Abstract
In meiosis I, two chromatids move to each spindle pole. Then, in meiosis II, the two are distributed, one to each future gamete. This requires that meiosis I chromosomes attach to the spindle differently than meiosis II chromosomes and that they regulate chromosome cohesion differently. We investigated whether the information that dictates the division type of the chromosome comes from the whole cell, the spindle, or the chromosome itself. Also, we determined when chromosomes can switch from meiosis I behavior to meiosis II behavior. We used a micromanipulation needle to fuse grasshopper spermatocytes in meiosis I to spermatocytes in meiosis II, and to move chromosomes from one spindle to the other. Chromosomes placed on spindles of a different meiotic division always behaved as they would have on their native spindle; e.g., a meiosis I chromosome attached to a meiosis II spindle in its normal fashion and sister chromatids moved together to the same spindle pole. We also showed that meiosis I chromosomes become competent meiosis II chromosomes in anaphase of meiosis I, but not before. The patterns for attachment to the spindle and regulation of cohesion are built into the chromosome itself. These results suggest that regulation of chromosome cohesion may be linked to differences in the arrangement of kinetochores in the two meiotic divisions.
Keywords: kinetochore arrangement, chromosome cohesion, chromosomes, meiosis, micromanipulation
Introduction
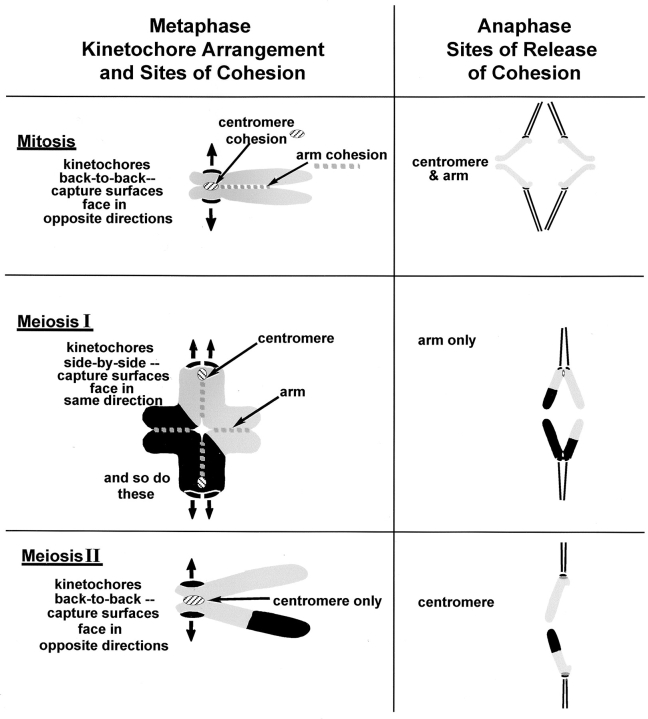
The reduction of chromosome number in meiosis is a central event in the lives of most eukaryotes, including humans. It makes diploidy possible because the gametes that are produced with half the chromosome number of their parent cells can then fuse to form a diploid zygote. The meiotic reduction in chromosome number depends on a distinctive attachment of chromosomes to the spindle as well as a distinctive regulation of the cohesion between sister chromatids (reviewed by Moore and Orr-Weaver 1998). The pattern of attachment in the first meiotic division is different from attachment in somatic mitosis. In mitosis, sister kinetochores lie back-to-back and capture microtubules from opposite poles; as a result, sister chromatids move to opposite poles in anaphase (Fig. 1). In the first meiotic division, however, sister chromatid kinetochores lie side-by-side, and they capture microtubules from the same spindle pole; as a result, sister kinetochores move to the same pole in anaphase I (Fig. 1). The meiosis II chromosome behaves like a mitotic chromosome; sister kinetochores are back-to-back in metaphase II, they capture microtubules from opposite poles and they move to opposite poles in anaphase II (Fig. 1).
Figure 1.
Kinetochore attachment to the spindle and chromosome cohesion in mitosis and meiosis. Mitosis, mitotic chromosomes consist of two sister chromatids. Kinetochore arrangement: one kinetochore faces one pole while its sister kinetochore faces the opposite pole and they move to opposite poles in anaphase. Chromosome cohesion: in metaphase, sister chromatids are held together by cohesion along chromosome arms and between centromeres. In anaphase both centromere and arm cohesion are released. Meiosis I, Bivalents consist of two homologous chromosomes, each of which is composed of a pair of sister chromatids. Kinetochore arrangement: both sister kinetochores face the same spindle pole, while the homologous pair of sister kinetochores faces the opposite spindle pole. Therefore, in anaphase I, both sister chromatids of one homologue move toward the same pole. Chromosome cohesion: sister chromatids in meiosis I are held together by cohesion between centromeres and between arms of sister chromatids. The bivalent is held together by both recombination and arm cohesion. Recombination sites are at the junction between black and grey chromosome segments. In anaphase of meiosis I, arm cohesion is released and half-bivalents separate. (Meiosis II) Each meiosis II chromosome consists of a pair of sister chromatids. Kinetochore arrangement: one kinetochore faces one pole, while its sister kinetochore faces the opposite pole. Consequently, sister kinetochores separate from one another in anaphase of meiosis II. Chromosome cohesion: in early stages of meiosis II, only the cohesion between centromeres remains; it is removed in anaphase II.
Chromosome cohesion and its timely release are just as important as kinetochore arrangement for correct reduction of chromosome number. If cohesion is absent, chromosomes segregate at random, so cohesion must be present before anaphase. Cohesion must be released at anaphase to allow chromosomes to move to opposite poles. In mitosis, chromosomes are held together along chromosome arms and between sister centromeres until anaphase, when cohesion lapses along the entire length of the chromosome (Fig. 1). In the first meiotic division, homologous chromosomes are linked together to form bivalents. This linkage is the result of cohesion along chromosome arms and recombination between the two homologous chromosomes (Fig. 1, Metaphase). In anaphase I, cohesion between chromatid arms is released, but cohesion between the centromeres of sister chromatids is maintained (Suja et al. 1992; Miyazaki and Orr-Weaver 1994; Bickel and Orr-Weaver 1996). The release of arm cohesion while centromere cohesion persists allows homologous chromosomes to separate from one another while the chromatids that make up each homologue remain glued together at the centromere (Fig. 1, Anaphase). In anaphase II, centromere cohesion is released and sister chromatids separate from one another (Fig. 1) (Miyazaki and Orr-Weaver 1994; Moore and Orr-Weaver 1998).
The special arrangement of kinetochores and regulation of cohesion in meiosis could arise from peculiarities of the cells, of the spindles, or of the chromosomes themselves. The ideal experiment to choose among these possibilities would be to inject a chromosome from a cell in the first meiotic division, for example, into a cell in the second division—does the chromosome then behave according to chromosome type or according to cell type? We have not succeeded in doing such an experiment, but we have fused a cell in meiosis I with one in meiosis II and have moved chromosomes from one spindle to the other.
One might expect that the critical differences between the two divisions are due to differences in the physiology of the cells or of the spindle (Lima-de-Faria 1958). If whole-cell differences are critical, then one would expect that a fused first and second division cell would be strange or dead. Alternatively, if spindle differences are controlling, then a transferred chromosome would behave according to the spindle to which it newly attached. For instance, a meiosis I chromosome on a meiosis II spindle would attach sister kinetochores to opposite poles and would lose centromere cohesion in anaphase. Finally, if the special properties are built into each chromosome, then the transferred meiosis I chromosome would behave normally, segregating sister kinetochores to the same pole while the nearby meiosis II chromosomes behave in their normal way, segregating sister kinetochores to opposite spindle poles. A few trial experiments showed that chromosomes do indeed attach according to chromosome type, not according to spindle type (Nicklas 1977). A key experiment (a meiosis II chromosome moved to a meiosis I spindle) was done only once, and the results were inconclusive. We have now learned how to do fusions more efficiently and have greatly extended the earlier results. Our new experiments focus not only on kinetochore attachment to the spindle, but also on chromosome cohesion. We resolved a critical ambiguity in the early study in which chromosomes that had already attached to one spindle were detached and moved to a different spindle. Once attached to a spindle, a chromosome is exposed to mitotic forces toward opposite poles, and it might well be imagined that such an attachment, even if brief, would rearrange kinetochores and cohesion sites. For instance, attachment of sister kinetochores to opposite poles in a meiosis II cell might pull the kinetochores so that they face in opposite directions. After such a chromosome is moved to a meiosis I spindle, the opposed kinetochores would preferentially capture microtubules from opposite poles, producing a meiosis II–type attachment. We have eliminated this ambiguity by moving chromosomes to the heterologous spindle before they could attach to their native spindle. We show that the chromosome contains all the information for how it will attach to the spindle and how it will separate in anaphase. Moreover, we show that the chromosome gains this information before nuclear envelope breakdown in meiosis I. We have also determined when a meiosis I chromosome changes so that it behaves like a meiosis II chromosome; the answer is surprisingly soon—by late anaphase of meiosis I.
Materials and Methods
Grasshopper Culture
Spermatocytes from laboratory colonies of the grasshoppers Melanoplus sanguinipes and Chortophaga australior were used in these experiments. Spermatocytes were cultured as previously described (Nicklas et al. 1982) at a temperature of 22.5–26°C.
Cell Observations and Micromanipulation
Cells were observed using phase contrast microscopy. Spermatocytes were fused by using a micromanipulation needle to vigorously massage the junction between adjacent cells. If membrane tearing in one cell was repaired by membrane flow in the adjacent cell, fusion occurred. After fusion, images of cells were recorded on an optical disk recorder (#2021; Panasonic). After fusion, chromosomes were manipulated with a microneedle.
Results
Chromosomes Attach to the Spindle and Divide According to Chromosome Type
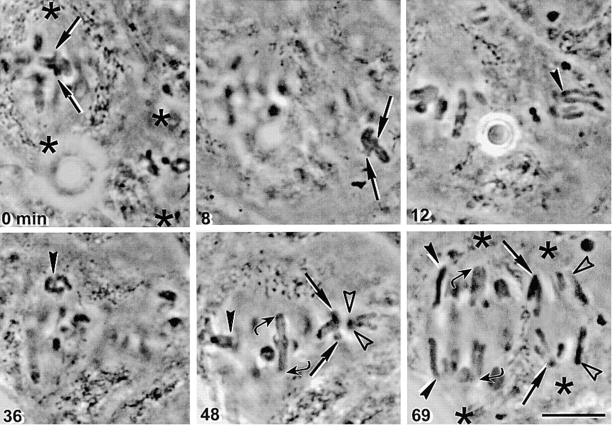
A total of six metaphase I/metaphase II fusions were studied. Fusion did not cause cell death, showing that the physiological differences between meiosis I and meiosis II cells do not make the two different cell types incompatible. The cells retained both a meiosis I and a meiosis II spindle in the same cell (Fig. 2, 0 min). We detached a bivalent from the meiosis I spindle. Such detachment is genuine—the old kinetochore microtubules are lost, so the chromosome must start fresh in forming microtubule attachments (Nicklas and Kubai 1985). The detached chromosome was then moved to the side of the meiosis II spindle farthest from the meiosis I spindle so that it would have no option but to attach to the meiosis II spindle. The bivalent promptly attached to the meiosis II spindle (Fig. 2, 8 min, straight arrows) and congressed to the spindle equator (Fig. 2, 48 min, straight arrows). In anaphase, sister chromatids behaved in the normal meiosis I fashion and moved together to their associated pole (Fig. 2, 69 min, straight arrows); meanwhile, the other chromosomes on that spindle behaved in their normal way with sister chromatids moving to opposite poles (Fig. 2 and 69 min, open arrowheads). Cohesion behavior was also chromosome intrinsic. Meiosis I chromosomes on meiosis II spindles lost cohesion only between chromatid arms (Fig. 2, 69 min, straight arrows), while the nearby meiosis II chromosomes lost cohesion between centromeres (Fig. 2, 69 min, open arrowheads).
Figure 2.
Determinants for the pattern of chromosome attachment to the spindle and release of chromosome cohesion are built into the chromosome. A metaphase I grasshopper spermatocyte was fused to a metaphase II spermatocyte. Spindle poles are indicated by asterisks, manipulated meiosis I chromosomes by straight arrows, unmanipulated meiosis I chromosomes by curved arrows, manipulated meiosis II chromosomes by filled arrowheads, and unmanipulated meiosis II chromosomes by open arrowheads. The fused cell contains two spindles. A bivalent was detached from the meiosis I spindle and placed near the meiosis II spindle (0 and 8 min, straight arrows). The bivalent attached to the meiosis II spindle with a pair of sister kinetochores facing each pole (48 min, straight arrows). Pairs of sister chromatids segregated to each pole (69 min, straight arrows). Unmanipulated bivalents on the meiosis I spindle had a pair of sister kinetochores facing each pole (48 min, curved arrows). In anaphase in unmanipulated bivalents, pairs of sister chromatids separated from one another (69 min, curved arrows). A meiosis II chromosome (12 min, filled arrowhead) was detached from the meiosis II spindle and placed near the meiosis I spindle (36 min, filled arrowhead). The meiosis II chromosome attached to the meiosis I spindle with a single sister kinetochore facing each pole (48 min, filled arrowhead), and single sister chromatids moved to opposite poles in anaphase (69 min, filled arrowheads). Unmanipulated meiosis II chromosomes attached with a single sister kinetochore facing each pole (48 min, open arrowheads) and moved to opposite poles in anaphase (69 min, open arrowheads). Bar, 10 μm.
We also did the reciprocal experiment in these six cells. A meiosis II chromosome was placed near the meiosis I spindle (Fig. 2, 36 min, filled arrowhead). The meiosis II chromosome attached to the meiosis I spindle and congressed to the spindle equator with sister kinetochores facing opposite poles (Fig. 2, 48 min, filled arrowhead). In anaphase, single chromatids moved apart to opposite poles (Fig. 2, 69 min, filled arrowheads), while the nearby meiosis I chromosomes behaved in their normal way (Fig. 2 and 69 min, curved arrows).
The results were the same in all 12 experiments in six fused cells. The chromosomes invariably attached and segregated in their normal manner according to chromosome type. However, it was possible that these characteristics of chromosome behavior were impressed on the chromosome as a result of its first spindle attachment. Therefore, we also did experiments in which chromosomes that had never attached to a spindle in one meiotic division were exposed to the unexpected condition of attaching to a spindle of the other meiotic division.
A cell in late prophase of meiosis I was fused with a cell in metaphase II (Fig. 3, 0 min). After nuclear envelope breakdown of the prophase I nucleus, one of the just-condensed meiosis I chromosomes was placed near the meiosis II spindle (Fig. 3 and 60 min, arrows). The meiosis I chromosome attached to the meiosis II spindle just as it normally would to a meiosis I spindle, with each pair of sister kinetochores associating with one spindle pole (Fig. 3, 85 min, arrows); the two pairs of sister kinetochores moved to opposite poles in anaphase (Fig. 3 and 182 min, arrows). Experiments of this type performed in three fused cells yielded identical results.
Figure 3.
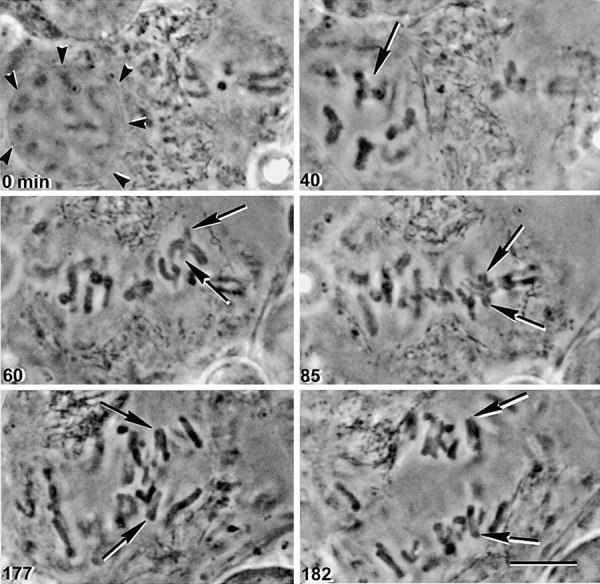
The way a meiosis I chromosome attaches to the spindle and releases cohesion does not depend on its initial spindle attachment. A late-prophase I spermatocyte and a metaphase II spermatocyte were fused (0 min). The prophase nuclear envelope was still present (0 min, arrowheads). After nuclear envelope breakdown, a bivalent that had not yet attached to the meiosis I spindle (40 min, arrow) was placed near the meiosis II spindle (60 min, arrows). The manipulated bivalent attached to the meiosis II spindle (85 min, arrows). Pairs of sister chromatids segregated to opposite poles in anaphase. The upper pair is more clearly visible in the 177 min image, while the lower pair is more clearly visible in the 182 min image. Bar, 10 μm.
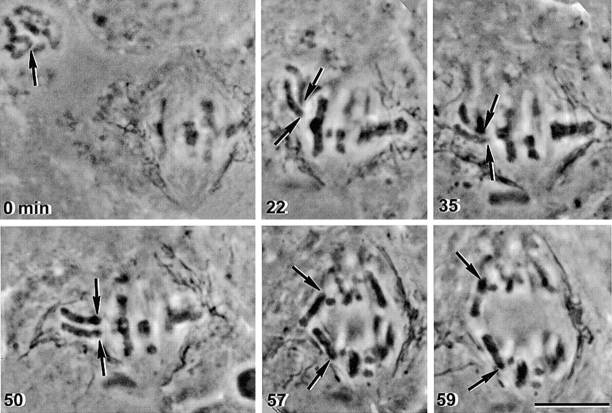
Complementary results were observed in a metaphase I/prophase II fusion (Fig. 4). Immediately after nuclear envelope breakdown in the prophase II nucleus, a meiosis II chromosome was placed near the meiosis I spindle (Fig. 4 and 22 min, arrows). It attached to the meiosis I spindle with a single sister kinetochore facing each pole (Fig. 4 and 50 min, arrows). Sister chromatids separated from one another in anaphase (Fig. 4 and 59 min, arrows). Experiments of this type performed in three fused cells yielded identical results.
Figure 4.
The way a meiosis II chromosome attaches to the spindle and releases cohesion does not depend on its initial spindle attachment. A prophase II spermatocyte and a metaphase I spermatocyte were fused (0 min). After nuclear envelope breakdown, a meiosis II chromosome that had not yet attached to the meiosis II spindle (0 min, arrow) moved near the meiosis I spindle (22 min, arrows). It attached with one chromatid facing each pole (22, 35, and 50 min, arrows), and single sister chromatids segregated to opposite poles in anaphase (57 and 59 min, arrows). Bar, 10 μm.
Meiosis I Chromosomes Can Behave as Meiosis II Chromosomes by Anaphase I, but Not Before
We wanted to determine when chromosomes become capable of attaching to the spindle in the meiosis II manner (with a single sister kinetochore facing each spindle pole) and releasing cohesion in the meiosis II manner (releasing centromere cohesion).
Bivalents in late metaphase I cells were induced to orient with sister kinetochores facing opposite poles (a meiosis II type of attachment). This was accomplished by detaching a chromosome from the spindle and repeatedly placing it so its centromeres faced the center of the spindle (Fig. 5A, 0 min, and B, 0 min, arrows). The chromosome would eventually attach to the spindle with one kinetochore facing one pole and the sister kinetochore facing the other (Fig. 5A, 0 min, and B, 0 min, arrows). These chromosomes invariably remained at the spindle equator for a long time after anaphase onset with both kinetochores greatly stretched toward the poles (Fig. 5A, Fig. 6 min, and B, 4 min). Two of the five chromosomes observed eventually separated sister chromatids to opposite poles (Fig. 5 A, 19 min, arrows). Three of the five chromosomes observed did not separate sister chromatids to opposite poles (Fig. 5 B, 17 min, arrows). This shows that chromosomes can exhibit meiosis II attachment characteristics by late metaphase I. However, sister chromatids cannot properly separate from one another in anaphase. Separation is either delayed (Fig. 5 A) or fails completely (B). Such rigorous experiments, in which kinetochores are repeatedly detached from the spindle, might be thought to damage the kinetochores, so that movement in anaphase is abnormal. That is not the case, however. The whole bivalent was manipulated in these experiments so that both pairs of sister kinetochores (one pair on each half-bivalent) were treated equally. In four of the five experiments, only one of the two half-bivalents attached with sister kinetochores to opposite poles (Fig. 5 C, 0 min, arrow). The kinetochores of the other half-bivalent attached to the same spindle pole; they moved normally in anaphase and without delay to that spindle pole (Fig. 5 C, 27 and 32 min, arrowheads). Thus it is the form of the attachment, not the manipulation, that determines how the chromosomes behave in anaphase.
Figure 5.
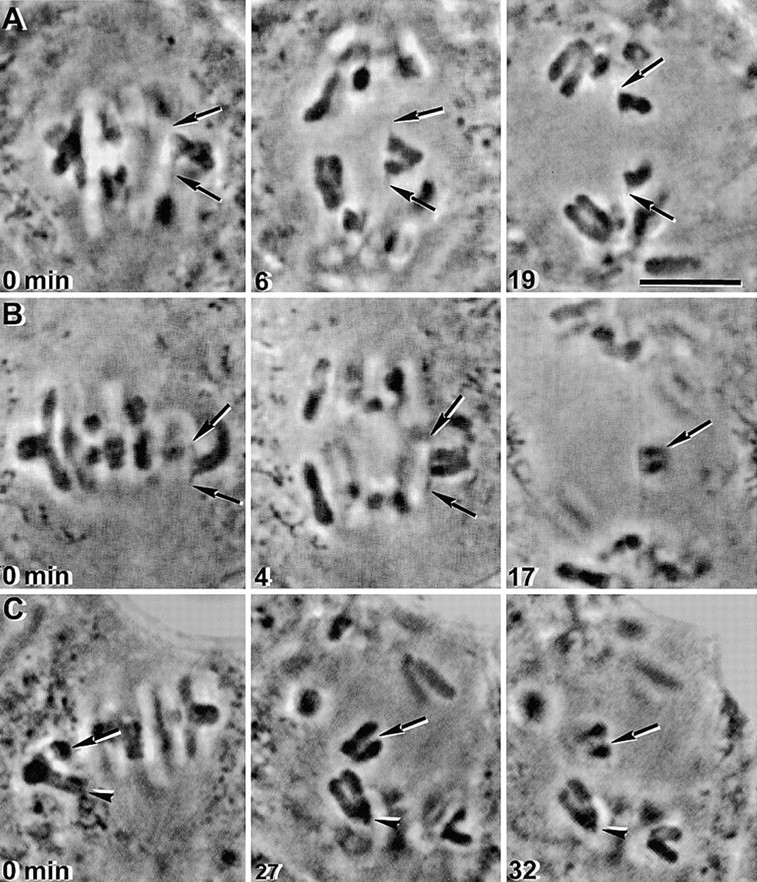
Bivalents can be induced to attach to the spindle with a single sister kinetochore facing each pole, but they neither attach nor separate in the normal meiosis II manner. Bivalents in unfused spermatocytes were micromanipulated. (A) One pair of sister kinetochores of the bivalent was induced to attach to opposite spindle poles (0 min, arrows). The chromosome remained, with stretched-out kinetochores, at the equator of the spindle after anaphase onset (6 min, arrows). 13 min after anaphase onset, the sister chromatids started to slowly separate from one another (19 min), moving towards the poles to which they were attached. (B) Another example of a bivalent in which one pair of sister kinetochores was induced to attach to opposite poles (0 min, arrows). In anaphase I, sister kinetochores were greatly stretched towards their spindle poles, but the sister chromatids did not separate from one another (4 and 17 min, arrows). (C) One pair of sister kinetochores of the bivalent was induced to attach to opposite poles (0 min, arrow), while the other pair of sister kinetochores attached to the same pole (0 min, arrowhead). In the pair that did attach to opposite poles, the sister chromatids did not separate from one another (27 and 32 min, arrows). The other pair of sister kinetochores attached to the lower spindle pole (0 and 27 min, arrowheads) and moved together to that pole in anaphase (27 and 32 min, arrowheads). Bar, 10 μm.
Figure 6.
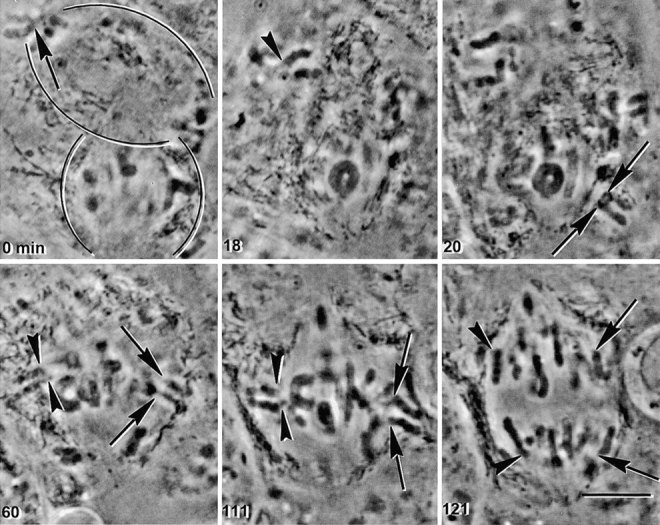
Chromosomes acquire meiosis II properties after anaphase I. A spermatocyte in anaphase I was fused to a spermatocyte in metaphase I (0 min). The spindles are outlined: anaphase I above, metaphase I below. Two different anaphase chromosomes were studied in this experiment, one indicated by an arrow and the other by an arrowhead. The chromosomes were detached from the anaphase I spindle (0 min, arrow; 18 min, arrowhead) and placed near the metaphase I spindle (20 min, arrows; 60 min, arrowheads). The chromosomes attached to the metaphase I spindle, with a sister kinetochore facing each pole (20 and 60 min, arrows; 60 min, arrowheads). The manipulated meiosis I chromosomes behaved just like meiosis II chromosomes when the cell entered anaphase, sending a single chromatid to each pole (111 and 121 min, arrowheads and arrows). Bar, 10 μm.
We determined when meiosis I chromosomes acquire the ability to behave like normal chromosomes in meiosis II; i.e., with sister kinetochores attaching to opposite poles and releasing cohesion between centromeres in anaphase. Prometaphase I or metaphase I cells were fused to anaphase I cells. After fusion, anaphase I chromosomes were detached from the anaphase spindle and placed near the metaphase I spindle (Fig. 6). 16 such anaphase I chromosomes in three fused cells were observed. These anaphase I chromosomes attached to the metaphase spindle with a single sister kinetochore facing each pole (Fig. 6 and 111 min), and a single sister chromatid moved to each pole in the subsequent anaphase (Fig. 6, 121 min). These data differ from what we saw in metaphase I cells (Fig. 5) in two respects. First, anaphase I chromosomes attached to the spindle with sister kinetochores facing opposite poles at the first attempt; metaphase I chromosomes attached in this manner only after repeated placement of the chromosome in such a position so as to force the meiosis II type of attachment. Second, anaphase I chromosomes on preanaphase spindles separate into single chromatids in the subsequent anaphase cleanly and with no lagging. Metaphase I chromosomes that were induced to attach to the spindle in the meiosis II manner suffered a considerable lag before separation of sister chromatids, if they did so at all. Thus, meiosis I chromosomes are capable of behaving like meiosis II chromosomes after anaphase I, but not before.
Discussion
The Properties Necessary for the Reduction of Chromosome Number in Meiosis Are Built into Each Chromosome
Spindle Attachment.
We have shown that the information for appropriate attachment to the spindle is contained within the chromosome itself, and not in the cytoplasm or the spindle. Similar results were obtained earlier (Nicklas 1977), but those limited trials included only one example of a meiosis II chromosome on a meiosis I spindle. We have also eliminated an important ambiguity by showing that the information for how to attach to the spindle is not imposed on the chromosome through its first spindle attachment, but, rather, it is built into the chromosome before the breakdown of the nuclear envelope.
Cohesion Release.
We found that the pattern of release of chromosome cohesion is also built into the chromosome itself. Our result differs from the single previous observation (Nicklas 1977) of a meiosis II chromosome on a meiosis I spindle, which did not separate properly in anaphase. That result was evidently due to the nonspecific stickiness sometimes seen in cultured spermatocytes. In the present nine experiments, all chromosomes separated cleanly in anaphase (e.g., Fig. 2 and Fig. 4). Also, like attachment, the pattern of cohesion release is built into the prophase chromosome: chromosomes that had not previously met with a spindle separated true to type, behaving as meiosis I (Fig. 3) or meiosis II (Fig. 4) chromosomes on the heterologous spindle.
Most revealing was the behavior of meiosis I chromosomes on a meiosis II spindle. One might easily have expected that in meiosis I cells the signal to start anaphase would be special: “release your arm cohesion only, not your centromere cohesion.” Instead, what was seen was that a meiosis I chromosome released only its arm cohesion while the meiosis II chromosomes all around it were losing cohesion at the centromere. Thus, the signal to start anaphase is a generic, cell-wide signal that generates a chromosome-specific response. The molecule responsible for cohesion between centromeres of sister chromatids is not destroyed or removed from bivalents at anaphase I, even in the presence of a meiosis II cytoplasm and on a meiosis II spindle. Either this centromere cohesion molecule is masked from recognition by some chemical modification, or it is inaccessible to degradation or removal due to its position within a folded bivalent.
Candidates for the centromere cohesion molecule are now being identified. In Drosophila, Mei-S332 is essential for maintaining sister chromatid cohesion at centromeres of meiosis I and II chromosomes (Kerrebrock et al. 1992, Kerrebrock et al. 1995; Bickel et al. 1998; Tang et al. 1998). It is present at the centromeres of meiotic chromosomes until anaphase II, at which point it is either removed from the centromeres or destroyed (Moore et al. 1998). In yeast, Rec8 is required to prevent precocious sister chromatid separation in meiosis (Klein et al. 1999; Watanabe and Nurse 1999). The Rec8 molecule is not removed from the centromere region until anaphase II, even though it is removed from chromosome arms at anaphase I (Klein et al. 1999; Watanabe and Nurse 1999). Our work emphasizes the importance of this key question: how are the centromere cohesion molecules modified or masked from the chromosome separation machinery in meiosis I chromosomes so that they are not affected when nearby meiosis II chromosomes are separating at the centromere?
Timing the Changes: Chromosomes Can Attach to the Spindle in the Meiosis II Manner by Metaphase I and Can Separate in the Meiosis II Manner by Anaphase I
The ability to induce attachment to opposite poles in metaphase I is correlated with a change in kinetochore structure. In prophase I bivalents, the kinetochores are not visibly double, but by metaphase I, two sister kinetochores are evident (Müller 1972; Goldstein 1981; Lin and Church 1982). Only at this time is it possible, though with great difficulty, to induce the sister kinetochores to attach to opposite spindle poles, by repeatedly detaching chromosomes from the spindle and placing the kinetochores so that they do not face either pole directly (as in Fig. 5A and Fig. B; 0 min). Univalents (unpaired meiosis I chromosomes) behave similarly. They attach to one spindle pole or the other until metaphase I, but thereafter they frequently reattach. By late metaphase, sister kinetochores often attach to opposite poles, but, like our late metaphase I chromosomes (Fig. 5), they do not separate cleanly and lag in anaphase (e.g., Rebollo and Arana 1995; Gimenez-Abian et al. 1997). Thus, the kinetochores of meiosis I chromosomes become structurally and functionally double by metaphase of the first meiotic division, but centromere cohesion cannot be removed properly.
Remarkably, chromosomes acquire the ability to attach easily in meiosis II as early as anaphase I, and this goes hand-in-hand with the ability to separate sister centromeres cleanly (Fig. 6). The contrast with metaphase I chromosomes (Fig. 5) is striking. It is surprising that meiosis II competence is acquired in anaphase I, before any substantial chromosome decondensation. We expected that the major structural remodeling of the kinetochore and centromere necessary for meiosis II behavior would require decondensation, and then recondensation, before the second meiotic division. Despite the absence of DNA synthesis, there is an interphase-like state between the two meiotic divisions and the chromosomes do decondense. However, our results suggest that no structural rearrangements are required beyond separation of homologous chromosomes from one another in anaphase I, and a subsequent attachment to a spindle. This changes our view of the time and nature of a major step in meiosis. The new question is: what happens to kinetochores, centromeres, and cohesion molecules in the brief interval between metaphase I and anaphase I that converts a somewhat malleable meiosis I chromosome (Fig. 5) into a chromosome that flawlessly executes meiosis II behaviors (Fig. 6)? We do not know, but the close correlation in timing of the changes in kinetochore and cohesion behavior suggests that they are related. A link between the two is supported by the work of Tanaka et al. 1999. They showed that the cohesin Scc1/Mcd1 requires the presence of functional kinetochore components to associate with newly activated centromeres (Tanaka et al. 1999). In addition, Megee et al. 1999 and Megee and Koshland 1999 showed that a centromere must be present on the chromosome for cohesion to be established properly, and for proper binding of Scc1/Mcd1 to the chromosome. The relation between kinetochore behavior and chromosome cohesion was also previously suggested by Orr-Weaver 1999 and Moore and Orr-Weaver 1998. They suggested that cohesion controls kinetochore behavior such that, in meiosis I, each kinetochore's position is constrained by the cohesion molecules so that it and its sister kinetochore must together act as a single kinetochore. The converse is also possible; i.e., the status of the kinetochore may control chromosome cohesion. The kinetochore undergoes a slow maturation between prometaphase I and meiosis II, starting as a single mass, and then becoming visibly double by late metaphase I, and finally becoming functionally double by meiosis II. We suggest that the cohesion molecules may be linked to the kinetochore in such a way that the maturation of the kinetochore makes the cohesion molecules accessible for release or destruction in anaphase II. The maturation of kinetochores and growing accessibility of cohesion molecules may be simply time-dependent processes, which would explain the difference that we see between metaphase I and anaphase I chromosomes. Perhaps the metaphase I chromosomes must spend a fixed period of time in meiosis before sister chromatids can properly separate from one another in anaphase. Our anaphase I chromosomes, because they had to reattach to a new spindle and await another anaphase, spent much longer in meiosis than did the metaphase I chromosomes. Alternatively, anaphase I may be required to trigger the ultimate switch between a meiosis I and a meiosis II chromosome. Further study of the structure of the kinetochore region may give clues as to the link between the regulation of cohesion and kinetochore status.
In conclusion, the reduction of chromosome number in meiosis is explained simply by properties that are built into meiotic chromosomes. Although chromosome structural peculiarities are the immediate cause of the distinctive behavior of chromosome in meiosis, these chromosomal properties must arise from earlier events in the differentiation of meiotic cells. The challenge for the future is to understand how the features of chromosome organization that distinguish chromosomes in the two meiotic divisions arise as a product of cell differentiation.
Acknowledgments
We thank Suzanne Ward and Jennifer King for discussions and major technical assistance, and Donna Maroni for vigorous editorial review. This paper is dedicated to the memory of Sten Fjellstedt.
This investigation was supported in part by grant GM-13745 from the Institute of General Medical Sciences, the National Institutes of Health.
References
- Bickel S.E., Moore D.P., Lai C., Orr-Weaver T.L. Genetic interactions between mei-S332 and ord in the control of sister-chromatid cohesion. Genetics. 1998;150:1467–1476. doi: 10.1093/genetics/150.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel S.E., Orr-Weaver T.L. Holding chromatids together to ensure they go their separate ways. Bioessays. 1996;18:293–300. doi: 10.1002/bies.950180407. [DOI] [PubMed] [Google Scholar]
- Gimenez-Abian J.F., Clarke D.J., Garcia de la Vega C., Gimenez-Martin G. The role of sister chromatid cohesiveness and structure in meiotic behaviour. Chromosoma. 1997;106:422–434. doi: 10.1007/s004120050264. [DOI] [PubMed] [Google Scholar]
- Goldstein L.S. Kinetochore structure and its role in chromosome orientation during the first meiotic division in male D. melanogaster . Cell. 1981;25:591–602. doi: 10.1016/0092-8674(81)90167-7. [DOI] [PubMed] [Google Scholar]
- Kerrebrock A.W., Miyazaki W.Y., Birnby D., Orr-Weaver T.L. The Drosophila mei-s332 gene promotes sister-chromatid cohesion in meiosis following kinetochore differentiation. Genetics. 1992;130:827–841. doi: 10.1093/genetics/130.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrebrock A.W., Moore D.P., Wu J.S., Orr-Weaver T.L. Mei-S332, a Drosophila protein required for sister-chromatid cohesion, can localize to meiotic centromere regions. Cell. 1995;83:247–256. doi: 10.1016/0092-8674(95)90166-3. [DOI] [PubMed] [Google Scholar]
- Klein F., Mahr P., Galova M., Buonomo S.B., Michaelis C., Nairz K., Nasmyth K. A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell. 1999;98:91–103. doi: 10.1016/S0092-8674(00)80609-1. [DOI] [PubMed] [Google Scholar]
- Lima-de-Faria A. Recent advances in the study of the kinetochore. Int. Rev. Cytol. 1958;7:123–157. [Google Scholar]
- Lin H.P., Church K. Meiosis in Drosophila melanogaster. III. The effect of orientation disruptor (ord) on gonial mitotic and the meiotic divisions in males. Genetics. 1982;102:751–770. doi: 10.1093/genetics/102.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megee P.C., Koshland D. A functional assay for centromere-associated sister chromatid cohesion. Science. 1999;285:254–257. doi: 10.1126/science.285.5425.254. [DOI] [PubMed] [Google Scholar]
- Megee P.C., Mistrot C., Guacci V., Koshland D. The centromeric sister chromatid cohesion site directs Mcd1 binding to adjacent sequences. Mol. Cell. 1999;4:445–450. doi: 10.1016/s1097-2765(00)80347-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki W.Y., Orr-Weaver T.L. Sister-chromatid cohesion in mitosis and meiosis. Annu. Rev. Genet. 1994;28:167–187. doi: 10.1146/annurev.ge.28.120194.001123. [DOI] [PubMed] [Google Scholar]
- Moore D.P., Orr-Weaver T.L. Chromosome segregation during meiosisbuilding an unambivalent bivalent. Curr. Top. Dev. Biol. 1998;37:263–299. doi: 10.1016/s0070-2153(08)60177-5. [DOI] [PubMed] [Google Scholar]
- Moore D.P., Page A.W., Tang T.T., Kerrebrock A.W., Orr-Weaver T.L. The cohesion protein MEI-S332 localizes to condensed meiotic and mitotic centromeres until sister chromatids separate. J. Cell. Biol. 1998;140:1003–1012. doi: 10.1083/jcb.140.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. Elektronenmikroskopische Untersuchungen zum Formwechsel der Kinetochoren während der Spermatocytenteilungen von Pales ferruginea (Nematocera) Chromosoma. 1972;38:139–172. doi: 10.1007/BF00326191. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. Chromosome distributionexperiments on cell hybrids and in vitro. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1977;277:267–276. doi: 10.1098/rstb.1977.0017. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B., Kubai D.F. Microtubules, chromosome movement, and reorientation after chromosomes are detached from the spindle by micromanipulation. Chromosoma. 1985;92:313–324. doi: 10.1007/BF00329815. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B., Kubai D.F., Hays T.S. Spindle microtubules and their mechanical associations after micromanipulation in anaphase. J. Cell Biol. 1982;95:91–104. doi: 10.1083/jcb.95.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T.L. The ties that bindlocalization of the sister-chromatid cohesin complex on yeast chromosomes. Cell. 1999;99:1–4. doi: 10.1016/s0092-8674(00)80055-0. [DOI] [PubMed] [Google Scholar]
- Rebollo E., Arana P. A comparative study of orientation at behavior of univalent in living grasshopper spermatocytes. Chromosoma. 1995;104:56–67. doi: 10.1007/BF00352226. [DOI] [PubMed] [Google Scholar]
- Suja J.A., Antonio C., Rufas J.S. Involvement of chromatid cohesiveness at the centromere and chromosome arms in meiotic chromosome segregationa cytological approach. Chromosoma. 1992;101:493–501. doi: 10.1007/BF00352472. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Cosma M.P., Wirth K., Nasmyth K. Identification of cohesin association sites at centromeres and along chromosome arms. Cell. 1999;98:847–858. doi: 10.1016/s0092-8674(00)81518-4. [DOI] [PubMed] [Google Scholar]
- Tang T.T.L., Bickel S.E., Young L.M., Orr-Weaver T.L. Maintenance of sister-chromatid cohesion at the centromere by the Drosophila MEI-S332 protein. Genes Dev. 1998;12:3843–3856. doi: 10.1101/gad.12.24.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Nurse P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature. 1999;400:461–464. doi: 10.1038/22774. [DOI] [PubMed] [Google Scholar]