Abstract
A central feature of cisternal progression/maturation models for anterograde transport across the Golgi stack is the requirement that the entire population of steady-state residents of this organelle be continuously transported backward to earlier cisternae to avoid loss of these residents as the membrane of the oldest (trans-most) cisterna departs the stack. For this to occur, resident proteins must be packaged into retrograde-directed transport vesicles, and to occur at the rate of anterograde transport, resident proteins must be present in vesicles at a higher concentration than in cisternal membranes. We have tested this prediction by localizing two steady-state residents of medial Golgi cisternae (mannosidase II and N-acetylglucosaminyl transferase I) at the electron microscopic level in intact cells. In both cases, these abundant cisternal constituents were strongly excluded from buds and vesicles. This result suggests that cisternal progression takes place substantially more slowly than most protein transport and therefore is unlikely to be the predominant mechanism of anterograde movement.
Keywords: secretion, cisternal maturation, coatomer, cargo, glycosyltransferase
Introduction
What is the role of the numerous COPI (coatomer)-coated transport vesicles (Orci et al. 1986) that bud from the cisternae at all levels of the Golgi stack? Two principal hypotheses have been proposed. In one view, these vesicles transport cargo across a stack of static cisternae in the cis-to-trans (anterograde) direction (Orci et al. 1986, Orci et al. 1989; Ostermann et al. 1993), among other roles they may play (Letourneur et al. 1994). Consistent with this, about half of these Golgi-derived vesicles contain such anterograde-directed cargo; the other half are retrograde-directed, containing receptors for retrieval of escaped ER residents from the Golgi stack (Orci et al. 1997).
An alternative view that recently has become prominent, cisternal maturation/progression (Glick et al. 1997; Glick and Malhotra 1998; Pelham 1998; Allan and Balch 1999), proposes that anterograde transport results from movement of the cisternae themselves; transport vesicles would have no role in anterograde transport, but rather would function exclusively in retrograde transport. Recent observations (Bonfanti et al. 1998) concerning the anterograde transport of procollagen aggregates (which are much larger than can be accommodated by standard size COPI vesicles) suggest that cisternal progression may occur, though on a time scale that seems too slow to account for the transport of most proteins (Bonfanti, L., O. Martella, A. Miranov, and A. Luini. 1999. Mol. Biol. Cell. 10:S114a; Volchuk et al. 2000).
To create a cisternal progression, new cisternae would have to be continuously added at the entry (cis) face of the stack, whereas at the exit (trans) face of the stack, the oldest cisterna would be shed as it is converted into post-Golgi and other vesicles. As a result, in the absence of a special mechanism for preventing this, the steady-state residents of the Golgi stack (including glycosyltransferases and other processing enzymes) would be rapidly lost from the trans face. Therefore, all current cisternal progression models (Glick et al. 1997; Glick and Malhotra 1998; Pelham 1998; Allan and Balch 1999) postulate that Golgi residents are continuously retrieved from later to earlier Golgi cisternae. Indeed, it is this essential feature that is connoted by the term maturation. Steady-state Golgi resident proteins are known to cycle through the ER on a time scale of several hours (Storrie et al. 1998), whereas anterograde transport across the stack typically occurs within 10–20 min (Green et al. 1981; Quinn et al. 1984). This means that if cisternal progression is to keep pace with this rapid anterograde transport, the proposed retrograde-directed recycling of Golgi residents would have to be much faster than return to the ER and be directed to earlier cisternae within the Golgi stack (Glick and Malhotra 1998).
Because they are the major, if not exclusive, class of vesicle budding throughout the stack, this retrieval is proposed to be carried out by Golgi-derived COPI vesicles moving in the retrograde direction (Glick et al. 1997; Glick and Malhotra 1998; Pelham 1998; Allan and Balch 1999). Furthermore, if transport vesicles have no role in anterograde transport, then most, if not all, of the COPI vesicles budding from the stack are predicted to carry retrograde-moving Golgi residents.
If cisternal progression were the exclusive mechanism of anterograde transport, then Golgi resident proteins must be present in the vesicles at a higher concentration than in the cisternal membranes from which these vesicles bud (Glick et al. 1997). This is the case because when the trans-most cisterna departs, the resident population must be effectively removed together with only a fraction of the membrane surface to leave the anterograde-directed cargo behind in maturing secretory vesicles. On the other hand, if cisternal progression were to occur at a slower rate than most protein transport (i.e., operating in parallel with a faster vesicle transport pathway), then this strict requirement is relaxed. Now, many of the vesicles should still have Golgi residents, but at lower concentrations than in cisternae; how low depending on how slow the progression is. The anterograde vesicle transport model is indifferent to this issue.
These basic predictions of cisternal progression/maturation models have not yet been adequately or convincingly tested. Certainly, it is known that steady-state residents of the cis-Golgi cisternae encounter trans-Golgi processing enzymes during their lifetime, implying that retrograde transport of residents occurs within the stack at some rate in vivo (Hoe et al. 1995; Harris and Waters 1996; Linstedt et al. 1997; Wooding and Pelham 1998). However, in the absence of more quantitative data, this fact does not speak to the level of resident proteins in anterograde- or retrograde-directed vesicles.
Measurements have been made of the concentration of certain resident proteins (glycosyltransferases and saccharide-processing enzymes) in COPI-coated vesicles produced in cell-free systems (Sönnichsen et al. 1996; Lanoix et al. 1999). In one study, COPI-coated vesicle fractions were isolated from cell-free incubations of Golgi membranes, and the concentration of resident and other proteins in the vesicles was compared with that in parental Golgi membranes using both electron microscope immunocytochemistry and biochemical determinations, with excellent agreement between these methods (Sönnichsen et al. 1996). The concentrations in the vesicles of the four resident proteins examined ranged from 14 to 30% of that present in cisternae, a result that is inconsistent with the concentration requirement for exclusive anterograde transport by cisternal progression.
However, questions have been raised about this study (Lanoix et al. 1999), because the COPI vesicles were formed in the presence of GTPγS, needed to keep the COPI coats attached. Lanoix et al. 1999 report that vesicles produced with GTP instead of GTPγS have a much higher concentration of residents than starting Golgi membranes. However, since Lanoix et al. 1999 studied an uncoated membrane fraction, it is difficult to prove conclusively that COPI vesicles were obtained, despite the many excellent controls that were reported.
Given the special importance of this measurement for evaluating the significance of cisternal progression for anterograde transport, it is important that such data now be obtained in intact cells, and we do so here. As matters stand, cell-free incubations used to produce COPI vesicles may not, for one or another reason (not limited to use of GTPγS), effectively reconstitute the recycling process for resident proteins; or there might be some systematic contamination artifact in subcellular fractionation used to obtain either coated or uncoated COPI vesicles. All of these uncertainties are avoided by obtaining data from intact cells.
Here, we provide measurements of the concentrations of mannosidase II (Man II) (Burke et al. 1982; Novikoff et al. 1983; Baron and Garoff 1990; Velasco et al. 1993), and N-acetylglucosaminyltransferase I (NAGT I; Kornfeld and Kornfeld 1985), both residents of medial Golgi cisternae (Nilsson et al. 1993; Rabouille et al. 1995).
Materials and Methods
Cells were fixed in phosphate-buffered glutaraldehyde and processed directly for cryoultramicrotomy after sucrose infiltration, following the protocol of Tokuyasu 1986. The cryosections were incubated with the respective antibodies, revealed by the protein A gold method using 10-nm gold particles (Roth et al. 1978). Golgi profiles showing stacked cisternae and associated vesicles were evaluated. The following antibodies were used: anti-Man II antibody supplied by Dr. K.W. Moremen (University of Georgia, Athens, Georgia; Moremen and Robbins 1991; Moremen et al. 1991), dilution 1:50; NAGT I tagged with myc was detected using the 9E10 mAb (Evan et al. 1985), dilution 1:2,000, followed by rabbit anti–mouse IgG, diluted 1:400. COPI was labeled with the anti-p36 (ε-COP) antibody (Hara-Kuge et al. 1994), dilution 1:400. Double-labeling was carried out by exposing the sections to a mixture of rabbit anti–Man II and mouse anti–β-COP (M3A5 from the late Dr. T. Kreis, University of Geneva, Geneva, Switzerland), followed by washing with PBS and labeling with goat anti–rabbit and goat anti–mouse IgG coupled to gold of 15 and 10 nm, respectively. Cryosections were examined and photographed in a Philips LS420 electron microscope with calibrated magnifications. Quantitative evaluation followed the procedures of Orci et al. 1997.
Results
To quantitatively establish the concentration of Golgi resident proteins in vesicles relative to cisternae in intact cells, we used immunogold particles directed against two well-characterized resident Golgi proteins, Man II and NAGT I.
As expected, Man II immunogold labeling in intact pancreatic β cells was essentially restricted to the Golgi stack (Fig. 1 and Table ). We limited the quantification of the density of gold particles (n/μm2) to those Golgi cisternae that were positive (as distinct from the Golgi area as a whole) and to those 70–90-nm vesicles that were immediately adjacent (lateral) to the labeled cisternae. The lateral vesicles do not migrate significantly in the cis-trans direction away from their budding sites (Orci et al. 1997, Orci et al. 1998) due to tethering (Orci et al. 1998; Sönnichsen et al. 1998), and based on earlier studies are almost exclusively COP I vesicles, many of which have already uncoated (Orci et al. 1997). The density of gold particles over vesicles lateral to Man II-staining cisternae (7 ± 3 gold particles/μm2) was not significantly above the background level of mitochondrial staining (8 ± 1 gold particles/μm2; see Table ), and only 3% of these lateral vesicles had a gold particle (including background labeling), setting an upper limit. By contrast, Man II was present at 91± 6 gold particles/μm2 in positive cisternae. Therefore, the concentration of Man II in lateral vesicles cannot be greater than (7/91) = 7.7% of that in parental cisternae.
Figure 1.
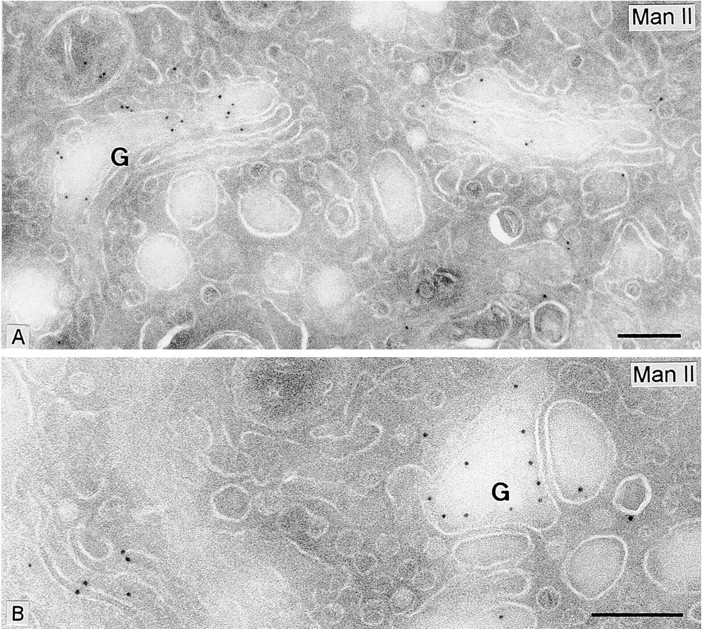
Immunogold labeling of Man II in insulin cells. A, The immunogold particles are predominantly associated with the cisternal profiles of the Golgi complex. Two neighboring Golgi complexes (G) are indicated. Surrounding cisternae-associated Golgi vesicles are virtually unlabeled. B, Detail of one dilated Golgi cisterna showing immunogold associated with the inner aspect of the limiting membrane; the surrounding vesicles are free of labeling. For quantitation, see Table . Bars, 0.2 μm.
Table 1.
Localization of Man II and NAGT I in Golgi Cisternae and Adjacent 70–90-nm Diameter Vesicles
| Density of labeling | ||
|---|---|---|
| Enzymatic/antigenic marker | Man II (insulin cells) | NAGT I (HeLa cells) |
| Gold particles/μm2 ± SEM | Gold particles/μm2 ± SEM | |
| Labeled Golgi cisternae | 91 ± 6 | 214 ± 11 |
| Vesicles lateral to labeled cisternae | 7 ± 3 | 17 ± 4 |
| Buds on labeled cisternae | 17 ± 10 | 24 ± 14 |
| Mitochondria (e.g., background) | 8 ± 1 | 10 ± 2 |
20 Golgi areas were evaluated in each case. A total of 3 ± 1% and 6 ± 2% of the vesicles lateral to the Golgi cisternae are labeled for Man II and NAGT I, respectively. For buds, the corresponding numbers were 7 ± 4% and 9 ± 6%.
The presence of Man II in cisternae and its virtual absence in lateral COPI vesicles is emphasized in Fig. 2A–C. A and B show Golgi stacks in a comparable orientation stained either for Man II or for ε-COP, respectively. The segregation of the overall labeling is evident. Double-labeling experiments (Fig. 2 C) confirmed that COPI vesicles have low levels of Man II, as compared with cisternae. Quantitative evaluation revealed that 42 ± 4% of the lateral vesicles are COPI positive and another 4 ±1% are Man II positive, but no vesicles labeled for both antigens (145 vesicles counted on 17 different Golgi stacks).
Figure 2.
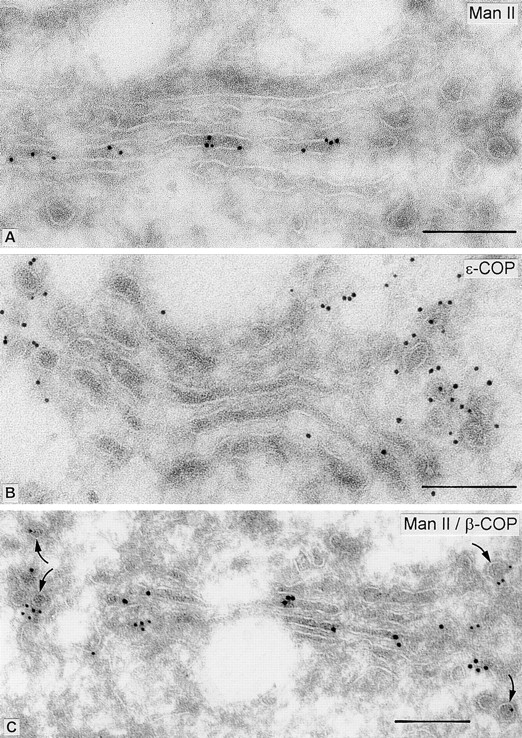
Immunogold-labeling of Man II and COPI (ε-COP) in fibroblasts. The comparable orientation of the Golgi stacks in cryosections A and B shows that Man II staining is present on medial cisternae, but is absent from vesicles lateral to the cisternal tips (A), whereas COPI-labeling has the reverse distribution, being localized to the vesicles lateral to the Golgi stack (B). C shows double-labeling with Man II and β-COP antibodies. The large (15 nm) gold particles labeling Man II are confined to cisternae, whereas the surrounding vesicles (arrows) stain for β-COP (10-nm gold). Quantitative evaluation reveals that 42 ± 4% of vesicles are COPI positive, 4 ± 1% are Man II positive, and no colocalization was observed (145 vesicles counted on 17 different Golgi stacks). Bars, 0.2 μm.
The virtual absence of staining for Man II in the lateral COPI vesicles is unlikely to be due to a systematic inability to detect antigens in these vesicles (versus in the cisternae) because of their much smaller size, since a number of antigens have previously been detected and quantified in COPI buds and vesicles (Sönnichsen et al. 1996; Orci et al. 1997), and because the labeling density for Man II in whole versus mitotically fragmented Golgi complex (the fragments frequently being in the size range of COP I vesicles) are similar (Sönnichsen et al. 1996).
A similar analysis was performed on sections of HeLa cells expressing an epitope-tagged NAGT I (Nilsson et al. 1993). Most of the labeling was restricted to the medial Golgi cisternae (Fig. 3 and Table ), and was present in these Man II-positive cisternae at a density of 214 ± 11 gold particles/μm2. Only 6 ± 2% of the lateral vesicles were labeled, and much of this labeling (17 ± 4 gold particles/μm2) was due to background (10 ± 2 gold particles/μm2 over mitochondria). The concentration of NAGT I in lateral vesicles is therefore not greater than (17/214) = 8.0% of that in parental cisternae.
Figure 3.
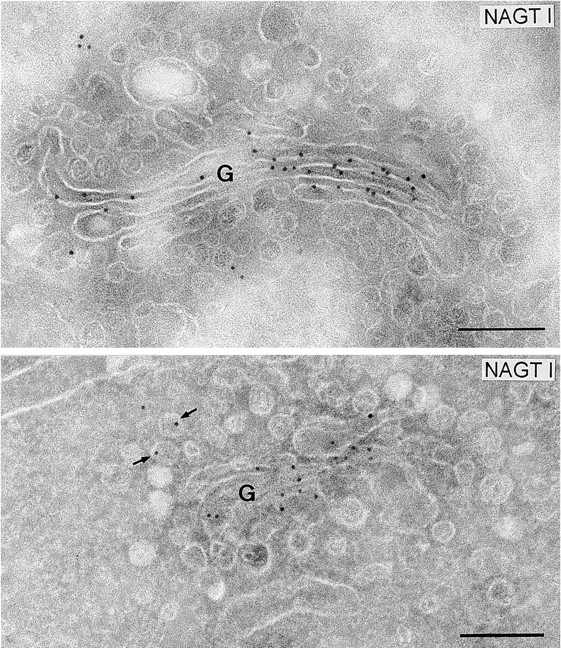
Gold labeling for myc-tagged NAGT I expressed in HeLa cells (Nilsson et al. 1993) is largely restricted to the cisternal profiles of the Golgi complex (top and bottom panel). The arrows indicate two labeled vesicular profiles in the bottom panel. For quantitation, see Table . Bars, 0.2 μm.
To be certain that these resident proteins are excluded from Golgi-derived COPI vesicles, we separately quantified immunostaining of buds forming from (but still continuous with) the Golgi cisternae (Table ). As would be predicted, the buds had dramatically less Man II and NAGT I than the cisternae that they are in the process of budding from. Earlier studies have established that all of these budding profiles are COPI-coated (Orci et al. 1997).
Discussion
Although we are unaware of previous studies using EM of sections of intact cells that quantify or specifically call attention to the presence or absence of steady-state resident proteins of the Golgi stack in lateral vesicles and buds, micrographs consistent with our result, a striking exclusion of residents from vesicles surrounding positive cisternae, are to be found in some earlier work. For example see: Figure 4 in Novikoff et al. 1977 and Figure 4 in Griffiths et al. 1989, both concerning cytochemical staining of TPPase; Figure 5 in Burke et al. 1982, concerning immunogold labeling of a then-unidentified 135-kD protein subsequently identified as ManII (Baron and Garoff 1990); and Figure 5 in Nilsson et al. 1993, concerning both NAGT I and galactosyltransferase.
Other studies have reported the presence of Man II and additional steady-state Golgi resident proteins in highly purified COPI-coated vesicle-containing fractions obtained from cell-free incubations of Golgi membranes (Ostermann et al. 1993; Sönnichsen et al. 1996) or partially purified from cell homogenates (Love et al. 1998), in the latter case without morphological evidence of purity. The concentration of resident proteins in COPI-coated vesicles relative to cisternae was measured in only one study (Sönnichsen et al. 1996) and did not exceed 30% of that in cisternae. Since COPI-coated vesicle preparations are invariably contaminated with sheared-off COP-coated buds that contain portions of parental cisternae (see Figure 3 D in Ostermann et al. 1993), which contribute resident proteins as an artifact of fractionation, the presence of resident proteins in bona fide COPI vesicles is likely overestimated by analyzing isolated COPI vesicles, whether by biochemical or morphological methods.
Recently, Lanoix et al. 1999 have analyzed the resident protein (glycosyltransferase) content of an uncoated membrane fraction produced from Golgi membranes in vitro (in the presence of GTP) that is thought to be derived from COPI-coated vesicles, and compared this with bona fide COPI-coated vesicles prepared with GTPγS. They report (Table IV in Lanoix et al., 1999) a 9.6-fold higher concentration (protein/phospholipid) of NAGT I and a 4.8-fold higher concentration of Man II, in the uncoated (GTP) vesicles than in the starting Golgi fraction and an exclusion of residents in the GTPγS -prepared coated vesicles. There was no corresponding enrichment in anterograde-directed cargo in the GTP-produced uncoated vesicles (1.7-fold for pIgR) or in bona fide COP I-coated vesicles made with GTPγS (1.2-fold). In contradiction to this, Nickel et al. 1998 analyzed bona fide coated COPI vesicles produced in the presence of GTP versus GTPγS, and report that anterograde-directed cargo is up to 50-fold more concentrated in GTP-prepared as compared with GTPγS-prepared coated vesicles. It is possible that the explanation for this serious discrepancy with regard to the concentration of anterograde cargo is that the GTP-prepared uncoated membrane fraction of Lanoix et al. 1999 is for some reason not derived mainly from once-coated COPI vesicles, despite reasonable arguments made to the contrary; the establishment of purity of morphologically distinct coated vesicles is inherently more reliable than uncoated vesicles that look in the electron microscope like many other types of vesicles. Also, since the steady-state resident protein population of the Golgi accounts for ∼95% of the total protein mass (Quinn et al. 1984; Griffiths et al. 1989), it is not evident how further concentration within COPI vesicles, as claimed by Lanoix et al. 1999, would be possible. This raises the possibility of a systematic error in quantitation in these studies that warrants further investigation.
All of these potential difficulties, including possible differences due to the use of GTP versus GTPγS (Lanoix et al. 1999), that may be inherent to fractionation or cell-free approaches are circumvented by measurements made on sections of whole cells, as we have done. Our results set a conservative upper limit for the concentration of steady-state Golgi residents in Golgi-associated COPI vesicles in vivo at ∼8% of their prevailing concentration in parental cisternae. This result is consistent with the previously established upper limit of ∼14–30% set by analyses of highly purified and morphologically pure COPI coated vesicle fractions (Sönnichsen et al. 1996).
The low level of resident proteins in COPI vesicles observed in vivo, and by Sönnichsen et al. 1996 in vitro is consistent with a role for these vesicles in bidirectionally redistributing steady-state residents within the stack (Pelham and Munro 1993; Hoe et al. 1995; Harris and Waters 1996; Linstedt et al. 1997; Wooding and Pelham 1998) to achieve their asymmetric steady-state distributions (Rabouille et al. 1995) via a dynamic equilibrium (Rothman and Wieland 1996), since in principle this equilibrium need not occur on the time scale of transport of cargo in the anterograde direction, except when cisternal progression is the sole means for anterograde transport.
In summary, our results are inconsistent with the view that cisternal progression is the sole or predominant means of anterograde transport, as this model does, as a matter of principle, require that the density of Golgi enzymes be at least as high in COPI vesicles as in the cisternal membranes (Glick et al. 1997). While our results are inconsistent with the possibility that cisternal progression occurs in lock-step with anterograde transport, they do not speak against the possibility that cisternae may progress on a substantially slower time scale than vesicle transport, as is independently indicated by other studies (Bonfanti et al. 1998; Bonfanti, L., O. Martella, A. Miranov, and A. Luini. 1999. Mol. Biol. Cell. 10:S114a; Volchuk et al. 2000).
Acknowledgments
We thank Dr. K.W. Moremen for kindly providing the Man II antibody. and Dr. G.B. Warren for kindly providing the HeLa cell line expressing epitope-tagged NAGT I.
This work was supported by grants from the Swiss National Science Foundation (to L. Orci) and from the National Institutes of Health (to J.E. Rothman).
Footnotes
Abbreviations used in this paper: Man II, mannosidase II; NAGT I, N-acetylglucosaminyltransferase I.
References
- Allan B.B., Balch W.E. Protein sorting by directed maturation of Golgi compartments. Science. 1999;285:63–66. doi: 10.1126/science.285.5424.63. [DOI] [PubMed] [Google Scholar]
- Baron M.D., Garoff H. Mannosidase II and the 135-kDa Golgi-specific antigen recognized monoclonal antibody 53FC3 are the same dimeric protein. J. Biol. Chem. 1990;265:19928–19931. [PubMed] [Google Scholar]
- Bonfanti L., Mironov A.A., Jr., Martinez-Menarguez J.A., Martella O., Fusella A., Baldassarre M., Buccione R., Geuze H.J., Mironov A.A., Luini A. Procollagen traverses the Golgi stack without leaving the lumen of cisternaeevidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/s0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- Burke B., Griffiths G., Reggio H., Louvard D., Warren G. A monoclonal antibody against a 135-K Golgi membrane protein. EMBO (Eur. Mol. Biol. Organ.) J. 1982;1:1621–1628. doi: 10.1002/j.1460-2075.1982.tb01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G.I., Lewis G.K., Ramsay G., Bishop J.M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B.S., Malhotra V. The curious status of the Golgi apparatus. Cell. 1998;95:883–889. doi: 10.1016/s0092-8674(00)81713-4. [DOI] [PubMed] [Google Scholar]
- Glick B.S., Elston T., Oster G. A cisternal maturation mechanism can explain the asymmetry of the Golgi stack. FEBS Lett. 1997;414:177–181. doi: 10.1016/s0014-5793(97)00984-8. [DOI] [PubMed] [Google Scholar]
- Green J., Griffiths G., Louvard D., Quinn P., Warren G. Passage of viral membrane proteins through the Golgi complex. J. Mol. Biol. 1981;152:663–698. doi: 10.1016/0022-2836(81)90122-4. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Fuller S.D., Back R., Hollinshead M., Pfeiffer S., Simons K. The dynamic nature of the Golgi complex. J. Cell Biol. 1989;108:277–297. doi: 10.1083/jcb.108.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Kuge S., Kuge O., Orci L., Amherdt M., Ravazzola M., Wieland F.T., Rothman J.E. En bloc incorporation of coatomer subunits during the assembly of COP-coated vesicles [published erratum appears in J. Cell Biol. 1994. 126:589] J. Cell Biol. 1994;124:883–892. doi: 10.1083/jcb.124.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S.L., Waters M.G. Localization of a yeast early Golgi mannosyltransferase, Och1p, involves retrograde transport. J. Cell Biol. 1996;132:985–998. doi: 10.1083/jcb.132.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe M.H., Slusarewicz P., Misteli T., Watson R., Warren G. Evidence for recycling of the resident medial/trans Golgi enzyme, N-acetylglucosaminyltransferase I, in ldlD cells. J. Biol. Chem. 1995;270:25057–25063. doi: 10.1074/jbc.270.42.25057. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lanoix J., Ouwendijk J., Stark A., Lin C.-C., Ostermann J., Nilsson T. GTP hydrolysis by arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COP I vesicles. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:4935–4948. doi: 10.1093/emboj/18.18.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F., Gaynor E.C., Hennecke S., Démollière C., Duden R., Emr S.D., Riezman H., Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Linstedt A.D., Mehta A., Suhan J., Reggio H., Hauri H.P. Sequence and overexpression of GPP130/GIMPcevidence for saturable pH-sensitive targeting of a type II early Golgi membrane protein. Mol. Biol. Cell. 1997;8:1073–1087. doi: 10.1091/mbc.8.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love H.D., Lin C.C., Short C.S., Ostermann J. Isolation of functional Golgi-derived vesicles with a possible role in retrograde transport. J. Cell Biol. 1998;140:541–551. doi: 10.1083/jcb.140.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K.W., Robbins P.W. Isolation, characterization, and expression of cDNAs encoding murine alpha-mannosidase II, a Golgi enzyme that controls conversion of high mannose to complex N-glycans. J. Cell Biol. 1991;115:1521–1534. doi: 10.1083/jcb.115.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K.W., Touster O., Robbins P.W. Novel purification of the catalytic domain of Golgi alpha-mannosidase II. Characterization and comparison with the intact enzyme. J. Biol. Chem. 1991;266:16876–16885. [PubMed] [Google Scholar]
- Nickel W., Malsam J., Gorgas K., Ravazzola M., Jenne N., Helms J.B., Wieland F.T. Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTPgammaS in vitro. J. Cell Sci. 1998;111:3081–3090. doi: 10.1242/jcs.111.20.3081. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Pypaert M., Hoe M.H., Slusarewicz P., Berger E.G., Warren G. Overlapping distribution of two glycosyltransferases in the Golgi apparatus of HeLa cells. J. Cell Biol. 1993;120:5–13. doi: 10.1083/jcb.120.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff A.B., Mori M., Quintana N., Yam A. Studies of the secretory process in the mammalian exocrine pancreas. I. The condensing vacuoles. J. Cell Biol. 1977;75:148–165. doi: 10.1083/jcb.75.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P.M., Tulsiani D.R., Touster O., Yam A., Novikoff A.B. Immunocytochemical localization of alpha-D-mannosidase II in the Golgi apparatus of rat liver. Proc. Natl. Acad. Sci. USA. 1983;80:4364–4368. doi: 10.1073/pnas.80.14.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Glick B.S., Rothman J.E. A new type of coated vesicular carrier that appears not to contain clathrinits possible role in protein transport within the Golgi stack. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Orci L., Malhotra V., Amherdt M., Serafini T., Rothman J.E. Dissection of a single round of vesicular transportsequential intermediates for intercisternal movement in the Golgi stack. Cell. 1989;56:357–368. doi: 10.1016/0092-8674(89)90239-0. [DOI] [PubMed] [Google Scholar]
- Orci L., Stamnes M., Ravazzola M., Amherdt M., Perrelet A., Söllner T.H., Rothman J.E. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Orci L., Perrelet A., Rothman J.E. Vesicles on stringsmorphological evidence for processive transport within the Golgi stack. Proc. Natl. Acad. Sci. USA. 1998;95:2279–2283. doi: 10.1073/pnas.95.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J., Orci L., Tani K., Amherdt M., Ravazzola M., Elazar Z., Rothman J.E. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- Pelham H.R. Getting through the Golgi complex. Trends Cell Biol. 1998;8:45–49. doi: 10.1016/s0962-8924(97)01185-9. [DOI] [PubMed] [Google Scholar]
- Pelham H.R., Munro S. Sorting of membrane proteins in the secretory pathway. Cell. 1993;75:603–605. doi: 10.1016/0092-8674(93)90479-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P., Griffiths G., Warren G. Density of newly synthesized plasma membrane proteins in intracellular membranes. II. Biochemical studies. J. Cell Biol. 1984;98:2142–2147. doi: 10.1083/jcb.98.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C., Hui N., Hunte F., Kieckbusch R., Berger E.G., Warren G., Nilsson T. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J. Cell Sci. 1995;108:1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J. Histochem. Cytochem. 1978;26:1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Rothman J.E., Wieland F.T. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sönnichsen B., Watson R., Clausen H., Misteli T., Warren G. Sorting by COP I-coated vesicles under interphase and mitotic conditions. J. Cell Biol. 1996;134:1411–1425. doi: 10.1083/jcb.134.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B., Lowe M., Levine T., Jamsa E., Dirac-Svejstrup B., Warren G. A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol. 1998;140:1013–1021. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., White J., Rottger S., Stelzer E.H., Suganuma T., Nilsson T. Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K.T. Application of cryoultramicrotomy to immunocytochemistry. J. Microsc. 1986;143:139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Velasco A., Hendricks L., Moremen K.W., Tulsiani D.R., Touster O., Farquhar M.G. Cell type-dependent variations in the subcellular distribution of alpha-mannosidase I and II. J. Cell Biol. 1993;122:39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchuk A., Amherdt M., Ravazzola M., Rivera V.M., Clackson T., Perrelet A., Söllner T.H., Rothman J.E., Orci L. Mega-vesicles implicated in the rapid transport of intra-cisternal aggregates across the Golgi stack. Cell. 2000;102:335–348. doi: 10.1016/s0092-8674(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Wooding S., Pelham H.R. The dynamics of Golgi protein traffic visualized in living yeast cells. Mol. Biol. Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]


