Abstract
Telomeres are unique chromatin domains located at the ends of eukaryotic chromosomes. Telomere functions in somatic cells involve complexes between telomere proteins and TTAGGG DNA repeats. During the differentiation of germ-line cells, telomeres undergo significant reorganization most likely required for additional specific functions in meiosis and fertilization. A telomere-binding protein complex from human sperm (hSTBP) has been isolated by detergent treatment and was partially purified. hSTBP specifically binds double-stranded telomeric DNA and does not contain known somatic telomere proteins TRF1, TRF2, and Ku. Surprisingly, the essential component of this complex has been identified as a specific variant of histone H2B. Indirect immunofluorescence shows punctate localization of H2B in sperm nuclei, which in part coincides with telomeric DNA localization established by fluorescent in situ hybridization. Anti–H2B antibodies block interactions of hSTBP with telomere DNA, and spH2B forms specific complex with this DNA in vitro, indicating that this protein plays a role in telomere DNA recognition. We propose that hSTBP participates in the membrane attachment of telomeres that may be important for ordered chromosome withdrawal after fertilization.
Keywords: sperm, telomere, histone H2B, nuclear membrane
Introduction
Telomeres have evolved to fulfill several essential roles: they protect chromosome ends, facilitate complete replication of chromosomal DNA molecules, and participate in chromosome positioning within nuclei (reviewed in Bryan and Cech 1999; Dandjinou et al. 1999). These functions depend on dynamic interactions between telomere DNA and various telomere-binding proteins. For example, double-stranded specific protein TRF1 partakes in negative regulation of telomerase activity, whereas the related TRF2 protects chromosomes from end-to-end fusion (van Steensel and de Lange 1997; van Steensel et al. 1998). Other known telomere-binding proteins in human somatic cells include the protein components of telomerase (Bryan and Cech 1999), the DNA-end binding protein Ku (Bianchi and de Lange 1999; Hsu et al. 1999), the hnRNA-binding protein hnRNPA1 (LaBranche et al. 1998), the tankyrase–ankyrin homologous protein with ribosylation activity (Smith and de Lange 1999), and an additional regulator of telomere length TIN2 (Kim et al. 1999).
Recently, new functions of telomeres start to emerge in germ-line cells. These chromosomal domains have been shown to participate in meiotic pairing in yeast (Cooper et al. 1998; Nimmo et al. 1998) and plants (Bass et al. 1997). Telomeres play a leading role in the formation of sperm-specific chromosome architecture in humans (Zalensky et al. 1995) and this architecture was proposed to be important for successful fertilization (Ward and Zalensky 1996). Telomere domains in germ-line cells are different from those in somatic cells in several respects. First, only in germ-line cells telomerase is highly active in vivo (Wright et al. 1996). This results in an almost twofold elongation of telomeric DNA during human spermatogenesis (de Lange et al. 1990). “Extended” sperm telomeres are brought to the zygote during fertilization, and serve as a species-specific zero-time length mark for the subsequent cell divisions in progeny. Second, a significant reorganization of the human telomere domain during spermatogenesis was demonstrated using cytological methods. At early meiotic stages, telomeres relocalize to the nuclear membrane and individual telomeres form clusters (Scherthan et al. 1996; Zalensky et al. 1997). Dimers and tetrameres of telomeres become more pronounced in spermatids and mature sperm (Zalensky et al. 1997; Meyer-Ficca et al. 1998).
Novel telomere-binding protein activities that have been identified in high-salt nuclear extracts of human (Zalensky et al. 1997) and bovine (Kozik et al. 2000) sperm may mediate these unique features of telomeres in spermatogenic cells. Human sperm telomere binding protein complex (hSTBP) binds double-stranded telomere DNA (Zalensky et al. 1997), while bovine protein interacts with single-stranded TTAGGG DNA (Kozik et al. 2000).
Here we report the identification, isolation, and characterization of a detergent-soluble protein complex interacting with double-stranded telomere DNA (dsTEL DNA) and therefore most probably responsible for association of human sperm telomeres with nuclear membrane. This complex does not contain known somatic-type telomere-binding proteins, but, remarkably, includes a sperm-specific variant of the histone H2B (spH2B) that is distinct from the major replication-dependent H2B. Immunofluorescence microscopy showed foci of spH2B in human sperm nuclei, which in part were colocalized with telomere DNA. In vitro binding experiments indicated that spH2B may play a role of the DNA-recognition element in hSTBP. Therefore, these data provide new insights into the unusual molecular organization of telomeres in human sperm that might be involved in spermiogenesis and fertilization.
Materials and Methods
Preparation of Nuclear Extracts and Gel-shift Assay
Human sperm cells were purified from ejaculates as described previously (Zalensky et al. 1993). Sperm cells were extracted using nonionic detergent buffer (0.5% Triton X-100, 100 mM NaCl, 7.5 mM Hepes, pH 7.9, 1 mM DTT, protease inhibitor cocktail; Boehringer) during 2–4 h at 4°C or, alternatively, using 0.5 M NaCl buffer (Zalensky et al. 1997). The hSTBP-containing supernatant was stored at −80°C until use. Crude HeLa nuclear extract was obtained essentially as described in Zhong et al. 1992. Telomere-binding activity was established using gel-shift assay. Extract containing 1–5 μg of total protein was incubated with 0.5–1 ng of the 32P-labeled double-stranded [TTAGGG]12 in binding buffer (Zhong et al. 1992) containing 100–500 ng of fragmented Escherichia coli DNA. The [TTAGGG]12 insert was isolated from the pTH12 plasmid provided by Dr. T de Lange (The Rockefeller University, New York, NY). In gel-shift experiments involving antibodies, 1 μl of corresponding serum was added to the standard binding reaction. After incubation for 30 min at room temperature, reaction mixture was separated in 6% PAGE prepared on 25 mM Tris-glycine-EDTA buffer.
Partial Purification of hSTBP
hSTBP activity was partially purified by gel filtration on Superdex 200HR column (Amersham Pharmacia Biotech) eluted with 7.5 mM Hepes, pH 7.9, 100 mM KCl, 1 mM DTT. Alternatively, purification was performed using ion-exchange column HiTrap S (Amersham Pharmacia Biotech) eluted with linear gradient (50 mM–1 M) of KCl in Hepes/DTT. Chromatographic fractions were assayed by gel-shift and analyzed by Western blotting using ECL detection.
Antibodies
Antibodies used in this work were provided by the following: anti–hTRF1 #5.2 and #371 by Dr. T. de Lange (The Rockefeller University, New York, NY), anti–hTRF2 by Dr. E. Gilson (CNRS/ENSL), polyclonal antibodies against calf thymus core histone fractions by Dr. E. Bers (St. Petersburg University, St. Petersburg, FL), and monoclonal antibodies against human H2B by Dr. B. Turner (University of Birmingham, Birmingham, AL). Anitprotamine antibodies were from Dr. R. Balhorn (LLNL). Anti–p80 Ku antibodies were from Santa Cruz Biotechnology, Inc. Secondary antibodies for ECL, immunofluorescence, and fluorescent in situ hybridization (FISH) were from Roche and Vector Laboratories.
Isolation and Purification of Histone H2B
Total basic proteins have been extracted from human sperm or HeLa nuclei as described earlier (Marvin et al. 1990; Zalensky et al. 1993) and histone fractions were purified using reverse phase HPLC on Vydac C4 column (Marvin et al. 1990).
Immunofluorescence Localization of Proteins and FISH
Human sperm cells were swollen using 0.05 mg/ml Heparin, 10 mM DTT during 30 min as described in detail earlier (Zalensky et al. 1995, Zalensky et al. 1997). Cells were fixed with cold methanol and rehydrated in washing solution. Primary antibodies were incubated overnight at 4°C. Secondary antibodies were Rhodamine or FITC labeled and used at 1:100 dilution. In our immunolocalization experiments different washing buffers (4× SSC, 0.1% Tween-20, PBS, and PBS with 01% Tween-20) were used with similar result. FISH localization of telomeres was carried out as described (Zalensky et al. 1997). For simultaneous localization of H2B and telomere DNA, immunofluorescence had been performed first, and then cells were fixed with 4% formaldehyde/PBS, washed, and subjected to standard FISH procedure. Finally, immunostaining was refreshed by incubation with secondary antibodies. Images were obtained using epifluorescence microscopy; photographic slides were converted to digital images using Nikon slide scanner and processed using Adobe Photoshop 5.0.
25 sperm nuclei were used for enumeration of telomere FISH and H2B immunostaining signals. Spots were counted as closely located if they were separated by a distance inferior to a signal radius.
Results and Discussion
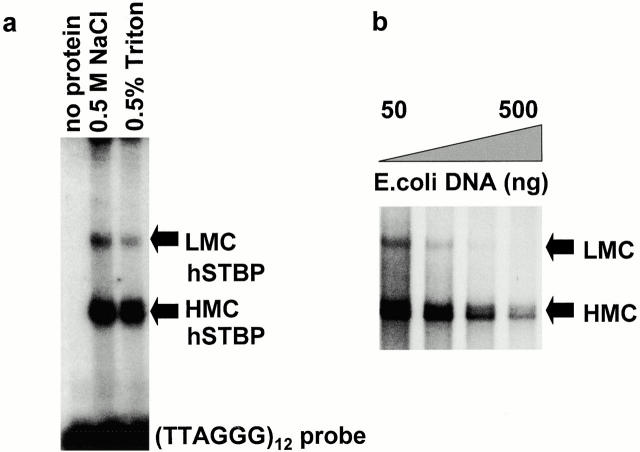
We were interested in characterizing proteins involved in telomere–membrane interactions in human sperm. To this end, nuclear membranes were partially solubilized by treatment with 0.5% Triton X-100 in buffer containing 100 mM NaCl. Earlier FISH data (Zalensky et al. 1995) demonstrated that such treatment destroyed association of human sperm telomeres with nuclear membrane. Usual methods for telomere-binding protein isolation involve nuclei extraction with salt buffers of higher molarity (e.g., 0.6 M KCl). Surprisingly, a Triton extract of human sperm was active in binding ds(TTAGGG) DNA, and this telomere-binding activity appeared to be identical to hSTBP previously described in 0.5 M NaCl nuclear extracts (Zalensky et al. 1997) as judged by a characteristic gel-retardation pattern (Fig. 1 a). The pretreatment of crude Triton-soluble hSTBP (hSTBPTR) with 6 M Urea, DNAase, and RNAase does not influence ds(TTAGGG) binding, at the same time activity is sensitive to temperature, and destroyed by 0.1% SDS or pepsin treatment (data not shown). Two shifted complexes are formed with ds(TTAGGG) (Fig. 1). Accumulation of a lower mobility complex (LMC) is more favorable at low concentrations of nonspecific competitor DNA, whereas the high mobility complex (HMC) is more stable at higher concentrations of competitor (Fig. 1 b). This feature is identical to that earlier described for hSTBP extracted with 0.5 M NaCl (Zalensky et al. 1997). Identical specificity towards nucleotide sequence in dsTEL substrate and kinetics of transition between LMC and HMC suggested that the lower mobility complex is a multimer of the higher mobility one and that this feature of hSTBP may be responsible for the microscopically observed telomere–telomere interactions (Zalensky et al. 1997).
Figure 1.
Identification of the hSTBP activity. (a) Proteins obtained from human sperm nuclei by extraction with high-salt or nonionic detergent were assayed for telomere binding using gel shift with the [TTAGGG]12 probe. (b) Transition between low and high mobility hSTBP-[TTAGGG]12 complexes induced by an increased concentration of nonspecific competitor DNA in binding reaction.
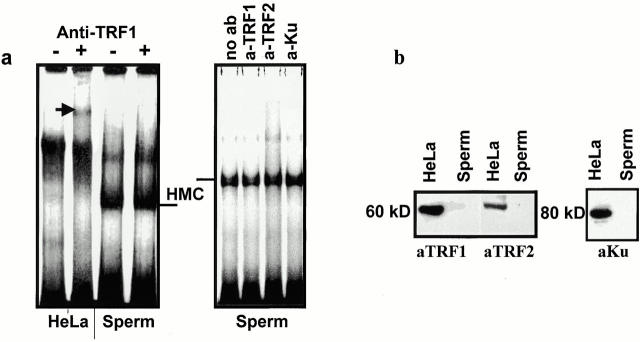
The hSTBPTR complex does not contain known somatic telomere-binding proteins. This has been proved first by DNA binding experiments performed in the presence of antibodies against TRF1, TRF2, and Ku proteins that are known components of somatic telomeres (Broccoli et al. 1997; Bianchi and de Lange 1999). Addition of the anti–TRF1 antibodies to the binding mixture containing TEL DNA substrate and HeLa nuclear extract results in the formation of a super-shifted complex (Fig. 2 a, left). This is an expected result because TRF1 is major telomere-binding protein in HeLa cells. In a similar reaction, but using hSTBPTR, the same antibodies are inactive (Fig. 2 a, left). Likewise, supershifted bands were not observed upon addition of anti–TRF2, and anti–Ku antibodies (Fig. 2 a, right). In addition, TRF1, TRF2, and Ku proteins were not detected in Western blots of either total sperm nuclear proteins (Fig. 2 b) or proteins from active Triton X-100 extracts (not shown). Therefore, hSTBPTR is a novel telomere-binding activity, different from that of somatic cells. Importantly, this activity was isolated by detergent treatment of sperm cells, and consequently most probably contributes to telomere attachment to the nuclear membrane.
Figure 2.
Somatic telomere-binding proteins are absent in hSTBPTR. (a) Crude nuclear extracts of HeLa cells and hSTBPTR were tested in a gel-shift assay in the presence of the indicated antibodies. The supershifted band formed by hTRF1-[TTAGGG]12 anti–hTRF1 complex is shown (arrow). (b) Western analysis of proteins from HeLa and human sperm nuclear lysates. Molecular weights of proteins were determined using prestained protein markers (not shown).
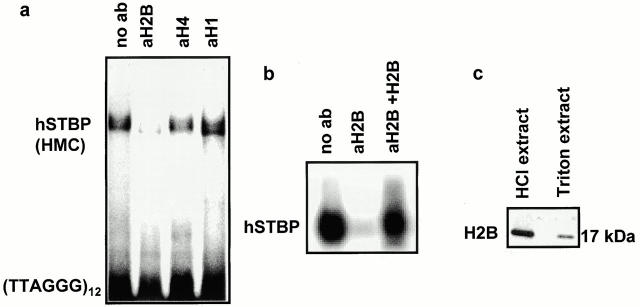
It has been shown (Gatewood et al. 1990) that mature human sperm nuclei contain ∼10–15% of residual histones of unknown function. Recent data from our group (Zalenskaya, I.A., E.M. Bradbury, and A.O. Zalensky, manuscript submitted for publication) has shown that although during human spermiogenesis the bulk of genome DNA is reorganized into nucleoprotamine, part of the telomere DNA remains packaged in nucleosomes. Therefore, a part of sperm histones are associated with telomeres. In view of this finding, the effect of several anti–histone antibodies on hSTBPTR binding to dsTEL DNA was tested. Antibodies against H4 and H1 fractions (Fig. 3 a), anti–H2A and –H3 (data not shown) did not alter the characteristic (TTAGGG)12 band shift by hSTBPTR. However, the antibodies against histone H2B from two different sources (anti–human H2B and anti–calf thymus H2B) were found to suppress binding (Fig. 3, a and b). When these antibodies were preincubated with the purified H2B, and then added to the binding mixture, the formation of the TEL DNA-hSTBP complex was restored (Fig. 3 b, right). Western blot analysis of total sperm nuclear proteins and hSTBPTR showed that the same anti–H2B antibodies recognized the only polypeptide with the apparent molecular weight of 17 kD (Fig. 3 c). Furthermore, in SDS gels, this protein comigrates with the HPLC-purified HeLa H2B (data not shown). Therefore, the 17-kD protein in hSTBPTR is histone H2B.
Figure 3.
Sperm-specific variant of human histone H2B is a component of hSTBPTR. (a) Antibodies against H2B, but not against other histone fractions suppress formation of hSTBP-[TTAGGG]12 complexes. Telomere binding activity was determined in gel-shift assay in the presence of the indicated antibodies. (b) Formation of the hSTBP-[TTAGGG]12 complex was restored after preincubation of anti–H2B serum with the purified HeLa H2B. (c) Western analysis of acid-soluble and Triton X-100–extracted proteins of human sperm. (d–g) Partial purification of hSTBPTR shows that spH2B histone coelutes with telomere-binding activity. Gel filtration: eluted fractions were assayed for telomere-binding activity (d), and Western blotting using anti–H2B antibodies (e). Ion-exchange chromatography: fractions were eluted by linear gradient of KCl, assayed for binding to [TTAGGG]12 probe (f) and in Western blotting (g).
We have partially purified hSTBPTR using either gel-filtration or ion-exchange chromatography. Fractions eluted from both columns were assayed for telomere-binding activity (Fig. 3d and Fig. f), and for H2B presence (Fig. 3e and Fig. g). In both experiments, the active fraction contains spH2B histone. From the combination of data presented in Fig. 3, we conclude that spH2B is a component of a telomere-binding complex in human sperm nuclei.
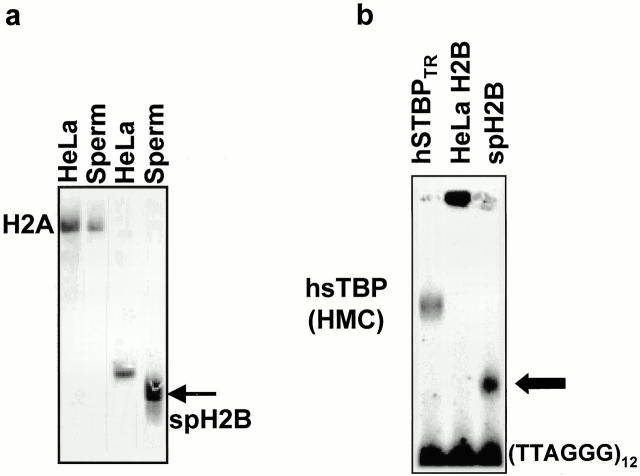
We have isolated and purified histones H2B from sperm and HeLa nuclei. Isolated proteins were analyzed in acetic acid/urea/Triton PAGE. This sensitive electrophoretic system allows to distinguish histone protein variants (Zweidler 1978). Fig. 4 a demonstrates that spH2B present in hSTBPTR differs from somatic counterpart and consequently is a variant protein. To determine whether spH2B might preferentially bind telomere DNA, we compared the ability of somatic and sperm H2B to form complexes with the ds(TTAGGG). H2B isolated from HeLa cells nonspecifically interacts with telomere DNA producing high-molecular weight aggregates (Fig. 4 b), which is the anticipated behavior for this DNA-binding protein. Quite interestingly, under identical conditions, spH2B formed a complex (Fig. 4 b) that is stable in the presence of up to 300× excess of nonspecific DNA in binding reaction. The electrophoretic mobility of the spH2B-TEL DNA complex is higher than that of the high mobility complex formed by hSTBPTR (Fig. 4 b) because the latter has a complex polypeptide composition (data not shown). We propose that amino acid sequences of sperm and somatic H2B are in part different, as suggested by the observed difference in electrophoretic mobility (Fig. 4 a). SpH2B may have an additional and unique sequence(s) with affinity towards ds(TTAGGG) DNA. At the same time, both H2B variants share an antibody epitope. It is possible that the DNA- and antibody-recognition sequences in spH2B are overlapping or closely located that would explain why antibodies block interactions between dsTEL DNA and hSTBPTR (Fig. 3).
Figure 4.
Human sperm H2B is a variant histone capable of forming complexes with telomeric DNA in vitro. (a) Separation of the purified sperm and somatic (HeLa) histone fractions H2B and H2A in Triton/acetic acid/urea PAGE. The arrow shows a sperm-specific variant of H2B. (b) 3 μg of crude hSTBPTR, 50 ng of HeLa H2B, or 50 ng of sperm H2B were incubated with 0.5 ng of labeled [TTAGGG]12 in binding buffer containing 100 μg/ml BSA and 100 ng fragmented E. coli DNA and separated in 6% PAGE. SpH2B-telomere DNA complex is shown by the arrow.
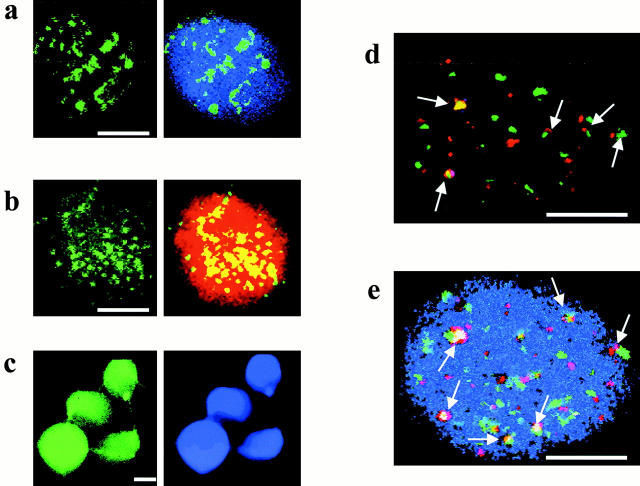
We determined the nuclear localization of histone H2B in sperm nuclei using indirect immunofluorescence and compared it with the localization of histone H4, protamine 2, and telomere DNA (Fig. 5). spH2B shows a punctate localization within a limited number of foci (average, Fig. 5, a and b). Use of two anti–H2B antibodies of different origin resulted in identical patterns of spH2B staining. In contrast, both protamine 2, the major structural protein, which organizes the bulk of sperm chromatin (Fig. 5 b, red signal, right) and histone H4 (c) display a dispersed even nuclear distribution. The punctated pattern of the spH2B localization resembles that of telomere DNA determined using FISH (Zalensky et al. 1997). Dual-labeling experiments reveal that some of the spH2B spots overlap (or are closely positioned) with telomere DNA (Fig. 5d and Fig. e). Average number of spH2B signals is 36 ± 6 per nuclei; from these, 9 ± 3 are superimposed and 17 ± 4 are closely positioned with telomere DNA loci.
Figure 5.
Localization of nuclear proteins and telomere DNA in human sperm nuclei. Typical patterns of localizations are presented. Note different magnifications (scale bars: 5 μm). (a and b) punctuated localization of histone H2B (yellow/green signal). (a) Indirect immunofluorescence using mouse monoclonal anti–human H2B; (right) relative localization of H2B and nuclear DNA [counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), blue] in the same cell. (b) Simultaneous localization of spH2B (using rabbit anti–H2B antibodies) and protamine 2 (using mouse anti–protamine antibodies). (Left) spH2B localization; (right) merged image: spH2B (yellow) and protamine (red). (c) Localization of histone H4 (left) and total nuclear DNA stained with DAPI (right). (d and e) Simultaneous localization of telomere DNA using FISH (red signals) and histone H2B (green signals). Images were registered using triple-band pass filter. Arrows mark some colocalized (or closely located) spH2B and TEL DNA. (d) No DNA counterstaining. (e) Nuclear DNA was counterstained with DAPI.
We assume that part of the spH2B, which is the most abundant histone fraction in human sperm (Zalensky et al. 1993), may be associated with yet unidentified chromosomal domains other than telomeres. Absence of a perfect colocalization may also be the result of the heparin pretreatment of sperm cells that is necessary to expose protein epitopes. Such treatment weakens the DNA-histone contacts and liberates some histones from DNA (Villeponteau 1992).
Our results demonstrate that a variant histone spH2B is part of the telomere-binding complex, which is implicated in telomere membrane attachment in human sperm cells. The involvement of histones in telomeric heterochromatin has been well documented in yeast (Grunstein 1997). In Saccharomyces cerevisiae, the telomeric complex includes the proteins RAP1 and SIR2-4, and the histone pair H3/H4, where RAP1 is the specific DNA-recognition protein. Our data indicate that in hSTBPTR this function may be performed by spH2B.
Testis- and sperm-specific variants of histones have been described in humans and other mammals, reviewed by Doenecke et al. 1997. It is accepted that these replacement histone variants contribute to the restructuring of chromatin during spermiogenesis, but their exact functions are not known. It is noteworthy that the majority of testis-specific histones are synthesized and incorporated into chromatin during meiotic stages (Doenecke et al. 1997), when the telomeres relocalize to the nuclear membrane and form telomere complexes (Scherthan et al. 1996; Zalensky et al. 1997). Testis H2B and H2A genes for histone subtypes that are different in part of the coding sequence from the major variants have been cloned from rat (Kim et al. 1987) and mouse (Choi et al. 1996). 17 human H2B genes have been identified and sequenced (Albig et al. 1999), but this complement does not contain the germ cell–specific gene(s). Peptide mapping of human testis H2B (Wattanaseree and Svasti 1983) showed that this protein differs from both human somatic and rat testis H2B in the presence of unique peptides. Direct structural information about the spH2B subtype that is described here is absent, and experiments are in progress to determine the partial amino acid sequence of human spH2B.
Identification of the hSTBPTR component(s) responsible for interactions with the nuclear membrane is in progress. Among possible candidates are lamins, since cell-specific lamin isoforms have been identified in the meiotic cells of rat (Alsheimer and Benavente 1996) and mice (Furukawa et al. 1994). Furthermore, lamins were shown to interact with telomere DNA in vitro (Shoeman and Troub, 1990).
We can speculate that spH2B might also participate in the membrane binding. Interestingly, a recent study of the interactions between nuclear lamina and chromatin (Goldberg et al. 1999) has shown that histones H2B/2A (but not H1, H3, or H4) specifically interact with Drosophila Dm0 lamin.
It is becoming increasingly apparent that histone subtypes, particularly replication-independent forms, have functions in addition to generation and stabilization of nucleosomes and chromatin structure. One recent example is the specific association of the H2A.X histone variant with sites of DNA double-stranded breaks (Rogakou et al. 1999). Our results show that an unusual histone H2B subtype of human sperm is an essential part of a telomere-binding complex in these cells. This and previous work (Zalensky et al. 1997) demonstrate that molecular organization of telomere chromosomal domain in somatic and germ-line human cells is different. We propose that hSTBP may participate in the functions of telomeres during spermiogenesis and after fertilization; however, further molecular and genetic studies are needed to elucidate the specific TEL DNA–protein interactions during these stages of development.
Acknowledgments
This work was supported by a University of California Health System grant and in part by the National Institutes of Health grant HD39830-01 to A.O. Zalensky.
Footnotes
Arunas A. Gineitis and Irina A. Zalenskaya contributed equally to the paper and should be considered co–first authors.
Abbreviations used in this paper: dsTEL DNA, double-stranded telomere DNA; FISH, fluorescent in situ hybridization; hSTBP, human sperm telomere binding complex; spH2B, sperm-specific variant of histone H2B.
References
- Albig W., Trappe R., Kardalinou E., Eick S., Doenecke D. The human H2A and H2B histone gene complement. Biol. Chem. 1999;380:7–18. doi: 10.1515/BC.1999.002. [DOI] [PubMed] [Google Scholar]
- Bass H.W., Marshall W.F., Sedat J.W., Agard D.A., Cande W.Z. Telomeres cluster de novo before the initiation of synapsisa three-dimensional spatial analysis of telomere positions before and during meiotic prophase. J. Cell Biol. 1997;137:5–18. doi: 10.1083/jcb.137.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A., de Lange T. Ku binds telomeric DNA in vitro. J. Biol. Chem. 1999;274:21223–21227. doi: 10.1074/jbc.274.30.21223. [DOI] [PubMed] [Google Scholar]
- Broccoli D., Smogorzewska A., Chong L., de Lange T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 1997;17:231–235. doi: 10.1038/ng1097-231. [DOI] [PubMed] [Google Scholar]
- Bryan T.M, Cech T.R. Telomerase and the maintenance of chromosome ends. Curr. Opin. Cell Biol. 1999;11:318–324. doi: 10.1016/S0955-0674(99)80043-X. [DOI] [PubMed] [Google Scholar]
- Choi Y.C., Gu W., Hecht N.B., Feinberg A.P., Chae C.B. Molecular cloning of mouse somatic and testis-specific H2B histone genes containing a methylated CpG island. DNA Cell Biol. 1996;15:495–504. doi: 10.1089/dna.1996.15.495. [DOI] [PubMed] [Google Scholar]
- Cooper J.P., Watanabe Y., Nurse P. Fission yeast Taz1 protein is required for meiotic telomere clustering and recombination. Nature. 1998;392:828–831. doi: 10.1038/33947. [DOI] [PubMed] [Google Scholar]
- Dandjinou A.T., Dionne I., Gravel S., LeBel C., Parenteau J., Wellinger R.J. Cytological and functional aspects of telomere maintenance. Histol. Histopathol. 1999;14:517–524. doi: 10.14670/HH-14.517. [DOI] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R.M., Cox D.R., Naylor S.L., Killery A.M., Varmus H.E. Structure and variability of human chromosome ends. Mol. Cell. Biol. 1990;10:518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenecke D., Albig W., Bode C., Drabent B., Franke K., Gavenis K., Witt O. Histonesgenetic diversity and tissue-specific gene expression. Histochem. Cell. Biol. 1997;107:1–10. doi: 10.1007/s004180050083. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Inagaki H., Hotta Y. Identification and cloning of an mRNA coding for a germ cell-specific A-type lamin in mice. Exp. Cell Res. 1994;212:426–430. doi: 10.1006/excr.1994.1164. [DOI] [PubMed] [Google Scholar]
- Gatewood J.M., Cook G.R., Balhorn R., Schmid C.W., Bradbury E.M. Isolation of four core histones from human sperm chromatin representing a minor subset of somatic histones. J. Biol. Chem. 1990;265:20662–20666. [PubMed] [Google Scholar]
- Goldberg M., Harel A., Brandeis M., Rechsteiner T., Richmond T.J., Weissm A.M., Gruenbaum Y. The tail domain of lamin Dm0 binds histones H2A and H2B. Proc. Natl. Acad. Sci. USA. 1999;96:2852–2857. doi: 10.1073/pnas.96.6.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M. Molecular model for telomeric heterochromatin in yeast. Curr. Opin. Cell Biol. 1997;9:383–387. doi: 10.1016/s0955-0674(97)80011-7. [DOI] [PubMed] [Google Scholar]
- Hsu H.L., Gilley D., Blackburn E.H., Chen D.J. Ku is associated with the telomere in mammals. Proc. Natl. Acad. Sci. USA. 1999;96:12454–12458. doi: 10.1073/pnas.96.22.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.J., Hwang I., Tres L.L., Kierszenbaum A.L., Chae C.B. Molecular cloning and differential expression of somatic and testis-specific H2B histone genes during rat spermatogenesis. Dev. Biol. 1987;124:23–34. doi: 10.1016/0012-1606(87)90455-6. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Kaminker P., Campisi J. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 1999;23:405–412. doi: 10.1038/70508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozik A., Bradbury E.M., Zalensky A.O. Identification and characterization of a bovine sperm protein that binds specifically to single-stranded telomeric deoxyribonucleic acid. Biol. Reprod. 2000;62:340–346. doi: 10.1095/biolreprod62.2.340. [DOI] [PubMed] [Google Scholar]
- LaBranche H., Dupuis S., Ben-David Y., Bani M.R., Wellinger R.J., Chabot B. Telomere elongation by hnRNP A1 and a derivative that interacts with telomeric repeats and telomerase. Nat. Genet. 1998;19:199–202. doi: 10.1038/575. [DOI] [PubMed] [Google Scholar]
- Marvin K.W., Yau P., Bradbury E.M. Isolation and characterization of acetylated histones H3 and H4 and their assembly into nucleosomes. J. Biol. Chem. 1990;265:19839–19847. [PubMed] [Google Scholar]
- Meyer-Ficca M., Muller-Navia J., Scherthan H. Clustering of pericentromeres initiates in step 9 of spermiogenesis of the rat (Rattus norvegicus) and contributes to a well-defined genome architecture in the sperm nucleus. J. Cell Sci. 1998;111:1363–1370. doi: 10.1242/jcs.111.10.1363. [DOI] [PubMed] [Google Scholar]
- Nimmo E.R., Pidoux A.L., Perry P.E., Allshire R.C. Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe . Nature. 1998;392:825–828. doi: 10.1038/33941. [DOI] [PubMed] [Google Scholar]
- Rogakou E.P, Boon C., Redon C., Bonner W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H., Weich S., Schwegler H., Heyting C., Harle M., Cremer T. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J. Cell Biol. 1996;134:1109–1125. doi: 10.1083/jcb.134.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeman R.L., Traub P. The in vitro DNA-binding properties of purified nuclear lamin proteins and vimentin. J. Biol. Chem. 1990;265:9055–9061. [PubMed] [Google Scholar]
- Smith S., de Lange T. Cell cycle dependent localization of the telomeric PARP, tankyrase, to nuclear pore complexes and centrosomes. J. Cell Sci. 1999;112:3649–3656. doi: 10.1242/jcs.112.21.3649. [DOI] [PubMed] [Google Scholar]
- van Steensel B., de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- van Steensel B., Smogorzewska A., de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- Villeponteau B. Heparin increases chromatin accessibility by binding the trypsin-sensitive basic residues in histones. Biochem. J. 1992;288:953–958. doi: 10.1042/bj2880953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanaseree J., Svasti J. Human testis-specific histone TH2Bfractionation and peptide mapping. Arch. Biochem. Biophys. 1983;225:892–897. doi: 10.1016/0003-9861(83)90103-0. [DOI] [PubMed] [Google Scholar]
- Ward W.S., Zalensky A.O. The unique, complex organization of the transcriptionally silent sperm chromatin. Crit. Rev. Eukaryot. Gene Expr. 1996;6:139–147. doi: 10.1615/critreveukargeneexpr.v6.i2-3.30. [DOI] [PubMed] [Google Scholar]
- Wright W.E., Piatyszek M.A., Rainey W.E., Byrd W., Shay J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Zalensky A.O., Allen M.J., Kobayashi A., Zalenskaya I.A., Bradbury E.M. Well-defined genome architecture in the human sperm nucleus. Chromosoma. 1995;103:577–590. doi: 10.1007/BF00357684. [DOI] [PubMed] [Google Scholar]
- Zalensky A.O., Tomilin N.V., Zalenskaya I.A., Teplitz R.L., Bradbury E.M. Telomere–telomere interactions and candidate telomere binding protein(s) in mammalian sperm cells. Exp. Cell Res. 1997;232:29–41. doi: 10.1006/excr.1997.3482. [DOI] [PubMed] [Google Scholar]
- Zalensky A.O., Yau P., Breneman J.W., Bradbury E.M. The abundant 19-kilodalton protein associated with human sperm nuclei that is related to seminal plasma alpha-inhibins. Mol. Reprod. Dev. 1993;36:164–173. doi: 10.1002/mrd.1080360207. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Shiue L., Kaplan S., de Lange T. A mammalian factor that binds telomeric TTAGGG repeats in vitro. Mol. Cell. Biol. 1992;12:4834–4843. doi: 10.1128/mcb.12.11.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]