Abstract
Association between the ER and mitochondria has long been observed, and the formation of close contacts between ER and mitochondria is necessary for the ER-mediated sequestration of cytosolic calcium by mitochondria. Autocrine motility factor receptor (AMF-R) is a marker for a smooth subdomain of the ER, shown here by confocal microscopy to be distinct from, yet closely associated with the calnexin- or calreticulin-labeled ER. By EM, smooth ER AMF-R tubules exhibit direct interactions with mitochondria, identifying them as a mitochondria-associated smooth ER subdomain. In digitonin-permeabilized MDCK cells, the addition of rat liver cytosol stimulates the dissociation of smooth ER and mitochondria under conditions of low calcium. Using BAPTA chelators of various affinities and CaEGTA buffers of defined free Ca2+ concentrations and quantitative confocal microscopy, we show that free calcium concentrations <100 nM favor dissociation, whereas those >1 μM favor close association between these two organelles. Therefore, we describe a cellular mechanism that facilitates the close association of this smooth ER subdomain and mitochondria when cytosolic free calcium rises above physiological levels.
Keywords: free calcium, autocrine motility factor receptor, AMF-R tubule, cytosol, semipermeabilized cell assay
Introduction
A close association between ER and mitochondria has been described in various cell types. Close contacts between the ER membrane and the mitochondrial outer membrane have been visualized by EM (Franke and Kartenbeck 1971; Morre et al. 1971; Montisano et al. 1982), and recent electron tomography studies have identified 15-nm diameter sites of close contact between the membranes of these two organelles (Perkins et al. 1997). Subcellular fractionation of cells identified an ER membrane fraction that copurifies with mitochondria (Lewis and Tata 1973; Shore and Tata 1977) and ER-derived mitochondria-associated membrane (MAM) fractions have been implicated in phospholipid transfer between the two organelles (Vance 1990; Shiao et al. 1998; Achleitner et al. 1999).
Interaction between ER and mitochondria has also been implicated in the sequestration and regulation of free cytosolic calcium (Ca2+; for review see Pozzan et al. 1994). Ca2+ release due to the generation of inositol 1,4,5-triphosphate (IP3) in response to receptor activation results in the formation of high local concentrations of Ca2+ at sites of close contact between ER and mitochondria that results in increases in mitochondrial Ca2+ (Rizzuto et al. 1993, Rizzuto et al. 1998; Simpson et al. 1997). ER-mediated Ca2+ uptake by mitochondria may activate mitochondrial metabolic activity by stimulating Ca2+-dependent mitochondrial dehydrogenases (Denton and McCormack 1990; Rutter et al. 1996; Jouaville et al. 1999), as well as regulate the subcellular pattern of Ca2+ oscillations generated by receptor activation (Rizzuto et al. 1994; Landolfi et al. 1998; Robb-Gaspers et al. 1998; Hajnoczky et al. 1999). Close contacts between ER and mitochondria, particularly under conditions of high local free cytosolic Ca2+, are therefore of functional importance.
We have identified a smooth ER subdomain specifically labeled for autocrine motility factor receptor (AMF-R; Benlimame et al. 1995). By EM, smooth AMF-R–labeled tubules exhibit continuity with the ribosome-studded tubules of the rough ER, and after treatment with ilimaquinone, form a highly fenestrated network of smooth tubules morphologically equivalent to the smooth ER of the hepatocyte (Benlimame et al. 1995; Wang et al. 1997). AMF-R tubules can be distinguished from the ER-Golgi intermediate compartment (ERGIC) by fluorescence microscopy and therefore represent a distinct smooth ER subdomain (Wang et al. 1997). We show here that smooth ER AMF-R tubules are intimately associated with mitochondria. Using a semipermeabilized cell assay, we further show that this association can be disrupted by cytosol in the presence of low cytosolic free Ca2+ levels. Elevated cytosolic free Ca2+ inhibits the ability of cytosol to dissociate the two organelles identifying a mechanism that facilitates the close association between the ER and mitochondria necessary for mitochondrial Ca2+ uptake.
Materials and Methods
Cells, Antibodies, and Chemicals
MDCK II cells were grown in DME supplemented with 10% FCS, nonessential amino acids, vitamins, glutamine, and a penicillin–streptomycin antibiotic mixture in an air 5% CO2 incubator at constant humidity.
Monoclonal IgM antibody against AMF-R was used in the form of concentrated hybridoma supernatant (Nabi et al. 1990). Polyclonal antibody to calreticulin was kindly provided by Dr. Luis Rokeach (Department of Biochemistry, Université de Montréal, Quebec, Canada) and to calnexin by Dr. John Bergeron (Department of Cell Biology and Anatomy, McGill University, Montreal, Quebec, Canada). Antibody to mitochondrial heat shock protein 70 (Mt-HSP70; clone JG1) was purchased from Affinity Bioreagents, Inc. Secondary antibodies conjugated to either Texas red or FITC were purchased from Jackson ImmunoResearch Laboratories. DTT was purchased from Sigma-Aldrich, and digitonin was purchased from ICN. The K2H2EGTA and K2CaEGTA solutions (Calibration Buffer Concentrate Kit) used to prepare the CaEGTA buffers. 5,5′-dimethyl BAPTA-AM, BAPTA-AM, 5,5′-difluoro BAPTA-AM, and 5,5′-dibromo BAPTA-AM were purchased from Molecular Probes.
Confocal and Electron Microscopy
Immunofluorescence labeling of 80/20% (vol/vol) methanol/acetone fixed MDCK cells for AMF-R, calnexin, calreticulin, and Mt-HSP70, and postembedding immunoelectron microscopic labeling for AMF-R were performed as previously described (Benlimame et al. 1995; Wang et al. 1997). Confocal images were obtained with a BioRad MRC-600 confocal microscope and EM images with a Phillips 300 electron microscope. The minimal ER-mitochondria distance was quantified by measuring from 14 randomly taken EM images (21,000×), the distance between the point on each ER tubule, smooth or rough, labeled or not for AMF-R, closest to a mitochondrion within the same cell. Rough ER tubules were identified by the presence of a linear array of membrane-associated ribosomes.
Preparation of Rat Liver Cytosol
Rat liver cytosol was prepared from cleaned rat liver cut into small pieces by scissors in buffer A (25 mM Tris, 25 mM KCl, 1 mM DTT, pH 7.4) supplemented with 85 mM sucrose and protease inhibitors at 4°C and homogenized at 4°C in 1 vol of the same buffer. The homogenate was centrifuged at 11,060 g for 20 min and the supernatant recentrifuged at 100,000 g for 90 min at 4°C. The supernatant was collected and assayed for protein concentration. Cytosol was heat-inactivated by boiling for 5 min.
In Vitro Assay for Smooth ER Mitochondria Association
MDCK cells plated at 25,000–50,000 cells/35-mm plate on glass cover slips for 2 d were quickly washed twice with cytoskeleton stabilizing buffer (CSB; 130 mM Hepes, 2 mM MgCl2, 10 mM EGTA, pH 6.9) prewarmed to 20°C and then incubated with 10 μg/ml digitonin in buffer A for 1 min at 20°C. The cells were then washed with CSB and incubated in buffer A, supplemented as indicated in the text, for 1 h at 37°C. The cells were washed twice with CSB containing 0.2% BSA, fixed with methanol/acetone and labeled for AMF-R (Texas red) and Mt-HSP70 (FITC). The gain and black level of the confocal images were adjusted for each slide for both the FITC and rhodamine channels such that the full dynamic range of the instrument was used. For each experiment, confocal images from all slides were obtained in one sitting using equivalent pinhole settings and the extent of overlap of AMF-R tubule labeling with mitochondria was determined using Northern Eclipse software (Empix Imaging). The nonspecific AMF-R labeling of the nuclei was first cut from the image and the total number of cytoplasmic pixels labeled for AMF-R determined. Using a convolution filter, the mitochondrial labeling was enlarged to ensure that shifts between the FITC and Texas red images and cellular variations in the intensity of the mitochondrial image did not influence the measure of the extent of AMF-R tubule overlap with mitochondria. Using the enlarged mitochondrial labeling as a mask, AMF-R labeling that coincided with the enhanced mitochondrial labeling was deleted and quantification of the remaining AMF-R–labeled pixels provided a measure of smooth ER AMF-R tubules that had dissociated from mitochondria. This value was divided by the total AMF-R–labeled cytoplasmic pixels to generate the percent dissociation of AMF-R tubules from mitochondria.
Ca2+ Measurements
To prepare the CaEGTA buffers, 100 mM stock solutions of K2H2EGTA and K2CaEGTA were diluted in buffer A to 25 mM. The resulting buffers were then mixed to generate 8 different 25-mM CaEGTA buffers containing 0, 5, 10, 15, 20, 22.13, 22.92, and 25 mM total Ca2+ (see Table ). Predicted free Ca2+ concentrations were calculated using WEBMAXCv1.10 at the CPatton MaxChelator website.
Table 1.
Free Calcium and Smooth ER–Mitochondria Dissociation
| Buffer | Total Ca2+ | Predicted free [Ca2+] | Measured free [Ca2+] | Cytosol added | Dissociation |
|---|---|---|---|---|---|
| mM | ± SEM | % ± SEM | |||
| Buffer A | − | 10.0 ± 0.8 (7) | |||
| Buffer A | + | 48.3 ± 3.8 (7) | |||
| CaEGTA buffer 1 | 0 | 0 | 8 ± 4 nM (3) | + | 51.2 ± 3.7 (5) |
| CaEGTA buffer 2 | 5 | 14 nM | 19 ± 5 nM (3) | + | 37.7 ± 3.9 (4) |
| CaEGTA buffer 3 | 10 | 37 nM | 39 ± 5 nM (3) | + | 36.2 ± 3.4 (4) |
| CaEGTA buffer 4 | 15 | 83 nM | 110 ± 20 nM (3) | + | 29.9 ± 5.1 (4) |
| CaEGTA buffer 5 | 20 | 221 nM | 350 ± 80 nM (3) | + | 25.4 ± 4.8 (4) |
| CaEGTA buffer 6 | 22.13 | 700 nM | 1.4 μM (1) | + | 20.3 ± 7.4 (3) |
| CaEGTA buffer 7 | 22.92 | 1.0 μM | 1.8 μM (1) | + | 19.0 ± 6.1 (3) |
| CaEGTA buffer 8 | 25 | 38.0 μM | 3.8 ± 0.2 μM (3) | + | 15.9 ± 2.1 (5) |
Free Ca2+ concentrations of 25 mM CaEGTA buffers containing the indicated amount of total Ca2+ as predicted by calculation and by measurement with Fura-2 (number of experiments indicated in parentheses) are indicated. Measured free [Ca2+] for CaEGTA buffer 8 is considered unreliable as it is outside Fura-2 sensitivity. The average percent dissociation of smooth ER and mitochondria was determined from the indicated number of experiments for each CaEGTA buffer.
The free Ca2+ concentration of the Ca2+-EGTA buffer solutions ([Ca2+]SOL) was estimated with the Ca2+ sensitive fluorophor Fura-2 used as a potassium salt at a concentration of 2.5 μM. Fluorescence measurements were performed using a dual-excitation spectrofluorometer (Spex Fluorolog II; Spex Industries Inc.). The excitation wavelengths were set at 350 and 380 nm and emission was monitored at 505 nm with a standard bandpass filter (Andover Corporation 500FS10).
[Ca2+]SOL was calculated using the following equation (Grynkiewicz et al. 1985): [Ca2+]SOL = K d × (R − RMIN)(RMAX − R) × Sf2/Sb2; with K d equal to 224 nM, R the ratio of the fluorescence measured at 350 and 380 nm, respectively, and Sf2/Sb2, the ratio of fluorescence at 380 nm in low and high Ca2+, respectively. The maximum fluorescence ratio (RMAX) was determined using a 10 mM CaCl2 solution to oversaturate the Fura-2, whereas RMIN was obtained with a Ca2+ free solution containing 10 mM EGTA. Measurements of [Ca2+]SOL were performed at a pH of 7.35 at room temperature (21–23°C).
Results
Close Association between Smooth ER AMF-R Tubules and Mitochondria
Specific labeling of smooth ER tubules with the anti–AMF-R antibody has been previously reported in MDCK and other cell types by postembedding immunoelectron microscopy (Benlimame et al. 1995, Benlimame et al. 1998; Wang et al. 1997). Using confocal microscopy, we compared the distribution of smooth ER AMF-R tubules with that of the ER markers calnexin and calreticulin. As seen in Fig. 1, the majority of the AMF-R label can be distinguished from that of the calnexin- and calreticulin-labeled ER although partial overlap of AMF-R tubules with the ER can be observed. AMF-R–labeled tubules are always seen in proximity of calnexin- or calreticulin-labeled elements of the ER and frequently appear as projections of calnexin- or calreticulin-labeled tubules (Fig. 1C and Fig. F, arrowheads), consistent with AMF-R labeling of smooth projections of rough ER tubules observed by EM (Benlimame et al. 1995; Wang et al. 1997).
Figure 1.
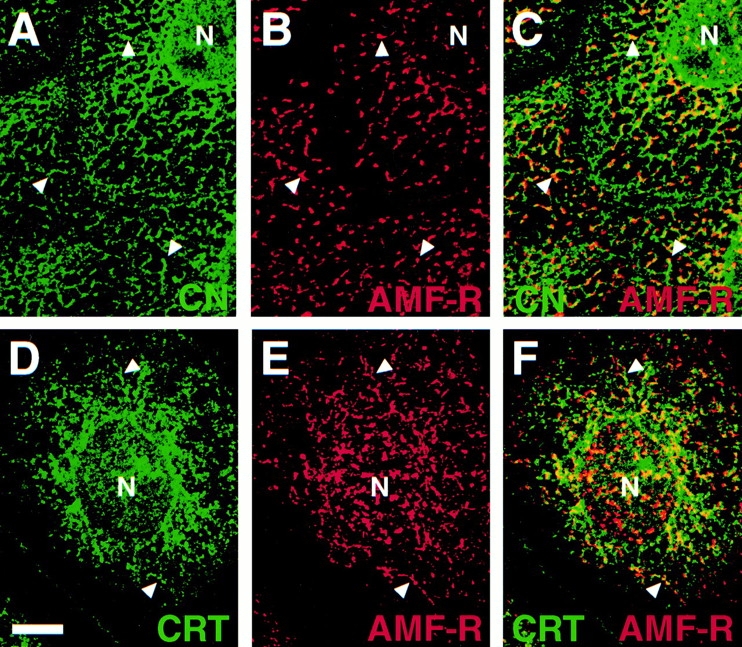
Smooth ER AMF-R tubules exhibit a close relationship to the calnexin- and calreticulin-labeled ER. MDCK cells were fixed and double-immunofluorescently labeled for either calnexin (A) or calreticulin (D) and AMF-R (B and E), and were imaged by confocal microscopy. Merged images (C and F) show that the distribution of AMF-R–labeled tubules is distinct from, but always in proximity to, the calnexin- or calreticulin-labeled ER (arrowheads). Bar, 10 μm.
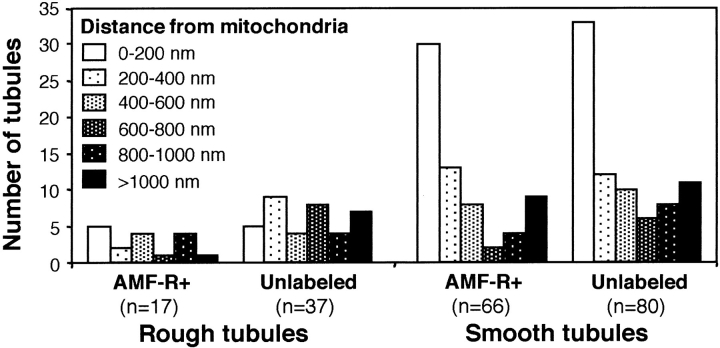
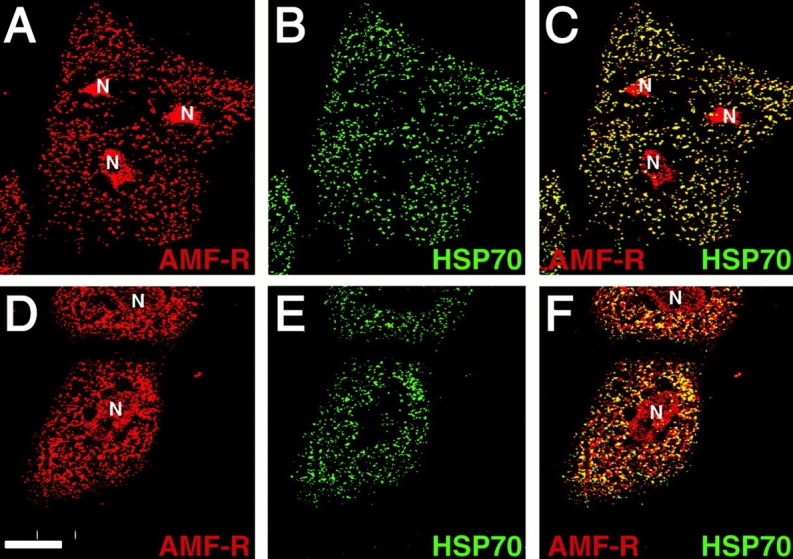
Double labeling of MDCK cells for AMF-R and Mt-HSP70 revealed a high degree of colocalization between the two signals (Fig. 2, A–C). In contrast, the calnexin- and calreticulin-labeled ER exhibits only partial overlap with the mitochondrial signal (Fig. 2, D–I). Postembedding EM labeling of smooth ER tubules for AMF-R is shown in Fig. 3. Smooth ER AMF-R tubules are observed to exhibit direct interaction with mitochondria and to associate with more than one mitochondrion (Fig. 3B and Fig. C). Quantification of the proximity of ER tubules to mitochondria on 14 EM micrographs of AMF-R–labeled MDCK cells taken in series with Fig. 3 A, revealed that AMF-R–labeled smooth ER tubules, and indeed smooth ER tubules in general, are more frequently observed within 200 nm of mitochondria than are rough ER tubules (Fig. 4). The fact that almost half of the AMF-R–labeled smooth ER tubules are located within 200 nm, the limit of optical resolution, of mitochondria in 80-nm ultrathin sections is consistent with the extensive colocalization of smooth ER AMF-R tubules with mitochondria observed by confocal microscopy. By subcellular fractionation, AMF-R is exclusively found within a high-speed membrane pellet and not within the 12,000 g mitochondrial pellet that contains Mt-HSP70, demonstrating that membranous smooth ER AMF-R tubules can be physically dissociated from mitochondria, similar to a previous study (Benlimame et al. 1995). Smooth ER AMF-R tubules are therefore a subdomain of the ER that is selectively associated with mitochondria.
Figure 2.
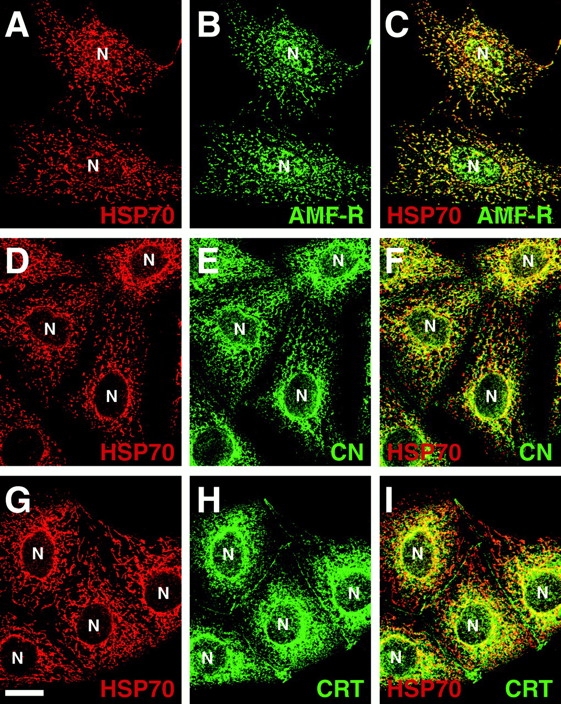
The AMF-R–labeled smooth ER subdomain is selectively associated with mitochondria. MDCK cells were fixed and double-immunofluorescently labeled for Mt-HSP70 (A,D, and G), together with AMF-R (B), calnexin (E), or calreticulin (N), and imaged by confocal microscopy. Merged images (C, F, and I) show that the association of smooth ER AMF-R tubules with mitochondria is significantly greater than either the calnexin- or calreticulin-labeled ER. Bar, 20 μm.
Figure 3.
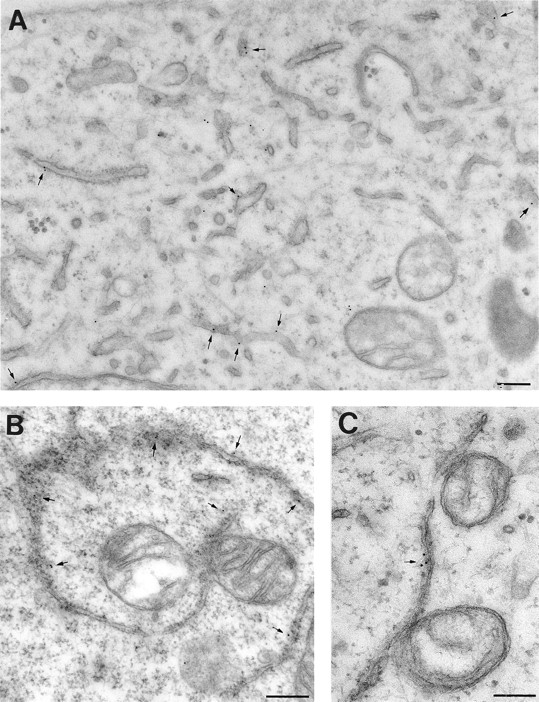
AMF-R tubules and mitochondria exhibit a close association by EM. MDCK cells were postembedding immunogold-labeled for AMF-R. AMF-R–labeling (arrows) is associated with smooth tubules closely associated with mitochondria. Bars: (A) 0.2 μM; (B and C) 0.1 μM.
Figure 4.
Quantitative analysis of the distance between ER tubules and mitochondria. The minimal distance of smooth and rough ER tubules labeled or not for AMF-R, as indicated, was determined from EM micrographs of AMF-R–labeled MDCK cells. The number of ER tubules located from 0–200, 200–400, 400–600, 600–800, 800–1,000, and >1,000 nm of a mitochondrion within the same cell is presented. The total number of tubules identified per condition (n) is indicated.
A Semipermeabilized Assay for Smooth ER–Mitochondria Dissociation
To better study the relationship between AMF-R tubules and mitochondria, we established a semipermeabilized assay based on digitonin permeabilization of subconfluent cultures of MDCK cells. Under the conditions of digitonin permeabilization used, upwards of 90% of the cells were permeabilized as determined by the ability to label cells with antibodies to cytoplasmic keratin filaments before fixation. Antibodies to calreticulin and to the lumenal domain of LAMP-2 did not label digitonin-permeabilized cells, indicating that intracellular organelles remained intact. Labeling with antibodies to tubulin revealed that the majority of microtubules were no longer intact. The association between smooth ER AMF-R tubules and mitochondria is maintained in semiintact MDCK cells since the two organelles exhibit essentially complete overlap in digitonin-permeabilized cells incubated only with buffer A (Fig. 5A, Fig. B, and Fig. C). However, in cells incubated with buffer A supplemented with 5 mg/ml rat-liver cytosol, the AMF-R label was no longer completely associated with mitochondria and distinct regions of nonoverlap between the two organelles were observed (Fig. 5D, Fig. E, and Fig. F) demonstrating the ability of cytosol to induce the dissociation of smooth ER from mitochondria.
Figure 5.
Rat liver cytosol dissociates AMF-R tubules and mitochondria in semipermeabilized MDCK cells. MDCK cells were permeabilized by digitonin and then incubated with buffer A (A, B, and C) or buffer A supplemented with 5 mg/ml rat liver cytosol (D, E, and F) at 37°C for 60 min. Fixed cells were immunofluorescently labeled for AMF-R (A and D; red) and Mt-HSP70 (B and E; green) and imaged by confocal microscopy. Dual color merged images are presented in C and F. Extensive overlap between smooth ER AMF-R tubules and mitochondria is observed in the presence of buffer alone (C), but following addition of cytosol (F) extensive AMF-R labeling not associated with mitochondria (in red) can be visualized. Bar, 20 μM.
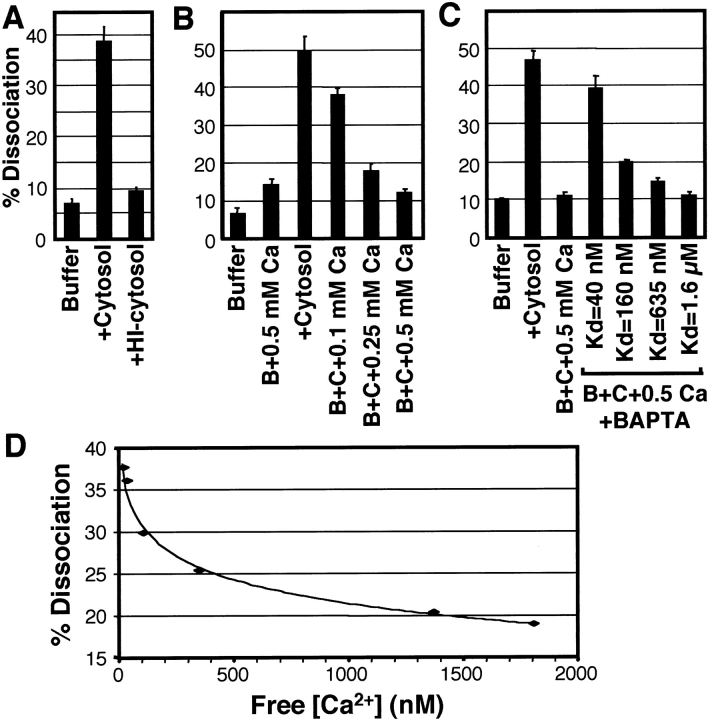
Using confocal images of cells double-labeled with anti–AMF-R (Texas red) and anti–Mt-HSP70 (FITC), a mask of the mitochondria labeling was generated and used to quantify the extent of overlap of AMF-R tubule labeling with mitochondria (see Materials and Methods). Using this protocol we obtained consistent data of the extent of dissociation of smooth ER AMF-R tubules from mitochondria (Fig. 6 A). In the presence of buffer A alone, the majority of AMF-R labeling overlapped with mitochondria and AMF-R labeling not associated with mitochondria was limited, normally from 5–10%. The addition of 5 mg/ml rat liver cytosol resulted in a significant increase in the extent of AMF-R labeling that did not overlap with mitochondria (40–50%). Heat inactivated cytosol was unable to dissociate mitochondria and smooth ER implicating cytosolic proteins in the dissociation activity. The ability of cytosol to dissociate AMF-R tubules and mitochondria is not energy dependent and occurs equally well in the absence or presence of 2 mM ATP, 2 mM GTP, or a creatine kinase based ATP regenerating system (ARS; 2 mM ATP, 37 U/ml creatine kinase, 8 mM creatine phosphate; data not shown).
Figure 6.
Ca2+ regulates cytosol-mediated dissociation of smooth ER AMF-R tubules and mitochondria. A, The percent dissociation of smooth ER tubules (± SEM) from mitochondria in semi-permeabilized MDCK cells was quantified from confocal images of cells double-labeled for AMF-R and Mt-HSP70 (for details see Materials and Methods). MDCK cells were permeabilized with digitonin and incubated for 60 min in the presence of buffer A alone or buffer A supplemented with 5 mg/ml rat liver cytosol or 5 mg/ml heat-inactivated cytosol. B, Semipermeabilized MDCK cells were incubated for 60 min with buffer A supplemented with 0.5 mM Ca2+ (+0.5 mM Ca), with cytosol (+cytosol), or with cytosol (C) plus 0.1, 0.25, or 0.5 mM Ca2+, as indicated. To assess the role of free Ca2+ in cytosol-mediated dissociation of smooth ER AMF-R tubules from mitochondria, semipermeabilized MDCK cells were incubated with buffer A alone, or with buffer A supplemented with cytosol (+cytosol), with cytosol containing 0.5 mM Ca2+ (+C+0.5 Ca), or with cytosol, 0.5 mM Ca2+, and 100 μM of various BAPTAs (B+C+0.5 Ca+BAPTA) with Ca2+ dissociation constants (K d) of 40 nM, 160 nM, 635 nM, and 1.6 μM, as indicated. Similar results were obtained from at least three independent experiments and data from representative experiments is presented. C, Using data presented in Table , free [Ca2+]SOL measured using Fura-2 in CaEGTA buffers 2-7 was plotted against the extent of dissociation of smooth ER AMF-R tubules from mitochondria. Free [Ca2+]SOL above 100 nM is associated with increasing association between the two organelles.
Ca2+-dependent Association of Smooth ER Tubules and Mitochondria
The addition of millimolar concentrations of Ca2+ inhibited the ability of cytosol to dissociate AMF-R tubules from mitochondria (Fig. 6 B). The effect was concentration-dependent and the addition of 0.5 mM Ca2+ inhibited cytosol-induced dissociation by ∼90%. In the absence of cytosol, Ca2+ alone did not affect the extent of association between smooth ER and mitochondria, demonstrating that Ca2+ specifically regulates the cytosolic dissociation activity. To determine the role of free Ca2+ in the regulation of the cytosol-dependent dissociation of smooth ER and mitochondria, we used BAPTA Ca2+ chelators with varying K ds for Ca2+ (Fig. 6 C). 5-5′-dimethyl BAPTA (K d = 40 nM) reversed by 70%, and BAPTA (K d = 160 nM) by ∼30% the ability of 0.5 mM Ca2+ to inhibit cytosol-dependent smooth ER-mitochondria dissociation. 5-5′-difluoro BAPTA (K d = 635 nM) and 5-5′-dibromo BAPTA (K d = 1.6 μM) reversed only minimally smooth ER–mitochondria dissociation. The ability of BAPTAs of various K d's to differentially influence smooth ER mitochondria association demonstrates that free Ca2+ levels regulate ER–mitochondria association. The fact that these effects are observed in the presence of 100 μM BAPTA indicates that free Ca2+ in the presence of cytosol and cells must be below 100 μM, in spite of the fact that 0.5 mM Ca2+ was added to the reaction. Indeed, no effect was observed using 50 μM of the various BAPTAs, indicating that protein Ca2+ chelators in the cells and in the cytosol reduce free Ca2+ to between 50–100 μM.
To further define the free Ca2+ concentrations that regulate cytosol-mediated dissociation of smooth ER and mitochondria, we added cytosol in the presence of CaEGTA buffers of defined free Ca2+ concentrations. Free [Ca2+]SOL was predicted using the WEBMAXCv1.10 program and measured using Fura-2 (Table ). Compared with the high degree of dissociation observed in the presence of free [Ca2+]SOL of less than 50 nM (CaEGTA buffers 2 and 3), free [Ca2+]SOL of ∼100–400 nM (CaEGTA buffers 4 and 5) were associated with partial inhibition and free [Ca2+]SOL concentrations >1 μM (CaEGTA buffers 6–8) associated with a significant inhibition of cytosol-mediated dissociation of smooth ER and mitochondria. Graphic representation of the data presented in Table (CaEGTA buffers 2–7) reveals a logarithmic relationship between free [Ca2+]SOL measured with Fura-2 and cytosol dissociation activity (Fig. 6 D). In particular, free [Ca2+]SOL below 100 nM are associated with extensive dissociation of the two organelles, whereas increasing the free [Ca2+]SOL above 100 nM results in the progressive inhibition of cytosol-mediated dissociation.
Discussion
The ER constitutes the largest intracellular membrane organelle of the cell, extends throughout the cytoplasm, and is composed of multiple functional domains (Sitia and Meldolesi 1992). Confocal images of MDCK cells double-labeled for AMF-R and calnexin or calreticulin show that while the AMF-R–labeled tubules can be clearly distinguished from the calnexin- and calreticulin-labeled ER, partial overlap is apparent and regions of close interaction between the two are evident. These images are therefore consistent with the identity of the AMF-R tubule as a smooth subdomain of the ER (Benlimame et al. 1995; Wang et al. 1997). The presence of AMF-R, the select sensitivity of smooth ER AMF-R tubules, but not the rough ER to ilimaquinone (Wang et al. 1997), and the fact that AMF-R labeling can be clearly distinguished from that of calnexin and calreticulin identify the smooth ER AMF-R tubule as a molecularly distinct subdomain of the ER. The high degree of association of AMF-R–labeled tubules with mitochondria by confocal and EM further identifies the AMF-R tubule as a mitochondria-associated subdomain of the ER.
The existence of ER subdomains that interact with mitochondria and which function to sequester cytosolic Ca2+ (Simpson et al. 1997; Rizzuto et al. 1998), together with the demonstrated role of cytosolic Ca2+ in regulating AMF-R tubule–mitochondria association, suggest a role for this smooth ER subdomain in the regulation of cytosolic Ca2+ levels. The diversity of Ca2+ concentrations and expression of Ca2+ binding proteins in different subdomains of the ER is well-established (Takei et al. 1992; Button and Eidsath 1996; Rooney and Meldolesi 1996; Golovina and Blaustein 1997; Montero et al. 1997; Pezzati et al. 1997). Not unlike the smooth ER AMF-R tubule, a smooth membrane-bound organelle of 50–250 nm apposed to the ER and mitochondria, called the calciosome, has been described to be enriched in a nonmuscle calsequestrin-like protein and to represent the major IP3-sensitive Ca2+ storage organelle (Volpe et al. 1988). Both AMF-R and the IP3 receptor are associated with cell surface caveolae (Fujimoto et al. 1992; Benlimame et al. 1998), and the caveolar-like clathrin-independent internalization pathway to smooth ER defined by AMF-R (Benlimame et al. 1998; Le et al. 2000) may serve to regulate the delivery of Ca2+ regulatory proteins to this mitochondria-associated smooth ER subdomain.
In semipermeabilized MDCK cells in the absence of cytosol or energy and independent of Ca2+, smooth ER AMF-R tubules and mitochondria maintain their close association, suggesting that interorganellar adhesion mechanisms are associated with the organelles. The ability of cytosol to dissociate the two organelles demonstrates the existence of a cytosolic mechanism that regulates ER–mitochondria association. The transfer of phosphatidyl serine between mitochondria and MAMs is prevented by proteolysis of mitochondrial surface proteins, but not of MAMs, suggesting that a mitochondrial surface protein is involved in the association between mitochondria and ER (Shiao et al. 1998). Whereas the relationship of smooth ER AMF-R tubules to MAMs remains unclear, cytosol may disrupt smooth ER AMF-R tubule–mitochondria association due to the presence of a Ca2+-regulated cytosolic protein that inhibits the adhesive function of such a mitochondrial surface protein.
Our results using BAPTAs of various affinities and CaEGTA buffers of varying free [Ca2+]SOL indicate that free Ca2+ regulates the ability of cytosol to stimulate dissociation of AMF-R tubules from mitochondria. While we hesitate to attribute precise free Ca2+ levels to our data, both the BAPTA and CaEGTA experiments are consistent with the ability of low free [Ca2+]SOL (<100 nM) to favor dissociation and of high free [Ca2+]SOL (>1 μM) to favor association of smooth ER tubules and mitochondria (Fig. 6; Table ). Therefore, these data argue that at physiological cytosolic Ca2+ levels, cytosol stimulates smooth ER-mitochondria dissociation. Whereas addition of cytosol increases the amount of smooth ER AMF-R tubules that do not colocalize with mitochondria, the two organelles still exhibit extensive association (Fig. 5 F) such that even in the presence of cytosol the two organelles still interact. Dissociation of AMF-R tubules from mitochondria induced by cytosol therefore reflects a partial disruption of direct contacts between the two organelles. The increased overlap of the two fluorescent signals observed in the presence of increasing Ca2+ may be indicative of the increased formation of close contacts between the two organelles.
Ca2+ transients of 1.6–1.9 μM have been identified at high density patches of the ER enriched in the IP3 receptor and calreticulin (Simpson et al. 1997), and the mitochondrial surface has been shown to be exposed to 3.4 μM Ca2+ after IP3 stimulation (Rizzuto et al. 1998). Our data showing increased ER–mitochondria association at millimolar cytosolic Ca2+ indicates that the elevated Ca2+ levels present at these ER–mitochondria close contacts can actively regulate ER–mitochondria association. Ca2+-dependent smooth ER–mitochondria association could therefore contribute to the establishment of segregated cytoplasmic domains between the two organelles in which the local elevated Ca2+ concentrations necessary for mitochondrial uptake can be established. A cellular mechanism that senses elevated cytosolic Ca2+ levels and then facilitates the establishment of ER–mitochondria contacts is therefore proposed to be implicated in the efficient sequestration of cytosolic Ca2+ to mitochondrial stores.
Acknowledgments
We thank Kevin Coonan and Nigel Banner of Empix Imaging (Mississuaga, Ontario) for developing the Northern Eclipse overlay program used to quantitate AMF-R tubule–mitochondria overlap. The photographic and digital reproductions were the work of Jean Leveillé and Gaston Lambert.
This study was supported by grants from the Medical Research Council of Canada.
Footnotes
Abbreviations used in this paper: AMF-R, autocrine motility factor receptor; IP3, inositol 1,4,5-triphosphate; MAM, mitochondria-associated membrane; Mt-HSP70: mitochondrial heat shock protein 70.
References
- Achleitner G., Gaigg B., Krasser A., Kainersdorfer E., Kohlwein S.D., Perktold A., Zellnig G., Daum G. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur. J. Biochem. 1999;264:545–553. doi: 10.1046/j.1432-1327.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- Benlimame N., Simard D., Nabi I.R. Autocrine motility factor receptor is a marker for a distinct tubular membrane organelle. J. Cell Biol. 1995;129:459–471. doi: 10.1083/jcb.129.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlimame N., Le P.U., Nabi I.R. Localization of autocrine motility factor receptor to caveolae and clathrin-independent internalization of its ligand to smooth endoplasmic reticulum. Mol. Biol. Cell. 1998;9:1773–1786. doi: 10.1091/mbc.9.7.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D., Eidsath A. Aequorin targeted to the endoplasmic reticulum reveals heterogeneity in luminal Ca++ concentration and reports agonist- or IP3-induced release of Ca++ . Mol. Biol. Cell. 1996;7:419–434. doi: 10.1091/mbc.7.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R.M., McCormack J.G. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu. Rev. Physiol. 1990;52:451–466. doi: 10.1146/annurev.ph.52.030190.002315. [DOI] [PubMed] [Google Scholar]
- Franke W.W., Kartenbeck J. Outer mitochondrial membrane continuous with endoplasmic reticulum. Protoplasma. 1971;73:35–41. doi: 10.1007/BF01286409. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Nakade S., Miyawaki A., Mikoshiba K., Ogawa K. Localization of 1,4,5-triphosphate receptor-like protein in plasmalemmal caveolae. J. Cell Biol. 1992;119:1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina V.A., Blaustein M.P. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hajnoczky G., Hager R., Thomas A.P. Mitochondria suppress local feedback activation of inositol 1,4, 5- trisphosphate receptors by Ca2+ . J. Biol. Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- Jouaville L.S., Pinton P., Bastianutto C., Rutter G.A., Rizzuto R. Regulation of mitochondrial ATP synthesis by calciumevidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfi B., Curci S., Debellis L., Pozzan T., Hofer A.M. Ca2+ homeostasis in the agonist-sensitive internal storefunctional interactions between mitochondria and the ER measured in situ in intact cells. J. Cell Biol. 1998;142:1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le P.U., Benlimame N., Lagana A., Raz A., Nabi I.R. Clathrin-mediated endocytosis and recycling of autocrine motility factor receptor to fibronectin fibrils is a limiting factor for cell motility. J. Cell Sci. 2000;113:3227–3240. doi: 10.1242/jcs.113.18.3227. [DOI] [PubMed] [Google Scholar]
- Lewis J.A., Tata J.R. A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J. Cell Sci. 1973;13:447–459. doi: 10.1242/jcs.13.2.447. [DOI] [PubMed] [Google Scholar]
- Montero M., Alvarez J., Scheenen W.J., Rizzuto R., Meldolesi J., Pozzan T. Ca2+ homeostasis in the endoplasmic reticulumcoexistence of high and low [Ca2+] subcompartments in intact HeLa cells. J. Cell Biol. 1997;139:601–611. doi: 10.1083/jcb.139.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montisano D.F., Cascarano J., Pickett C.B., James T.W. Association between mitochondria and rough endoplasmic reticulum in rat liver. Anat. Rec. 1982;203:441–450. doi: 10.1002/ar.1092030403. [DOI] [PubMed] [Google Scholar]
- Morre D.J., Merritt W.D., Lembi C.A. Connections between mitochondria and endoplasmic reticulum in rat liver and onion stem. Protoplasma. 1971;73:43–49. doi: 10.1007/BF01286410. [DOI] [PubMed] [Google Scholar]
- Nabi I.R., Watanabe H., Raz A. Identification of B16-F1 melanoma autocrine motility-like factor receptor. Cancer Res. 1990;50:409–414. [PubMed] [Google Scholar]
- Perkins G., Renken C., Martone M.E., Young S.J., Ellisman M., Frey T. Electron tomography of neuronal mitochondriathree-dimensional structure and organization of cristae and membrane contacts. J. Struct. Biol. 1997;119:260–272. doi: 10.1006/jsbi.1997.3885. [DOI] [PubMed] [Google Scholar]
- Pezzati R., Bossi M., Podini P., Meldolesi J., Grohovaz F. High-resolution calcium mapping of the endoplasmic reticulum–Golgi exocytic membrane system. Electron energy loss imaging analysis of quick frozen-freeze dried PC12 cells. Mol. Biol. Cell. 1997;8:1501–1512. doi: 10.1091/mbc.8.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Bastianutto C., Brini M., Murgia M., Pozzan T. Mitochondrial Ca2+ homeostasis in intact cells. J. Cell Biol. 1994;126:1183–1194. doi: 10.1083/jcb.126.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Robb-Gaspers L.D., Burnett P., Rutter G.A., Denton R.M., Rizzuto R., Thomas A.P. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney E., Meldolesi J. The endoplasmic reticulum in PC12 cells. Evidence for a mosaic of domains differently specialized in Ca2+ handling. J. Biol. Chem. 1996;271:29304–29311. doi: 10.1074/jbc.271.46.29304. [DOI] [PubMed] [Google Scholar]
- Rutter G.A., Burnett P., Rizzuto R., Brini M., Murgia M., Pozzan T., Tavare J.M., Denton R.M. Subcellular imaging of intramitochondrial Ca2+ with recombinant targeted aequorinsignificance for the regulation of pyruvate dehydrogenase activity. Proc. Natl. Acad. Sci. USA. 1996;93:5489–5494. doi: 10.1073/pnas.93.11.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao Y.J., Balcerzak B., Vance J.E. A mitochondrial membrane protein is required for translocation of phosphatidylserine from mitochondria-associated membranes to mitochondria. Biochem. J. 1998;331:217–223. doi: 10.1042/bj3310217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore G.C., Tata J.R. Two fractions of rough endoplasmic reticulum from rat liver. I. Recovery of rapidly sedimenting endoplasmic reticulum in association with mitochondria. J. Cell Biol. 1977;72:714–725. doi: 10.1083/jcb.72.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P.B., Mehotra S., Lange G.D., Russell J.T. High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. J. Biol. Chem. 1997;272:22654–22661. doi: 10.1074/jbc.272.36.22654. [DOI] [PubMed] [Google Scholar]
- Sitia R., Meldolesi J. Endoplasmic reticuluma dynamic patchwork of specialized subregions. Mol. Biol. Cell. 1992;3:1067–1072. doi: 10.1091/mbc.3.10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K., Stukenbrok H., Metcalf A., Mignery G.A., Sudhof T.C., Volpe P., De Camilli P. Ca2+ stores in Purkinje neuronsendoplasmic reticulum subcompartments demonstrated by the heterogeneous distribution of the InsP3 receptor, Ca(2+)-ATPase, and calsequestrin. J. Neurosci. 1992;12:489–505. doi: 10.1523/JNEUROSCI.12-02-00489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance J.E. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- Volpe P., Krause K.H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D.P. Calciosome, a cytoplasmic organellethe inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc. Natl. Acad. Sci. USA. 1988;85:1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-J., Benlimame N., Nabi I.R. The AMF-R tubule is a smooth ilimaquinone-sensitive subdomain of the endoplasmic reticulum. J. Cell Sci. 1997;110:3043–3053. doi: 10.1242/jcs.110.24.3043. [DOI] [PubMed] [Google Scholar]