cycles on the hip) than humans was clearly not borne out by the data, this similarity in activity levels may prove useful in terms of future comparisons between the two species.
Goldsmith et al.15 and Schmalzried et al.16 both remarked on the high levels of inter-subject and intra-subject variability in their studies. The ratios of maximum to minimum number of steps per day in these two studies were 5:1 and 45:1 respectively. (The latter high number is likely due to the very low number of steps by the least-active individual in that particular study). The present study in emus shows only a 3.6:1 ratio, and suggests that emus have less variable activity levels than do humans.
While the average number of loading cycles per day for normal emus approximated that seen in humans, it is important to note that the human data described above were for normal (healthy) subjects. As regards human osteonecrosis patients, we are not aware of any studies that have examined their daily loading regime. While osteonecrosis patients tend to be young “high demand” individuals, many have co-morbidities, and almost certainly their activity level tends to decrease when the hip becomes symptomatic.
Concern was allayed as regards operated-upon animals potentially having reduced activity levels, either due to increased pain or due to the change in environmental conditions (indoors versus outdoors). While the initial sample size described here (two animals for a two-hour period) is insufficient to draw formal statistical conclusions about the overall activity level of post-operative versus pre-operative animals, it does provide anecdotal evidence that activity levels after surgery remain reasonably high.
Goldsmith et al.,15 whose study methodology utilized pedometry, noted that activity levels varied seasonally for the younger of two subjects whom they monitored over the course of a year: activity was highest during the summer. The videotaping in the present emu study was performed in midsummer during clear weather. Variations in emu activity due to weather would obviously affect these estimates of emu yearly activity. These animals are typically housed outdoors year-round. It seems a reasonable assumption that they would spend more time in the covered area of their pen during wet or colder weather, which presumably would involve less activity than that presently reported. Although daily life in an emu pen seemingly holds less variety of hip joint loadings than do the lifestyles of most humans (e.g., stair-climbing, sit-to-stand, stooping to pick something up, etc.19), the emu videotapes nevertheless showed a range of occasional activities seemingly involving high hip demand. These included episodes of running, jumping, confrontations between birds (kicking, scratching, etc.) plus the numerous (average 37 per day) sit-to-stand transitions. Our impression is that the variety of emu hip loadings is not substantially different from that of humans, and probably exceeds that of sedentary individuals.
Of the various indices of activity level here measured, the number of daily loading cycles seems most relevant to studying osteonecrosis, since femoral head collapse is probably a fatigue-related process, driven by cyclic fluctuation of stress in the at-risk osseous lattice. The measured average number of emu daily loading cycles, which extrapolates to 1.8 million per year, is remarkably similar to the recent estimates for healthy humans of 1.7 million per year by Seedholm and Wallbridge20 and of up to 2.0 million per year estimate by Silva et al.21
Finally, the above observations and data of course apply only to emus housed in a farm-type pen enclosure. However, the activity level of emus in the wild, while presumably higher, is of lesser practical interest in the context of this animal serving as a model for necrotic femoral head collapse, because any individual animal potentially used for surgical research would probably be housed in such a (sheltered) pen, or for short durations in an indoor enclosure, rather than being free-ranging.
Acknowledgments
Funding was provided by the Roy J. Carver Foundation, NIH Grants #AR46601 and #AR49919 (TDB and MGC), and an NSF Graduate Research Fellowship (KLT). Thanks to Dr. Wanda Gordon for assistance with videotaping.
References
- 1.Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop. 1998;S355:S314–335. [PubMed] [Google Scholar]
- 2.Chryssanthou C. Animal model of human disease: Dysbaric osteonecrosis. Am J Pathol. 1981;103:334–336. [PMC free article] [PubMed] [Google Scholar]
- 3.Conzemius MG, Brown TD. Animal Models in Osteonecrosis. Techniques in Orthopaedics. 2001;16:90–97. [Google Scholar]
- 4.Glimcher MJ. Cell biology during repair of osteonecrosis: implications for rational treatment. Acta Orthop Belg. 1999;65:17–22. [PubMed] [Google Scholar]
- 5.Levin D, norman D, Zinman C, Misselevich I, Reis DN, Boss JH. Osteoarthritis-like disorder in rats with vascular deprivation-induced necrosis of the femoral head. Pathol Res Pract. 1999;195:637–647. doi: 10.1016/S0344-0338(99)80129-0. [DOI] [PubMed] [Google Scholar]
- 6.Muller-Vahl H, Pabst R. An animal model for aseptic necrosis after intramuscular injections. Int J Tissue React. 1984;6:251–244. [PubMed] [Google Scholar]
- 7.Nadel SN, Debatin JF, Richardson WJ, Hedlund LW, Senft C, Rizk WS, Malizos KN, Stahl DL, Martinez S. Detection of acute avascular necrosis of the femoral head in dogs: dynamic contrast-enhanced MR imaging vs spin-echo and STIR sequences. Am J Roentgenol. 1992;159:1255–1261. doi: 10.2214/ajr.159.6.1442396. [DOI] [PubMed] [Google Scholar]
- 8.Newton B, Crawford CJ, Powers DL, Allen BL., Jr The immature goat as an animal model for Legg-Calve-Perthes disease. J Invest Surg. 1994;7:417–430. doi: 10.3109/08941939409016508. [DOI] [PubMed] [Google Scholar]
- 9.Welch RD, Hogan PM, Groom IJ, Jones JP., Jr . Osteonecrosis of the Femoral Head Associated with Colitis in a Horse and Treatment. In: Urbaniak JR Jr, Jones JP, editors. Etiology. Rosemont: IL (AAOS); 1997. Chapter 24 in Osteonecrosis: Etiology, Diagnosis. [Google Scholar]
- 10.Conzemius MG, Brown TD, Zhang Y, Robinson RA. A New Animal Model of Femoral Head Osteonecrosis: One that Progresses to Human-Like Mechanical Failure. J. Orthop. Res. 2002;20:303–309. doi: 10.1016/S0736-0266(01)00108-5. [DOI] [PubMed] [Google Scholar]
- 11.Bradway J, Morrey BF. The Natural History of the Silent Hip in Bilateral Atraumatic Osteonecrosis. J. Arthroplasty. 1993;8:383–387. doi: 10.1016/s0883-5403(06)80036-7. [DOI] [PubMed] [Google Scholar]
- 12.Siegel S. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- 13.Adams DJ, Spirt AA, Brown TD, Fritton SP, Rubin CT, Brand RA. Testing the daily stress stimulus theory of bone adaptation with natural and experimentally controlled strain histories. J. Biomech. 1997;30:671–678. doi: 10.1016/s0021-9290(97)00004-3. [DOI] [PubMed] [Google Scholar]
- 14.Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech. 2000;33:317–325. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- 15.Goldsmith AA, Dowson D, Wroblewski BM, Siney PD, Fleming PA, Lane JM, Stone MH, Walker R. Comparative study of the activity of total hip arthroplasty patients and normal subjects. J. Arthroplasty. 2001;16:613–619. doi: 10.1054/arth.2001.23568. [DOI] [PubMed] [Google Scholar]
- 16.Schmalzried TP, Szuszczewicz ES, Northfield MR, Akizuki KH, Frankel RE, Belcher G, Amstutz HC. Quantitative assessment of walking activity after total hip or knee replacement. J. Bone and Joint Surg. 1998;80A:54–59. [PubMed] [Google Scholar]
- 17.Wallbridge NC, Dowson D. The walking activity of patients with artificial hip joints. Eng Med. 1982;11:95–96. doi: 10.1243/emed_jour_1982_011_023_02. [DOI] [PubMed] [Google Scholar]
- 18.Sequeira MM, Rickenbach M, Wietlisbach V, Tullen B, Schutz Y. Physical activity assessment using a pedometer and its comparison with a questionnaire in a large population survey. Am. J. Epidem. 1995;142:989–999. doi: 10.1093/oxfordjournals.aje.a117748. [DOI] [PubMed] [Google Scholar]
- 19.Nadzadi ME, Pedersen DR, Yack HJ, Callaghan JJ, Brown TD. Kinematics, kinetics and finite element analysis of commonplace maneuvers at risk for total hip dislocation. J Biomech. 2003;36:577–591. doi: 10.1016/s0021-9290(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 20.Seedhom BB, Wallbridge NC. Walking activities and wear of prostheses. Ann Rheum Dis. 1985;44:838–843. doi: 10.1136/ard.44.12.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva M, Shepherd EF, Jackson WO, Dorey FJ, Schmalzried TP. Average patient walking activity approaches 2 million cycles per year: Pedometers under-record walking activity. J. Arthroplasty. 2002;17:693–697. doi: 10.1054/arth.2002.32699. [DOI] [PubMed] [Google Scholar]


