Abstract
The γ-tubulin ring complex (γTuRC), purified from the cytoplasm of vertebrate and invertebrate cells, is a microtubule nucleator in vitro. Structural studies have shown that γTuRC is a structure shaped like a lock-washer and topped with a cap. Microtubules are thought to nucleate from the uncapped side of the γTuRC. Consequently, the cap structure of the γTuRC is distal to the base of the microtubules, giving the end of the microtubule the shape of a pointed cap. Here, we report the cloning and characterization of a new subunit of Xenopus γTuRC, Xgrip210. We show that Xgrip210 is a conserved centrosomal protein that is essential for the formation of γTuRC. Using immunogold labeling, we found that Xgrip210 is localized to the ends of microtubules nucleated by the γTuRC and that its localization is more distal, toward the tip of the γTuRC-cap structure, than that of γ-tubulin. Immunodepletion of Xgrip210 blocks not only the assembly of the γTuRC, but also the recruitment of γ-tubulin and its interacting protein, Xgrip109, to the centrosome. These results suggest that Xgrip210 is a component of the γTuRC cap structure that is required for the assembly of the γTuRC.
Keywords: Xgrip210, centrosome, microtubule nucleation, γ-tubulin ring complex, Xenopus
Introduction
The microtubule (MT) cytoskeleton is assembled from highly dynamic polymers of α- and β-tubulin heterodimers. It is found in all eukaryotic cells and is vital for many cellular functions such as intracellular organization, membrane trafficking, and cell division. The dynamic nature (Mitchison and Kirschner 1984a) and spatial control of MT assembly allows this cytoskeleton to change its shape and mass efficiently in response to cell cycle and developmental cues (Desai and Mitchison 1998).
Spatial and temporal control of MT nucleation in different organisms is carried out by specialized organelles collectively called the MT organizing centers (MTOC). The major MTOC found in animal cells is the centrosome, which was first observed >100 yr ago (Wilson 1925). The centrosome, which consists of a pair of centrioles and a pericentriolar material that contains the MT nucleating activity (Gould and Borisy 1977), is found juxtaposed to the nucleus of most fibroblastic cells. The centrosome not only nucleates MTs more efficiently than spontaneous MT nucleation (Mitchison and Kirschner 1984b), but also participates in organizing the interphase MT arrays that are critical for intracellular trafficking (Kellogg et al. 1994). Progression through the cell cycle into mitosis is accompanied by many changes in the MT cytoskeleton including increased MT nucleation by the centrosome (Kellogg et al. 1994). The increase in MT nucleation is believed to be important for spindle assembly and organization (Heald et al. 1997; Hyman and Karsenti 1998). Thus, to understand how the MT cytoskeleton is regulated throughout the cell cycle, it is critical to understand how the centrosome promotes MT nucleation.
The discovery of γ-tubulin (Oakley 1992) initiated a new era of studying MT nucleation by the centrosome (Wiese and Zheng 1999). Identified as a suppressor of a β-tubulin mutation in the filamentous fungus, Aspergillus nidulans (Oakley and Oakley 1989), γ-tubulin is a highly conserved protein localized to all MT nucleating sites examined thus far (Wiese and Zheng 1999). Genetic studies in A. nidulans (Oakley et al. 1990), Saccharomyces cerevisiae (Sobel and Synder 1995; Marschall et al. 1996; Spang et al. 1996), Schizosaccharomyces pombe (Horio et al. 1991), and Drosophila melanogaster (Sunkel et al. 1995; Tavosanis et al. 1997) suggest that γ-tubulin is involved in MT nucleation. Biochemical studies of γ-tubulin in Xenopus eggs and Drosophila embryos led to the purification of a 2-MD γ-tubulin ring complex (γTuRC) that can nucleate MT assembly in vitro (Zheng et al. 1995; Oegema et al. 1999). Most γ-tubulin in animal cells appears to exist as γTuRC (Wiese and Zheng 1999), and several studies indicated that γTuRC is recruited to the centrosome to function as a MT nucleator (Felix et al. 1994; Martin et al. 1998; Moritz et al. 1998; Schnackenberg et al. 1998). For example, electron tomographic reconstruction of isolated Drosophila and Spisula centrosomes revealed that the pericentriolar material of these centrosomes contains hundreds of γTuRC-like structures (Moritz et al. 1995; Schnackenberg et al. 1998). In addition, in vitro centrosome reconstitution assays reveal that γTuRC is essential (but not solely sufficient) for the formation of a functional centrosome (Martin et al. 1998; Moritz et al. 1998).
Analysis of γ-tubulin complexes purified from Drosophila embryos has offered important insights into the organization of the γTuRC. The Drosophila γTuRC can be dissociated into a smaller γ-tubulin–containing complex, the γ-tubulin small complex (γTuSC), that is a tetramer of two γ-tubulins and one each of the Drosophila gamma ring protein (Dgrip) 91 and Dgrip84. Stoichiometric analyses have suggested that each γTuRC consists of approximately six γTuSCs that make up the ring wall of the γTuRC as revealed by cryoelectron microscopy (Oegema et al. 1999; Wiese and Zheng 1999). Recent electron tomographic reconstruction of the Drosophila γTuRC shows that it consists of a lock-washer–shaped ring that is covered with a cap on one face (Moritz et al. 2000). When viewed from the side, the γTuRC ring consists of repeated, hairpin-shaped subunits that were proposed to correspond to γTuSCs (Moritz et al. 2000). The cap structure possibly consists of the non-γTuSC subunits named Dgrips163, 128, and 75s (Keating and Borisy 2000; Moritz et al. 2000). This structural organization suggests that the cap structure may be important for the assembly of multiple γTuSCs into one γTuRC.
Xenopus γTuRC is similar to Drosophila γTuRC in its subunit composition, structure, and function (Zheng et al. 1995; Oegema et al. 1999; Wiese and Zheng 1999). Here we report the characterization of a Xenopus gamma ring protein (Xgrip) 210 as a potential subunit for the cap structure that is required for the assembly of the γTuRC and its recruitment to the centrosome.
Materials and Methods
Buffers
Hepes 100 (mM): 50 Hepes, pH 8, 1 MgCl2, 1 EGTA, and 100 KCl. Hepes 1M: the same as Hepes 100 except that the concentration of KCl is 1 M instead of 100 mM. Cytostatic factor (CSF)–XB (mM): 10 potassium Hepes, pH 7.7, 100 KCl, 2 MgCl2, 0.1 CaCl2, 50 sucrose, and 5 EGTA. BRB80 (mM): 80 potassium Pipes, pH 6.8, 1 MgCl2, 1 EGTA. MT-stabilizing buffer (mM): 100 Pipes, pH 6.9, 5 EGTA, 10 MgCl2, 10 μg/ml Taxol.
Cloning of Xgrip210
Mouse polyclonal ascites against Xgrip210 were generated as described (Martin et al. 1998). The antibodies were used to screen a λZAP cDNA library of Xenopus oocytes (Stratagene) as described (Sambrook et al. 1989), with modifications (Hirano and Mitchison 1994). To obtain the missing 5′ end, we carried out 5′ rapid amplification of cDNA ends using a 5′/3′ RACE Kit (Boehringer). Three partially overlapping primers (gsp1, GGT GAG AAG AGT CAA TGA TGC; gsp2, TGC AGG AGT TGA TAA AAC ACA; and gsp3, CTG CAG ATA TTT CCT AAG GCC) corresponding to the 5′ region of the longest cDNA clone were used in the RACE reaction. The longest RACE product contained 234 amino acids that were missing from the original clone. We subcloned the RACE product into the 5′ end of the original cDNA construct and used the resulting construct as well as the original cDNA construct to translate proteins in rabbit reticulocyte lysates (Promega). The translated proteins were fractionated on a 7% SDS polyacrylamide gel together with Xenopus egg extract and analyzed by Western blot, probing with antibodies against Xgrip210.
Antibodies and Immunoblotting
To raise rabbit polyclonal antibodies against Xgrip210, a fusion protein was made between glutathione S-transferase (GST) and a fragment of Xgrip210 corresponding to amino acids 874–1097. This fusion protein was purified as described (Martin et al. 1998), and used to generate rabbit polyclonal antibodies. After depleting anti–GST antibodies from the antisera, the antibodies were affinity purified against the fusion protein. The antibodies against Xgrip109 and Xenopus γ-tubulin used here were described previously (Martin et al. 1998). The monoclonal antibody against γ-tubulin was commercially available (GTU-88; Sigma-Aldrich).
Immunoblotting was carried out using affinity-purified antibodies at a concentration of ∼1 μg/ml using either an ECL detection system (Amersham Pharmacia Biotech) or an alkaline phosphatase detection system (Promega).
Xenopus Egg Extract, Xenopus Demembranated Sperm, Sucrose Gradient Sedimentation, Immunoprecipitation, and Other Assays
Crude or clarified Xenopus egg extracts and demembranated Xenopus sperm were prepared as described (Martin et al. 1998). The preparation of a 30% ammonium sulfate pellet, immunodepletion, and all of the assays used in this manuscript were described previously (Martin et al. 1998). Sucrose gradient sedimentation was carried out on either 5–40% or 10–50% continuous gradients, as described (Martin et al. 1998) with minor modifications in making the 5–40% sucrose gradient. To make the 5–40% continuous sucrose gradient, step gradients were made with equal volumes of 5, 13.75, 22.5, 31.25, and 40% sucrose in Hepes 100 and allowed to diffuse into continuous gradients. This modification allowed better resolution of the Xgrip210, Xgrip109, and γ-tubulin–containing protein complexes than the gradients used previously. Fractions were collected manually from top to bottom with a cut-off pipette tip.
Cell Culture and Immunofluorescence Staining
The Xenopus cell line, XLK-WG, derived from a primary culture of Xenopus kidney cells (provided by Drs. Z. Wu and J.G. Gall, Carnegie Institution of Washington, Washington, DC) was used for immunofluorescence staining as described (Martin et al. 1998). Xgrip210 was localized to the sperm centrosome according to a previously described method (Martin et al. 1998). When double labeling γ-tubulin and Xgrips 109 or 210 was carried out, secondary antibodies against mouse or rabbit (Molecular Probes, Inc.) conjugated with Alexa Fluor 488 or 594, respectively, were used.
Photomicrographs were obtained using a cooled CCD camera (Princeton Scientific Instruments, Inc.) on a Nikon E800 microscope. Images were processed digitally using Adobe Photoshop (Adobe Systems Inc.).
Immunogold Labeling and Electron Microscopy
Recombinant dynamitin was added to Xenopus CSF egg extract at a final concentration of 0.5 mg/ml. MT nucleation in the extract was induced by DMSO (5% vol/vol final) at room temperature for 30 s, at which time the extract was diluted 50-fold with MT-stabilizing buffer plus 0.1% Triton X-100. Diluted extract was immediately loaded onto a 4-ml cushion of MT-stabilizing buffer plus 10% glycerol in a 15-ml Corex tube. At the bottom of the tube was a plastic support holding a 12-mm round coverslip with Formvar-coated EM grids attached. The Corex tubes were centrifuged in a Sorvall HB-4 swinging bucket rotor at 6,000 g for 30 min at 25°C. The coverslips with attached grids were removed and rinsed with MT-stabilizing buffer and individual grids were immunostained with Xgrip210 antibodies conjugated to 10-nm gold particles as described (Keating and Borisy 2000). Conjugation of Xgrip210 antibodies to 10-nm gold particles was performed using a method (Hughes and Beesley 1998) modified as previously described (Keating and Borisy 2000). Grids were examined with a Philips 300 TEM at 80 kV and photographed at 64,000×. Negatives were scanned at 1,200 dpi and analyzed using Adobe Photoshop. We chose microtubules that have only one gold particle at the capped ends to compare the Xgrip210 localization with γ-tubulin localization that we determined previously using the same conditions (Keating and Borisy 2000).
Results
Molecular Characterization of Xgrip210
To identify it components, Xenopus γTuRC was purified and used for protein sequencing and mouse polyclonal antibody production (Martin et al. 1998). We successfully generated mouse polyclonal antibody against Xgrip210, the largest Xgrip found in the Xenopus γTuRC. We referred to Xgrip210 as Xgrip195 in previous publications (Zheng et al. 1995; Martin et al. 1998; Oegema et al. 1999), but propose to change its name to Xgrip210 to more accurately reflect its apparent molecular mass of 210 kD.
Using the mouse polyclonal antibody, we cloned a number of cDNAs from a Xenopus oocyte cDNA expression library. The longest cDNA was joined with the additional 5′ coding sequences of Xgrip210 obtained by 5′ rapid amplification of cDNA ends. When translated in vitro, the joint cDNA clone produced a protein similar in size to Xgrip210 found in the Xenopus egg extract (data not shown). This suggests that we have the complete coding sequence for Xgrip210. The cDNA sequence (accession No. AJ291606) predicts a protein of 1,625 amino acids with a pI of 5.6.
The COOH-terminal ∼1,000 amino acids of Xgrip210 has sequence homology (>40% identity) to a partial human expressed sequence tag (No. AL022328). Therefore, we suggest that this human protein is the putative homologue of Xgrip210, which we name Hgrip210. Xgrip210 also shares homology with Dgrip163, the largest grip found in Drosophila γTuRC (Fig. 1). Both Xgrip210 and Dgrip163 contain two sequence motifs (grip motifs 1 and 2, see the underlined sequences in Fig. 1) that are conserved in all grips identified thus far (Gunawardane et al. 2000).
Figure 1.
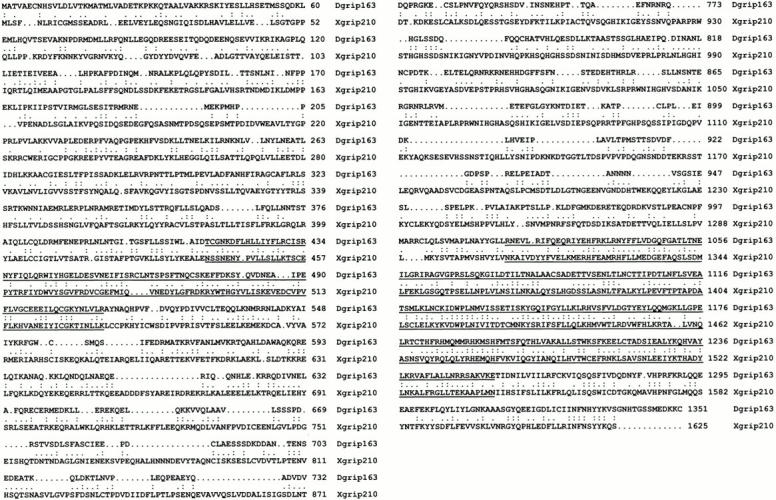
Xgrip210 defines a new family of grips of γTuRC. Sequence comparison of Xgrip210 and Dgrip163. The two sequences share over 20% amino acid identity. Double dots indicate identical amino acids, and single dot denotes the conserved changes. The sequences corresponding to grip motifs 1 and 2 found in the amino and carboxyl half of the proteins, respectively, are underlined.
Xgrip210 Is a Component of the Xenopus γTuRC and Is Localized to the Centrosomes
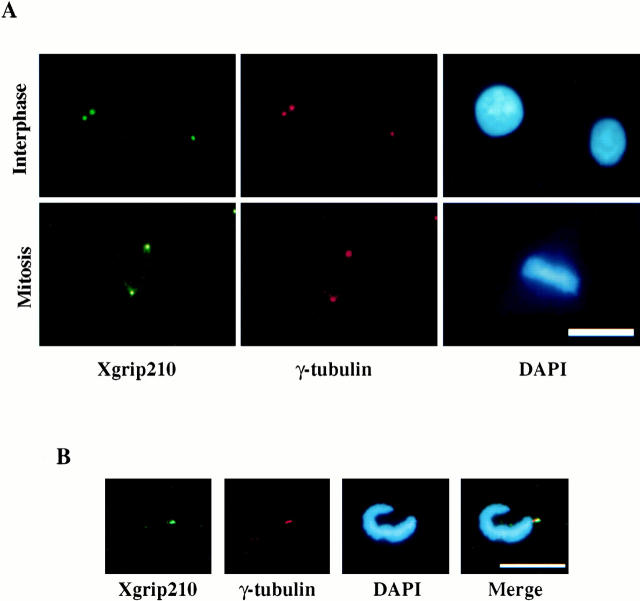
To further characterize Xgrip210, we generated rabbit polyclonal antibodies against the protein. The affinity-purified antibodies recognize a single protein in Xenopus egg extracts with the expected size for Xgrip210 (Fig. 2 A). When antibodies against Xgrip210, Xgrip109, or Xenopus γ-tubulin were used to immunoprecipitate the corresponding proteins from Xenopus egg extracts, all three antibodies immunoprecipitated the same set of Xenopus γTuRC proteins, showing that Xgrip210 is a component of the γTuRC (Fig. 2 B). In addition, Xgrip210 and γ-tubulin cosedimented on sucrose gradients (Fig. 2 C), further demonstrating that Xgrip210 is a subunit of the γTuRC. Using immunofluorescence microscopy, we localized Xgrip210 to the centrosomes of Xenopus tissue culture cells and Xenopus sperm incubated in egg extracts (Fig. 3). Therefore, like its homologue Dgrip163 (Gunawardane et al. 2000), Xgrip210 is a centrosomal protein.
Figure 2.
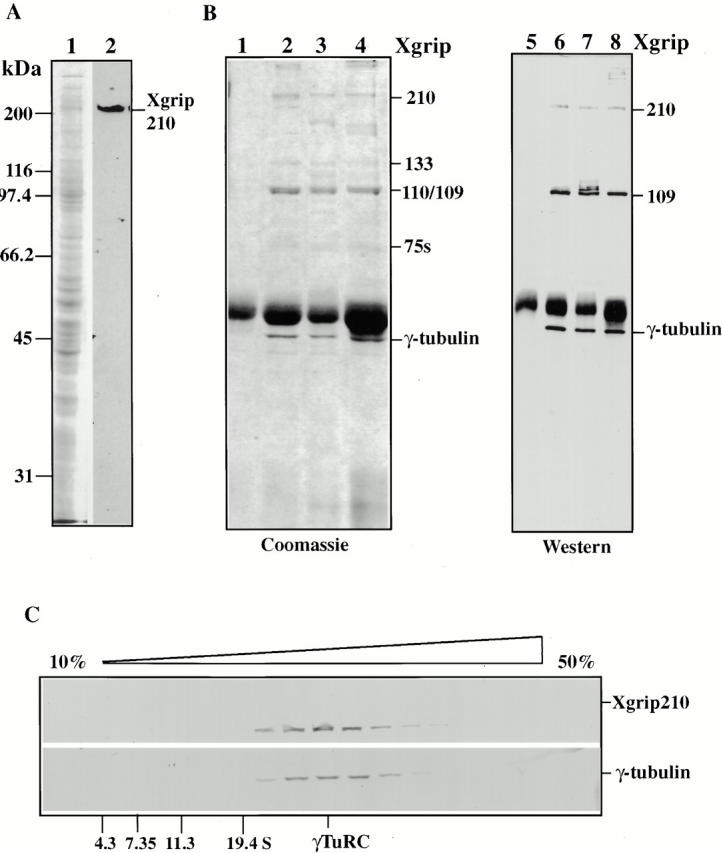
Xgrip210 is a component of Xenopus γTuRC. (A) Crude Xenopus egg extracts were analyzed by Coomassie blue staining (1) or by Western blot, probing with anti–Xgrip210 antibodies (2). The antibodies specifically recognized a protein of the expected size for Xgrip210. (B) Random rabbit IgG (1 and 5) and antibodies against Xgrips210 (2 and 6) or 109 (3 and 7) or γ-tubulin (4 and 8) were used to immunoprecipitate the respective proteins from Xenopus egg extracts. The immunoprecipitates were analyzed by SDS-PAGE followed by Coomassie blue staining (1–4) or Western blot, probing with each of these antibodies (5–8). All three antibodies immunoprecipitated the same γTuRC subunits. Although the Xgrip210 antibody specifically recognized Xgrip210 in the egg extracts (A, 2), the antibody recognized several high molecular weight proteins in the Xgrip210 immunoprecipitate (B, 8). The nature of these cross-reactive proteins is currently unknown. (C) Clarified Xenopus egg extract or protein standards with S values of 4.3 S (bovine serum albumin), 7.35 S (rabbit muscle aldolase), 11.3 S (bovine liver catalase), and 19.4 S (porcine thyroglobulin) were run on parallel 10–50% sucrose gradients. The fractions were analyzed by Western blot, probing for γ-tubulin or Xgrip210 or by Coomassie blue staining for the protein standards.
Figure 3.
Xgrip210 colocalizes with γ-tubulin at the centrosome. (A) Immunofluorescence staining of Xenopus tissue culture cells with antibodies against Xenopus γ-tubulin and Xgrip210. (B) Immunofluorescence staining of Xenopus sperm centrosomes with antibodies against Xenopus γ-tubulin and Xgrip210. Nuclear DNA in A and B was stained by DAPI. Scale bars: 20 μm.
Xgrip210 Localizes to the Distal Portion of the γTuRC-capped MT End
Structural studies showed that the minus ends of MTs nucleated from the γTuRC have a distinct cap (Keating and Borisy 2000; Moritz et al. 2000; Wiese and Zheng 2000). The distal portion of this cap is thought to correspond to the cap structure of the γTuRC (Moritz et al. 2000). We have previously localized γ-tubulin and Xgrip109 to the ends of MT capped by γTuRC using immunogold labeling (Keating and Borisy 2000). To define the ultra-structural localization of Xgrip210, we used the same method and identical conditions to localize Xgrip210 (Keating and Borisy 2000). MT were nucleated in CSF-arrested Xenopus egg extracts and immunostained using antibodies against Xgrip210 conjugated directly to 10-nm gold particles (Keating and Borisy 2000). Of the γTuRC-capped MT (n = 136 from three experiments), 42 ± 7% had one or more gold particles at the capped end.
To obtain finer resolution, we selected only those capped MT that had one gold particle at the end (Fig. 4 A). This procedure avoided including gold particles that were present as part of a cluster and not directly contacting Xgrip210. For each MT, the position of the center of the gold particle was measured relative to the end of the MT lattice. The positions of the gold particles were plotted and are shown superimposed on the image of a typical capped MT end (Fig. 4 B). Examination of individual MT ends revealed that a large proportion of gold particles were found at or near the pointed end of the capped MT (Fig. 4 B), a position corresponding to the cap structure of the γTuRC. More than two thirds (71%) of the gold particles were within 15 nm of the tip of the γTuRC cap, a distance at which the projecting antibodies could still contact Xgrip210 at the tip of the cap, suggesting that Xgrip210 is in this region.
Figure 4.
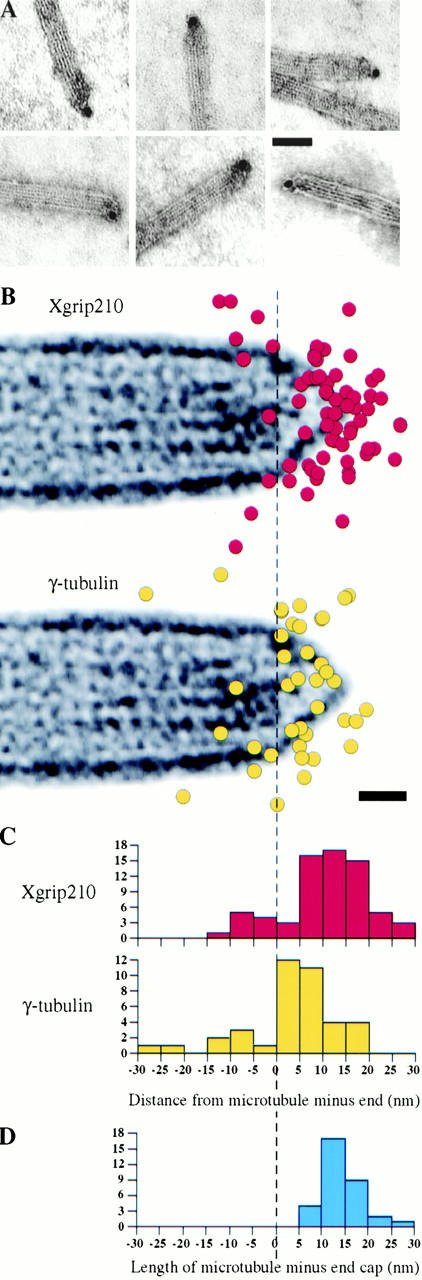
Immunogold localization of Xgrip210. (A) Examples of MTs with single 10-nm anti–Xgrip210 gold particles at the MT ends capped by γTuRC. In all cases shown, the center of the gold particle was within 15 nm of the tip of the end cap. Scale bar: 50 nm. (B) Distribution of anti–Xgrip210 or anti–γ-tubulin gold particles superimposed on an image of a typical γTuRC-capped MT end. Scale bar: 10 nm. (C) Distance distribution of the gold particles to the base of the MT lattice (dashed line), measured along the long axis of the MTs (red, Xgrip210; yellow, γ-tubulin). The average distance of the anti–Xgrip210 and anti–γ-tubulin gold particles from the base of the MT end are 10.4 ± 8.8 nm (n = 69) for Xgrip210 and 3.7 ± 9.9 nm (n = 39; Keating and Borisy 2000) for γ-tubulin. (D) Distribution of lengths of the microtubule end cap measured from the end of the microtubule lattice (B, dashed line) to the tip, from a representative sample of microtubules. The average length of the cap was 14.3 ± 3.7 nm (n = 33).
We then compared the gold particle distribution of Xgrip210 to that of γ-tubulin, determined previously (Keating and Borisy 2000; Fig. 4 B). The anti–Xgrip210 gold particles were further from the end of the MT lattice and closer to the central axis of the MT than those directed against γ-tubulin (Fig. 4B and Fig. C). The average distance of the Xgrip210 gold particles from the end of the MT lattice was 10.4 ± 8.8 nm (n = 69), which is significantly different (P < 0.05, independent t test) from the average distance for the γ-tubulin gold particles (3.7 ± 9.9 nm, n = 39; Keating and Borisy 2000). This can also be seen by comparing the histograms of the distances of the gold particles from the base of the MT lattice (Fig. 4 C), which shows that the distribution of anti–Xgrip210 particles is shifted further away from the end of the MT lattice than the anti–γ-tubulin particles. We have also measured the distance between the base of the microtubule lattice and the tip of the γTuRC cap from 33 microtubules and found it to be 14.3 ± 3.7 nm. The distance distribution of the tip of the cap overlapped with the majority of the Xgrip210 gold particles (Fig. 4C and Fig. D). Structural studies of γTuRC suggested that MTs nucleated by γTuRC grow from the face of the γTuRC that is not covered by the cap structure (Keating and Borisy 2000; Moritz et al. 2000). Therefore, the localization of Xgrip210 is consistent with the idea that Xgrip210 is a component of the γTuRC cap structure that is distal to the base of MTs.
Xgrip210 Is Essential for MT Nucleation from the Sperm Centrosome
To study whether Xgrip210 is essential for γTuRC function, we used assays described previously (Martin et al. 1998) to examine whether Xenopus γTuRC can support centrosome assembly and MT nucleation in the absence of Xgrip210. Xenopus egg extracts support the assembly of a functional sperm centrosome in the presence of an ATP regenerating system in vitro (Felix et al. 1994; Stearns and Kirschner 1994). We previously showed that immunodepleting γTuRC from the egg extract completely inhibits sperm centrosome assembly. This can be reversed by adding purified Xenopus γTuRC or partially purified Xenopus γTuRC obtained in the pellet after precipitation of egg extract proteins with 30% ammonium sulfate (Martin et al. 1998). γTuRC precipitated with 30% ammonium sulfate could be dissociated with 1 M salt and reassembled upon removal of the salt, producing functional γTuRC capable of complementing the γTuRC-depleted egg extract to support sperm centrosome assembly (Martin et al. 1998).
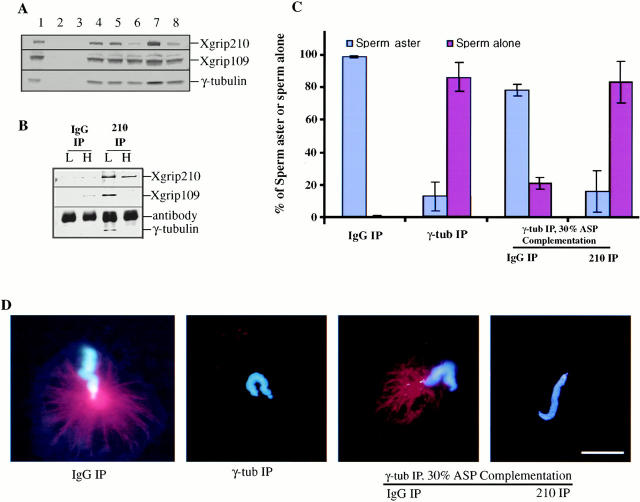
If Xgrip210 is essential for γTuRC function, its removal from the γTuRC should abolish the ability of the remaining γTuRC components to complement the γTuRC-depleted egg extract in the centrosome assembly assays. To test this, we precipitated Xenopus γTuRC from the egg extract with 30% ammonium sulfate, resuspended the pellet in buffer containing 1 M salt, and immunodepleted Xgrip210 with anti–Xgrip210 antibodies. Control reactions were immunoprecipitated with random rabbit IgG. Following salt removal and concentration, the protein mixture was used to complement the γTuRC-depleted Xenopus egg extract in centrosome assembly assays. Western blot analysis of the supernatant and pellet of the immunoprecipitations showed that most of the Xgrip210 (>70%) was removed by anti–Xgrip210 antibodies. There was no significant reduction of γ-tubulin or Xgrip109 compared with random IgG control, because Xgrip210 antibody did not coimmunoprecipitate Xgrip109 or γ-tubulin in high salt (Fig. 5A and Fig. B). Centrosome assembly assays showed that removal of Xgrip210 almost completely blocked the formation of sperm asters (Fig. 5C and Fig. D). This suggests that Xgrip210 was required for γTuRC function.
Figure 5.
Immunodepletion of Xgrip210 inhibits sperm centrosome formation. (A) Western blot analysis of immunodepleted egg extracts and 30% ammonium sulfate fractions. Xenopus egg extract immunodepleted with either random IgG (1) or antibodies against Xenopus γ-tubulin (2). 30% ammonium sulfate supernatant (3) and pellet (4). 30% ammonium sulfate pellet (30% ASP) resuspended in Hepes 1M and immunodepleted with either random IgG (5) or antibodies against Xgrip210 (6). 7 and 8 are the same as 5 and 6, respectively, except that the proteins were desalted into Hepes 100 and concentrated ∼10-fold. (B) Immunoprecipitates of random IgG (IgG IP) and Xgrip210 antibody (210 IP) from high (H) or low (L) salt-treated 30% ASP were analyzed by Western blot, probing with antibodies against Xgrips210 or 109, or γ-tubulin. Xgrip210 antibody did not immunoprecipitate significant amounts of Xgrip109 or γ-tubulin in high salt conditions. (C) The blue and red columns represent the percentages of sperm with or without associated MT asters, respectively. Immunodepletion of the egg extract with random IgG did not inhibit the formation of sperm centrosomes (IgG IP). The inhibition of sperm aster formation by immunodepleting γ-tubulin (γ-tub IP) was relieved by complementing with the 30% ASP that was depleted with random IgG (IgG IP), but not by the 30% ASP that was depleted with antibodies against Xgrip210 (210 IP). Error bars represent standard deviations calculated from three independent experiments. (D) Representative sperm nuclei with or without a MT aster from the assays in C are shown. Scale bar: 20 μm. The MTs were labeled by inclusion of a small amount of rhodamine-tubulin in the assays, and the sperm DNA was stained with DAPI.
Xgrip210 Is Essential for the Recruitment of γ-Tubulin and Xgrip109 to the Centrosome
To determine whether γ-tubulin and Xgrip109 were recruited to the centrosome in the absence of Xgrip210, we carried out the immunoprecipitation and centrosome assembly assays as described above except that nocodazole was added to the reactions to prevent MT formation. We found that it was easier to visualize the immunofluorescence staining of sperm centrosomes in the absence of MT asters. Neither γ-tubulin nor Xgrip109 localized to the sperm tips (where the sperm centrosomes are located) in the absence of Xgrip210 (Fig. 6).
Figure 6.
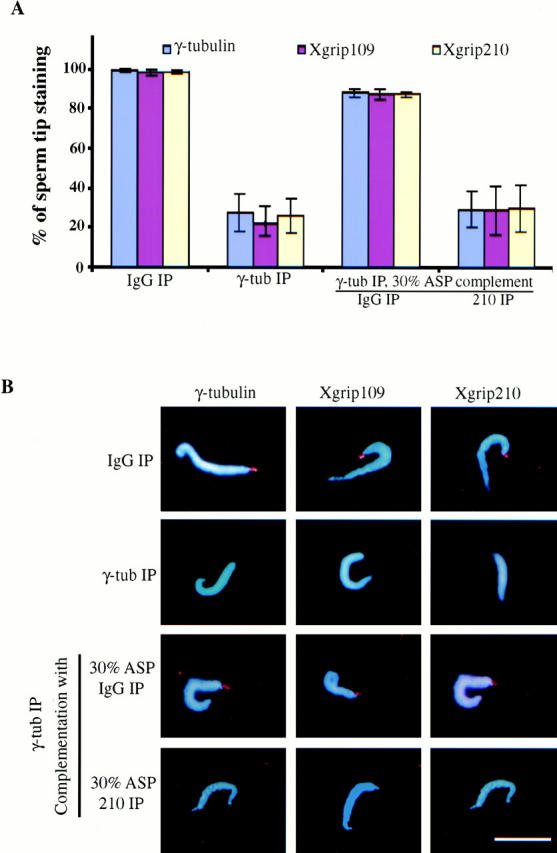
Immunodepleting Xgrip210 inhibits the recruitment of Xgrip109 and γ-tubulin to the sperm centrioles. (A) Blue, red, and yellow columns represent the percentage of sperm tips having γ-tubulin, Xgrip109, or Xgrip210 staining, respectively. Immunodepletion of γ-tubulin from Xenopus egg extracts (γ-tub IP) resulted in ∼70% reduction of the recruitment of γ-tubulin, Xgrip109, or Xgrip210 to the sperm tips, while the same treatment with random IgG (IgG IP) had no effect. Complementing the γ-tubulin–depleted egg extract with the 30% ASP immunodepleted with random IgG (IgG IP) restored the localization of γ-tubulin, Xgrip109, and Xgrip210 to the sperm tips, while the same treatment with anti–Xgrip210 antibodies (210 IP) had no effect. Error bars: standard deviations calculated from three independent experiments. (B) Merged images of sperm incubated in extract immunodepleted with either random IgG (IgG IP) or anti–γ-tubulin antibodies (γ-tub IP), and γ-tubulin–depleted extract complemented with either mock-depleted (30% ASP IgG IP) or Xgrip210-depleted (30% ASP 210 IP) 30% ASP. The images were pseudocolored. Sperm DNA was stained with DAPI and merged with the antibody staining of the sperm tip. In cases where the images appear to be similar, it is due to double labeling of γ-tubulin (mouse monoclonal antibody; Sigma-Aldrich) and Xgrip210 (rabbit polyclonal antibodies). Scale bar: 20 μm.
Xgrip210 Is Not Localized at the Sperm Centrosome in the Absence of Xgrip109
Next, we asked whether Xgrip210 could localize to the centrosome in the absence of Xgrip109. The experiment was performed as described above, except that Xgrip109 was depleted. We found that there was no significant coprecipitation of Xgrip210 with Xgrip109 (Fig. 7A and Fig. B). However, consistent with our previous finding (Martin et al. 1998), ∼50% of total γ-tubulin coprecipitated with Xgrip109 (Fig. 7A and Fig. B). Immunofluorescence staining with anti–Xgrip210 antibodies revealed that Xgrip210 failed to localize to the sperm tip in the absence of Xgrip109 (Fig. 7C and Fig. D). Therefore, Xgrip109 is necessary for the localization of Xgrip210 to the sperm centrosome.
Figure 7.
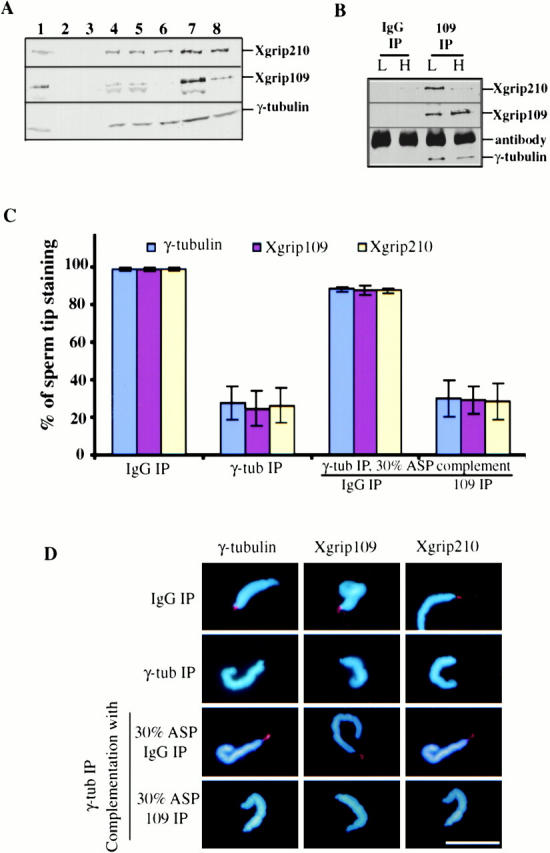
Immunodepletion of Xgrip109 inhibits the recruitment of Xgrip210 to the sperm centrioles. (A) Western blot analysis of the extracts and fractions used in the assays. Xenopus egg extract immunodepleted with either random IgG (1) or antibodies against Xenopus γ-tubulin (2). 30% ammonium sulfate supernatant (3) and pellet (4). 30% ASP resuspended in Hepes 1M and immunodepleted with either random IgG (5) or antibodies against Xgrip109 (6). 7 and 8 are the same as 5 and 6, respectively, except that the proteins were desalted into Hepes 100 and concentrated ∼10-fold. (B) Immunoprecipitates of random IgG (IgG IP) and Xgrip109 antibody (109 IP) from high (H) or low (L) salt-treated 30% ASP were analyzed by Western blot, probing with antibodies against Xgrips210, 109, or γ-tubulin. Xgrip109 antibody did not coimmunoprecipitate a significant amount of Xgrip210 in the high salt conditions. (C) Blue, red, and yellow columns represent the percentage of sperm that have γ-tubulin, Xgrip109, and Xgrip210 staining at the tips, respectively. Immunodepletion of γ-tubulin from Xenopus egg extracts (γ-tub IP) resulted in >70% reduction of the recruitment of γ-tubulin, Xgrip109, or Xgrip210 to the sperm tips, while the same treatment with random IgG (IgG IP) had no effect. Complementing the γ-tubulin–depleted egg extract with the 30% ASP immunodepleted with random IgG (IgG IP) restored the localization of γ-tubulin, Xgrip109, and Xgrip210 to the sperm tips, while the same treatment with anti–Xgrip109 antibodies (109 IP) had no effect. Error bars: standard deviations calculated from three independent experiments. (D) Representative sperm from different treatments stained with antibodies against γ-tubulin, Xgrip109, or Xgrip210 are shown. In cases where the images appear to be similar, it is due to double labeling of γ-tubulin and Xgrip210. Scale bar: 20 μm.
Xgrip210 Is Essential for the Assembly of the γTuRC
We then examined whether Xgrip210 is essential for γTuRC assembly. γTuRC was partially purified by 30% ammonium sulfate and dissociated with high salt. Xgrip210 was immunoprecipitated from the salt-dissociated γTuRC with random IgG precipitation as controls. After immunoprecipitation, the protein solutions were desalted and analyzed by sucrose gradient sedimentation followed by Western blotting to detect different γTuRC components. Treatment with 1 M salt completely disassembled the γTuRC (compare Fig. 8A with E). Immunodepletion of Xgrip210 blocked γTuRC reassembly from the salt-dissociated components (Fig. 8 D). In contrast, random IgG treatment allowed the reassembly of a fraction of the γTuRC (Fig. 8 C). These results suggest that Xgrip210 is required for the formation of the γTuRC. A fraction of Xgrip210 was aggregated by the high salt treatment (Fig. 8 A). However, under the same conditions, neither γ-tubulin nor Xgrip109 aggregated; instead, they cosedimented with an S value expected for that of the γTuSC (Fig. 8A and Fig. B). This suggests that Xgrip210 was not required for the formation of γTuSC (Fig. 8B and Fig. D, see also Discussion).
Figure 8.
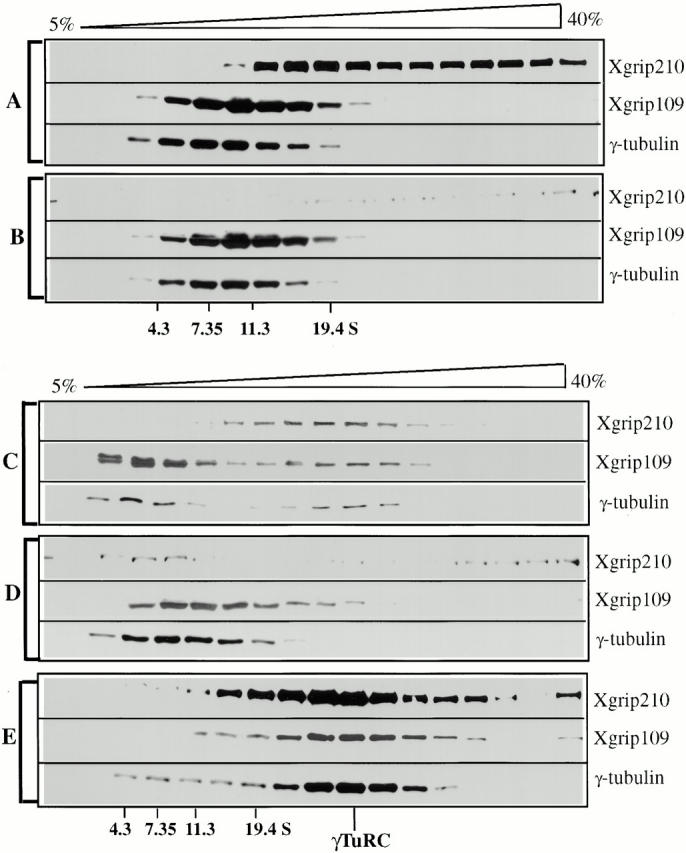
Xgrip210 is required for γTuRC assembly. The γTuRC present in 30% ASP was dissociated by Hepes 1M and immunodepleted with either random IgG (A) or Xgrip210 antibody (B) followed by analysis on 5–40% continuous sucrose gradients made in Hepes 100. Alternatively, after immunodepletion, salt concentrations were reduced by passing the immunodepleted mixtures through desalting columns equilibrated with Hepes 100, and then analyzed by sucrose gradient sedimentation (C and D). Random IgG depletion allowed the reassembly of a fraction of the Xgrip210, Xgrip109, and γ-tubulin into the γTuRC-size complex (C), whereas immunodepleting Xgrip210 completely blocked reassembly (D). (E) 30% ASP was resuspended in Hepes 100 and fractionated on identical sucrose gradients as a control. All gradient fractions were analyzed by Western blot, probing with antibodies against Xgrips210 or 109, or γ-tubulin. The intact γTuRC in E migrated to the same position as that of the reassembled γTuRC shown in C. Protein standards used (S values indicated) were the same as described in Fig. 3 C.
Discussion
Since the discovery of γTuRC, a general picture has emerged regarding the organization of this complex. The tetrameric subcomplex γTuSC appears to be the major building block of the lock washer–shaped ring that is covered by a distinct cap on one face of the ring (Oegema et al. 1999; Keating and Borisy 2000; Moritz et al. 2000). The cap structure was hypothesized to consist of the non–γTuSC grips (Moritz et al. 2000). In Xenopus γTuRC, these grips are Xgrips210, 133, and 75s. Clearly, characterizing the function of these grips is essential to understanding γTuRC assembly and function. However, little progress has been made because the protein sequences of most of these grips were unknown. We have now cloned one of these subunits, Xgrip210. Probing the functions of Xgrip210 biochemically and structurally has provided important insights into the mechanism of γTuRC assembly and recruitment.
Xgrip210 and γTuRC Assembly
When Xenopus γTuRC was dissociated with 1 M salt, Xgrip109 and γ-tubulin comigrated on sucrose gradients with an S value of ∼11 S, similar to that of Drosophila γTuSC (Fig. 8). Since approximately six molecules of γTuSC are assembled into one γTuRC, the multiple γTuSCs might be held together by the non-γTuSC subunits, Xgrips210, 133, and 75s to form the γTuRC. Indeed, we found that Xgrip210 is required for γTuRC assembly (Fig. 8). However, based on our studies of its homologue, Dgrip163, we believe that Xgrip210 alone would not be sufficient for the assembly of multiple γTuSCs into γTuRC (Gunawardane et al. 2000). Therefore, we propose that Xgrips210, 133, and 75s are all required for the formation of the cap structure and that this structure is important for γTuRC assembly. Consistent with this idea, our ultrastructural studies revealed that Xgrip210 was most likely localized to the cap structure of the γTuRC (Fig. 4).
Xgrip210 and the Recruitment of γTuRC to the Centrosome
Several studies have strongly suggested that γTuRC is recruited to the centrosome to nucleate MTs. However, how γTuRC is recruited and docked at the centrosome remains unknown. We showed that Xgrip109 and γ-tubulin cosedimented with an S value expected for a γTuSC on sucrose gradients in the absence of Xgrip210. However, neither Xgrip109 nor γ-tubulin was localized to the centrosome in the absence of Xgrip210, revealing, for the first time, that γTuSC cannot bind to the centrosome on its own. Therefore, γTuSC is unlikely to be responsible for tethering the γTuRC to the centrosome. We also found that Xgrip210 cannot localize to the centrosome in the absence of Xgrip109. This suggests that Xgrip210 does not contain the localization signal or that the signal is insufficient for its localization.
Based on these findings, we propose the following two models to account for the recruitment and docking of the γTuRC to the centrosome. In the first model, the recruiting/docking factor(s) could recognize the shape or a large surface area of the intact γTuRC, but not the individual components. In this model, perturbations that disrupt the γTuRC structure would block the recruiting/docking of all the γTuRC subunits to the centrosome. Alternatively, one of the remaining uncharacterized γTuRC subunits, Xgrip133 or Xgrip75s, could be involved in recruiting and docking the γTuRC to the centrosome. In this case, Xgrip133 or Xgrip75s should be localized to the centrosome independent of the other γTuRC subunits. Clearly, further studies of Xgrips133 and 75s will help us to differentiate these γTuRC recruitment models.
Acknowledgments
We thank Drs. Z. Wu and J. Gall for providing the Xenopus cell lines, and the members of the Zheng lab (C. Wiese, R. Gunawardane, O. Martin, and S. Lizarraga) for critical reading of the manuscript.
This work was supported by grants from National Institutes of Health (RO1-GM56312-01) and the Pew Scholar's Award to Y. Zheng.
Footnotes
Dr. Keating's and Dr. Borisy's present address is Department of Cell and Molecular Biology, Northwestern University Medical School, Chicago, IL 60611.
Abbreviations used in this paper: CSF, cytostatic factor; Dgrip, Drosophila gamma ring protein; γTuRC, γ-tubulin ring complex; γTuSC, γ-tubulin small complex; MT, microtubule; Xgrip, Xenopus gamma ring protein.
References
- Desai A., Mitchison T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1998;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- Felix M.A., Antony C., Wright M., Maro B. Centrosome assembly in vitrorole of γ-tubulin recruitment in Xenopus sperm aster formation. J. Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R.R., Borisy G.G. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J. Cell Biol. 1977;73:601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane R.N., Martin O.C., Cao K., Zhang L., Dej K., Iwamatsu A., Zheng Y. Characterization and reconstitution of Drosophila γ-tubulin ring complex subunits. J. Cell Biol. 2000;151:1513–1523. doi: 10.1083/jcb.151.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Habermann A., Karsenti E., Hyman A. Spindle assembly in Xenopus egg extractsrespective roles of centrosomes and microtubule self-organization. J. Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Mitchison T. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell. 1994;79:449–458. doi: 10.1016/0092-8674(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Horio T., Uzawa S., Jung M.K., Oakley B.R., Tanaka K., Yanagida M. The fission yeast gamma-tubulin is essential for mitosis and is localized at microtubule organizing centers. J. Cell Sci. 1991;99:693–700. doi: 10.1242/jcs.99.4.693. [DOI] [PubMed] [Google Scholar]
- Hughes D., Beesley J. Preparation of colloidal gold probes. Methods Mol. Biol. 1998;80:275–282. doi: 10.1007/978-1-59259-257-9_29. [DOI] [PubMed] [Google Scholar]
- Hyman A., Karsenti E. The role of nucleation in patterning microtubule networks. J. Cell Sci. 1998;111:2077–2083. doi: 10.1242/jcs.111.15.2077. [DOI] [PubMed] [Google Scholar]
- Keating D., Borisy G. Immunostructural evidence for the template mechanism of microtubule nucleation. Nat. Cell Biol. 2000;2:352–357. doi: 10.1038/35014045. [DOI] [PubMed] [Google Scholar]
- Kellogg D.R., Moritz M., Alberts B.M. The centrosome and cellular organization. Annu. Rev. Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- Marschall L., Jeng R., Mulholland J., Stearns T. Analysis of Tub4p, a yeast γ-tubulin–like proteinimplications for microtubule-organizing center function. J. Cell Biol. 1996;134:443–454. doi: 10.1083/jcb.134.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin O.C., Gunawardane R., Iwamatsu A., Zheng Y. Xgrip109a γ-tubulin–associated protein with an essential role in γ-tubulin ring complex (γTuRC) assembly and centrosome function. J. Cell Biol. 1998;141:675–687. doi: 10.1083/jcb.141.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T.J., Kirschner M.W. Dynamic instability of microtubule growth Nature. 312 1984. 237 242a [DOI] [PubMed] [Google Scholar]
- Mitchison T.J., Kirschner M.W. Microtubule assembly nucleated by isolated centrosomes Nature. 312 1984. 232 237b [DOI] [PubMed] [Google Scholar]
- Moritz M., Braunfeld M., Guenebaut V., Heuser J., Agard D. Structure of the γ-tubulin ring complexa template for microtubule nucleation. Nat. Cell Biol. 2000;2:365–370. doi: 10.1038/35014058. [DOI] [PubMed] [Google Scholar]
- Moritz M., Braunfeld M.B., Fung J.C., Sedat J.W., Alberts B.M., Agard D.A. Three dimensional structural characterization of centrosomes from early Drosophila embryos. J. Cell Biol. 1995;130:1149–1159. doi: 10.1083/jcb.130.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Zheng Y., Alberts B., Oegema K. Recruitment of the γ-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol. 1998;142:775–786. doi: 10.1083/jcb.142.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B.R. γ-Tubulinthe microtubule organizer? Trends Cell Biol. 1992;2:1–5. doi: 10.1016/0962-8924(92)90125-7. [DOI] [PubMed] [Google Scholar]
- Oakley B.R., Oakley C.E., Yoon Y., Jung M.K. γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans . Cell. 1990;61:1289–1301. doi: 10.1016/0092-8674(90)90693-9. [DOI] [PubMed] [Google Scholar]
- Oakley C.E., Oakley B.R. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans . Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Oegema K., Wiese C., Martin O.C., Milligan R., Iwamatsu A., Mitchison T., Zheng Y. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 1999;144:721–733. doi: 10.1083/jcb.144.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E., Maniatis T. Molecular CloningA Laboratory Manual. Vol. 2 1989. Cold Spring Harbor Laboratory Press, ; Cold Spring Harbor, NY: C. Nolan, editor. 8.46. pp. p [Google Scholar]
- Schnackenberg B., Khodjakov A., Rieder C., Palazzo R. The disassembly and reassembly of functional centrosomes in vitro. Proc. Natl. Acad. Sci. USA. 1998;95:9295–9300. doi: 10.1073/pnas.95.16.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel S., Synder M. A highly divergent gamma-tubulin gene is essential for cell growth and proper microtubule organization in Saccharomyces cerevisiae . J. Cell Biol. 1995;131:1775–1788. doi: 10.1083/jcb.131.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A., Geissler S., Grein K., Schiebel E. Gamma-tubulin–like Tub4p of Saccharomyces cerevisiae is associated with the spindle pole body substructures that organize microtubules and is required for mitotic spindle formation. J. Cell Biol. 1996;134:429–441. doi: 10.1083/jcb.134.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Kirschner M. In vitro reconstitution of centrosome assembly and functionthe role of γ-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Sunkel C., Gomes R., Sampaio P., Perdigao J., Gonzalez C. Gamma-tubulin is required for the structure and function of the microtubule organizing centre in Drosophila neuroblasts. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:28–36. doi: 10.1002/j.1460-2075.1995.tb06972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavosanis G., Llamazares S., Goulielmos G., Gonzalez C. Essential role for gamma tubulin in the acentriolar female meiotic spindle of Drosophila . EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:1809–1819. doi: 10.1093/emboj/16.8.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C., Zheng Y. Gamma-tubulin complexes and their interaction with microtubule-organizing centers. Curr. Opin. Struct. Biol. 1999;9:250–259. doi: 10.1016/S0959-440X(99)80035-9. [DOI] [PubMed] [Google Scholar]
- Wiese C., Zheng Y. A new function for the γ-tubulin ring complex as a microtubule minus-end cap. Nat. Cell Biol. 2000;2:358–364. doi: 10.1038/35014051. [DOI] [PubMed] [Google Scholar]
- Wilson E.B. The Cell in Development and Heredity 1925. Garland Publishing, ; New York, NY: J.A. Moore, editor. pp. 1987 pp [Google Scholar]
- Zheng Y., Wong M., Alberts B., Mitchison T. Nucleation of microtubule assembly by a gamma-tubulin–containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]