Abstract
Cell–cell and cell–matrix interactions play a critical role in tissue morphogenesis and in homeostasis of adult tissues. The integrin family of adhesion receptors regulates cellular interactions with the extracellular matrix, which provides three-dimensional information for tissue organization. It is currently thought that pancreatic islet cells develop from undifferentiated progenitors residing within the ductal epithelium of the fetal pancreas. This process involves cell budding from the duct, migration into the surrounding mesenchyme, differentiation, and clustering into the highly organized islet of Langerhans. Here we report that αvβ3 and αvβ5, two integrins known to coordinate epithelial cell adhesion and movement, are expressed in pancreatic ductal cells and clusters of undifferentiated cells emerging from the ductal epithelium. We show that expression and function of αvβ3 and αvβ5 integrins are developmentally regulated during pancreatic islet ontogeny, and mediate adhesion and migration of putative endocrine progenitor cells both in vitro and in vivo in a model of pancreatic islet development. Moreover, we demonstrate the expression of fibronectin and collagen IV in the basal membrane of pancreatic ducts and of cell clusters budding from the ductal epithelium. Conversely, expression of vitronectin marks a population of epithelial cells adjacent to, or emerging from, pancreatic ducts. Thus, these data provide the first evidence for the contribution of integrins αvβ3 and αvβ5 and their ligands to morphogenetic events in the human endocrine pancreas.
Keywords: pancreatic islets, endocrine progenitors, αvβ3, integrins, cell migration
Introduction
Interactions of cells with their environment play a critical role in directing embryonic development and in maintaining tissue integrity in adult life. The structure/function paradigm dictates that the architectural elements that determine tissue structure also affect tissue function. In this context, the integrin family of adhesion receptors regulates many of the cellular interactions with the extracellular matrix (ECM), which provide critical elements of three-dimensional information for tissue organization. Integrins are remarkably multifunctional since they mediate not only adherence to specific ECM proteins, but also regulate the migration and spatial segregation of different cell types within tissues (Hynes 1987; Sonnenberg 1993; Gullberg and Ekblom 1995), cell proliferation (Schwartz and Ingber 1994), establishment and maintenance of cytodifferentiation (Walker et al. 1989; Strange et al. 1992; Talhouk et al. 1992; Lin and Bissell 1993), cell polarity (Jones and Watt 1993; Watt and Jones 1993; DiPersio et al. 1997), and ultimately cell survival (Giancotti 1997).
A distinctive feature of developing epithelia is the cell type–specific pattern of cell adhesion receptors whose expression and function is quantitatively and temporally regulated in a very precise manner. Integrins αvβ3 and αvβ5 are among those receptors that contribute to the coordinated cell adhesion and movement of diverse cell types on various ECM components including vitronectin (VN), fibronectin (FN), and collagen IV (Coll-IV) (Pelletier et al. 1996; George et al. 1997). Interestingly, invasive endothelial cells express high levels of αvβ3 that are downregulated upon cell differentiation (Brooks et al. 1994; Clark et al. 1996). Similarly, usage of αvβ5 has been found to be dynamically regulated in migrating versus stationary epithelial cells (Marchisio et al. 1991).
Cell growth and differentiation of endocrine progenitors in the pancreas provides a model of programmed epithelial morphogenesis. It is currently thought that pancreatic endocrine cells originate from a subpopulation of undifferentiated progenitors residing within the ductal epithelium of the fetal pancreas (Pictet and Rutter 1972; Teitelman and Lee 1987; Alpert et al. 1988; Gu and Sarvetnick 1993). This process involves cell budding from the duct, migration into the surrounding mesenchyme, differentiation, and clustering into the highly organized islet of Langerhans (Pictet et al. 1972) representing the endocrine compartment of the mammalian pancreas (Langerhans 1869; Orci and Unger 1975; Orci 1982). The parallel creation of a complex vascular network and the orientation of the endocrine cells around this network completes the development of the adult islet (Jansson 1995; Lombardi et al. 1985; Orci et al. 1989).
Here we report the novel observation that pancreatic ductal cells and clusters of undifferentiated cells emerging from the ductal epithelium of the developing human pancreas are characterized by the expression of high levels of αvβ3 and αvβ5 integrins. The expression and function of αvβ3 and αvβ5 integrins are developmentally regulated during pancreatic islet ontogeny, and mediate adhesion and migration of undifferentiated pancreatic epithelial cells. Using an in vivo model of human fetal islet development, we demonstrate that inhibiting the function of the αvβ3 and αvβ5 integrins results in a dramatic perturbation of islet cell emergence from the ductal epithelium. Thus, these data provide the first evidence for the contribution of these two integrins to morphogenetic events in the human pancreas.
Materials and Methods
Tissues
Mid-gestation human fetal pancreata ranging from 18 to 20 wk of gestational age were obtained through a nonprofit organ procurement program (Advanced Bioscience Resources), which also obtained consent for tissue donation. Samples of human adult pancreas and pancreatic islets were prepared at The Diabetes Research Institute (University of Florida, Miami, FL) as previously described (Ricordi et al. 1988). Use of human tissues was approved by the appropriate Institutional Review Boards.
Immune Fluorescence, Confocal Microscopy, and Morphometric Analysis
Triple immune fluorescent labeling was performed on 8-μm-thick cryostat sections prepared from snap-frozen fetal pancreata (18–20 wk of gestation), as previously described (Cirulli et al. 1998). Primary antibodies used were sheep IgGs anti–human insulin (The Binding Site), rabbit anti–human glucagon (Chemicon International, Inc.), mouse anti–human glucagon (Sigma-Aldrich), mouse anti-αvβ3 (clone LM609; kind gift of Dr. David Cheresh, The Scripps Research Institute), mouse anti-αvβ5 (clone 15F11; kind gift of Dr. Ingrid Stuiver, The Scripps Research Institute), and anti-β1 (clone mAb 13; kind gift of Dr. Steve Akiyama, National Institute of Dental Research, Bethesda, MD). Anti-ECM antibodies were anti–Coll-IV, anti-VN, and anti-FN (all from GIBCO BRL). Goat anti–platelet endothelial cell adhesion molecule (PECAM-1; Santa Cruz Biotechnology, Inc.). All fluorophore-labeled secondary antibodies were used as F(ab)2 fractions and were from Jackson Immunoresearch Laboratories: lissamine rhodamine (LRSC)–conjugated affinity-purified donkey anti–sheep IgGs (5 μg/ml; preadsorbed on chicken, guinea pig, hamster, horse, human, mouse, rabbit, and rat serum proteins); LRSC-conjugated affinity-purified donkey anti–goat IgGs (5 μg/ml; preadsorbed on chicken, guinea pig, hamster, horse, human, mouse, rabbit, sheep, and rat serum proteins); FITC-conjugated affinity-purified donkey anti-rabbit IgGs (5 μg/ml; preadsorbed on bovine, chicken, goat, guinea pig, hamster, horse, human, mouse, rat and sheep serum proteins); indodicarbocyanine (Cy5)-conjugated affinity-purified donkey anti–mouse IgGs (5 μg/ml; preadsorbed on bovine, chicken, goat, guinea pig, hamster, horse, human, rabbit, rat and sheep serum proteins). In all experiments, separate sections were incubated with a mixture of normal sheep (or goat), rabbit, and mouse IgGs, followed by incubations with appropriate fluorophore-labeled secondary antibodies to be used as control reference for specificity of primary antibodies.
After extensive washes, sections were mounted in slow fade medium (Molecular Probes), and viewed on a Zeiss Axiovert 35M microscope equipped with a laser scanning confocal attachment (MRC-1024; Bio-Rad Laboratories), using an oil immersion ×40 1.3 NA objective lens. Fluorescent images relative to each marker were collected by using the 488, 568, and 647 nm excitation lines from an argon/krypton mixed gas laser. Color composite images were generated using Adobe Photoshop 5.5 (Adobe Systems Inc.) running on a UMAX SuperMac S910/250 computer (UMAX Computer Corporation), and printed with a Fujix Pictography 3000 color printer (Fuji Photo Film U.S.A., Inc.).
Microscopic fields acquired from the fetal pancreatic sections (n = 50) immune stained for αvβ3 and αvβ5 were analyzed for pixel intensity of αvβ3- and αvβ5-specific immune reactivity using the NIH Image software (National Institutes of Health, Bethesda, MD). Pixels' intensity units (0–255) were recorded from a total of 250 domains of cell–cell and/or cell–matrix contact for each cell type (ductal, endocrine, epithelial undifferentiated, and stromal).
Generation of Islet-like Cell Clusters and Cell Monolayers
Human fetal pancreata at 18–20 wk of gestation were minced in small pieces and digested in a collagenase solution (6 mg/ml/HBSS; Collagenase-P; Boehringer) for 15 min at 37°C in a shaking water bath (150 cycles/min) as previously described (Otonkoski et al. 1993). Cell clusters resulting from this procedure were washed in cold HBSS and placed in culture in RPMI-1640 (Sigma-Aldrich) containing 11 mM glucose and supplemented with 10% normal human serum. This culture condition, applied for 3–5 d in nonadherent culture dishes, allows the formation of smooth islet-like cell clusters. These cell clusters contain mostly undifferentiated epithelial cells and ∼5% endocrine cells (mainly insulin- and glucagon-producing cells; Beattie et al. 1994; Otonkoski et al. 1994, Otonkoski et al. 1996). Culture of fetal pancreatic cell clusters in Petri dishes coated with the ECM 804G (Langhofer et al. 1993) supplemented with 10 ng/ml recombinant human hepatocyte growth factor/scatter factor, promotes the generation of cell monolayers. Under these culture conditions cells forming the islet-like cell clusters adhere, migrate and expand to produce large monolayers (Beattie et al. 1996). This in vitro system was used as a model to monitor cell migration from a three-dimensional configuration (cell clusters) into the bidimensional arrangement of cell monolayers. To allow the identification of identical microscopic fields at different incubation times we used alpha-numeric hatched coverslips, precoated with the 804-G matrix. In brief, 804-G cells were cultured to confluence on these coverslips, after which they were lysed with NH4OH (20 mM), washed with PBS and stored at 4°C until use.
Adhesion Assay
We used a micro-adhesion assay method to optimize the use of limited tissue samples using 60-position micro templates. Each well has a volume of 20 μl and requires only 2,000 cells (Calof and Lander 1991). In brief, the cells were labeled with [35S]methionine/cysteine and washed, and 2 × 103 cells were plated onto wells precoated with human Coll-IV, FN, laminin (LN; GIBCO BRL), or VN (Promega) at the specified concentrations. After a 30-min incubation, the nonadherent cells were spun off at low centrifugal force and the template was exposed overnight to a phosphorimaging plate and analyzed with a phosphorimaging scanner (Applied Biosystems). Results are expressed as the integrated volume in pixels read from the imaging plate over a user-defined area representing the adhered isotope-labeled cells. This integrated value is linearly related to the number of isotope decays per pixel per unit time. Adhesion to a substance extracted from a marine mussel, Cell-Tak (Collaborative Biomedical) was used as a positive control since poly-l-lysine did not work for the fetal cells, indicating differences in cell surface glycosylation of fetal cells as compared with adult islet cells. Adhesion to BSA was used as a negative control.
PCR
10 ng of total RNA from either fetal or adult human islets were reverse transcribed into cDNA. Then 2.5 and 5 μl of cDNA were used for each PCR reaction using either αv-specific or cyclophilin-specific primes. Each of the cDNA samples was amplified 20 cycles with the same 3′ primer (DS54, sense: atggcttttccgccgcggcgacggctg) and a 5′ primer (DS60, anti-sense: gcactatcagcagtaaagccttgg) located upstream of the signal sequence (total amplified fragment, 3,259 bp). Nested PCR reactions were then performed with a series of αv-specific primer pairs amplifying sequences from 800 to 1,200 bp in length located in different regions of the αv sequence (DS57, sense: gccttcaacctagacgtggacagtc; DS58, anti-sense: tgcagccatctgctcgccagt); GenBank title: HUMVTNR; sequence data are available from EMBL/GenBank/DDBJ under accession no. M14648. PCR conditions were as follows: 5 min at 94°C for hot start, followed by up to 35 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 4 min, with a final extension of 10 min at 72°C. The control was amplification of cyclophilin. To estimate the linear range of the nested reactions, we analyzed the PCR products at 10, 15, 20, 25, 30, and 35 cycles.
Transplantation Model
Explants of early gestational age human fetal pancreas are known to continue their differentiation program when transplanted under the kidney capsule of immune incompetent mice (Tuch et al. 1984). We took advantage of this model of pancreatic islet development to study the in vivo roles of αvβ3 and αvβ5 integrins in the emergence and migration of endocrine cells from the pancreatic ductal epithelium. Fragments (3–5 mm) of 15-wk-old human fetal pancreas were transplanted under the kidney capsule of four NODlt/szSCID mice. 2 wk after transplantation (to allow time for the engraftment and revascularization of these tissue grafts), two of the mice were implanted subcutaneously with osmotic pumps loaded with the integrin-blocking “27O” arginine, glycine, aspartic acid (RGD) peptide analogue, and the other two mice were implanted with pumps loaded with a control peptide analogue, “39M”. The RGD peptide analogue 27O [sequence: AcPen(OmeY) ARGDN(Tic)CNH2] and the control peptide 39M [sequence: GKGESP] were provided by Integra Life Sciences. Peptide 27O and the control peptide 39M were supplied in 50% DMSO/water at a concentration of 11.2 mM and 34.3 mM, respectively. Miniosmotic pumps (model 2001; Alza Pharmaceuticals; mean delivery rate 1 μl/h × 7 d) were loaded with 200 μl of peptides at a concentration of 11 μM. The osmotic pumps were changed weekly thereafter for 12 consecutive weeks. At the completion of the experiments the grafts were removed from the two groups of animals and analyzed by immune fluorescence staining for the four islet hormones. Pumps were checked at the time of explantation and consistently found empty by visual inspection.
Morphometric Analysis of Pancreatic Grafts
A total of 275 slides per graft were stained by immune fluorescence for the four islet hormones and analyzed at a confocal microscope for the identification of islet cell types, their architectural organization, and their relationship with ductal structures. Images acquired for each specific immune reactivity were then analyzed for measurements of each islet cell type–specific surface area using Image-Pro Plus software (Media Cybernetics). Islet cells were considered “ductal associated” when directly adjacent to the ductal epithelium, or clustered and contiguous with ducts within a range of 30 μm.
Statistics
Where applicable, data were analyzed using Stat View 4.01 software (Abacus Concepts, Inc.) for determination of mean, standard deviation, and parametric statistics (paired t test).
Results
Ductal Cells in the Developing Human Pancreas Express High Levels of αvβ3 and αvβ5 Integrin Receptors
In humans, the development of islets of Langerhans occurs during fetal life, whereas growth and expansion of the exocrine tissue is a perinatal event (Pictet and Rutter 1972). In rodents, these two functionally distinct compartments, endocrine and exocrine, derive from common precursors present in the pancreatic ductal epithelium engaging separate differentiation programs at distinct developmental stages (Pictet et al. 1972; Alpert et al. 1988; Herrera et al. 1991; Gu et al. 1994). The epithelial compartment of the human pancreas at a mid-gestational age (16–20 wk) consists of a branching ductal tree giving rise to numerous clusters of cells that protrude into the surrounding mesenchyme (Cirulli et al. 1998).
Little is known about the mechanisms that regulate emergence and migration of endocrine progenitor cells from the ductal epithelium into the surrounding mesenchyme. Based on the known roles of integrin receptors in the regulation of cell interactions with the extracellular environment during development, we performed a series of studies using confocal microscopy on human fetal pancreata (16–20 wk of gestation), to identify the integrin receptors highlighting the ductal epithelium and/or endocrine cells emerging from it. We found that a unique pattern of expression characterizes both αvβ3 and αvβ5 integrin receptors: high in ductal cells and decreased in developing islet cell clusters.
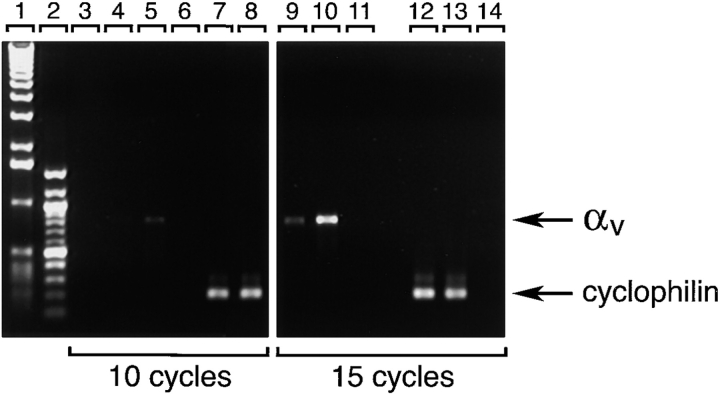
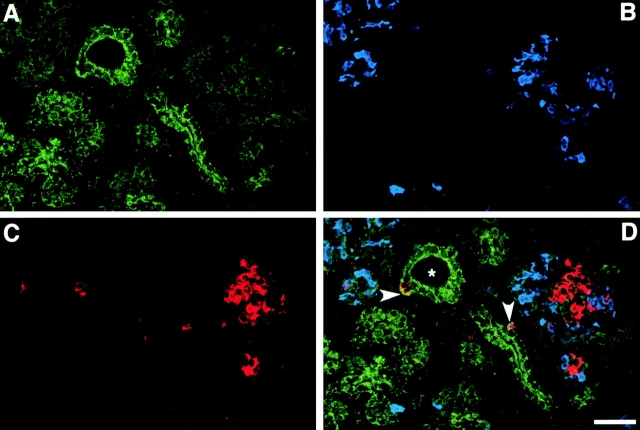
As shown in Fig. 1, αvβ3-specific immune reactivity (green) highlights the pancreatic ductal epithelium and clusters of cells branching from the ducts (A; arrows). The expression pattern of αvβ3 is restricted to regions of cell–cell and cell–matrix contacts. Most cell clusters budding from the ductal epithelium are contiguous with (or adjacent to) developing islets as identified by the insulin- (red) and glucagon-specific (blue) immune reactivity (D; arrowheads). We consistently observed that the brightness of αvβ3-specific immune reactivity (green) is decreased in these developing endocrine cells when compared with the levels of αvβ3 expression in ductal cells, including ductal cells expressing insulin and/or glucagon. Consistent with the current view that endocrine cells arise from precursors present within the ductal epithelium (Pictet et al. 1972; Teitelman and Lee 1987; Alpert et al. 1988; Gu and Sarvetnik, 1993), these results suggest that undifferentiated endocrine progenitors may use αvβ3 during early events determining migration from the ductal epithelium into the surrounding mesenchyme. Our studies further indicate that, after differentiation and clustering of endocrine cells into the highly organized islets of Langerhans, the expression of αvβ3 is downregulated. Consistent with this observation, we found that expression of the αv transcripts is decreased in adult islet cells as compared with fetal islet cells. Thus, PCR analysis of αv-specific transcripts detected in the linear range of amplification demonstrated that although barely detectable levels of αv transcripts are observed after 10 cycles of PCR on mRNA of human adult islets (Fig. 2, lane 4), significantly higher levels of αv-specific transcripts can be readily amplified from samples of fetal islets (Fig. 2, lane 5). This quantitative difference becomes more evident after 15 cycles of PCR (compare lanes 9 and 10). Cyclophilin-specific cDNA amplified from both fetal and adult islets mRNA samples served as an intra-assay control reference cDNA at all cycles (Fig. 2, lanes 7, 8, 12, and 13). Flow cytometric studies validated the results of this transcriptional analysis by demonstrating that lower levels of both αvβ3 and αvβ5 integrin proteins are expressed at the cell surface of human adult islet cells as compared with fetal cells (data not shown). This pattern suggests that the expression of these two integrins is developmentally regulated during islet ontogeny.
Figure 1.
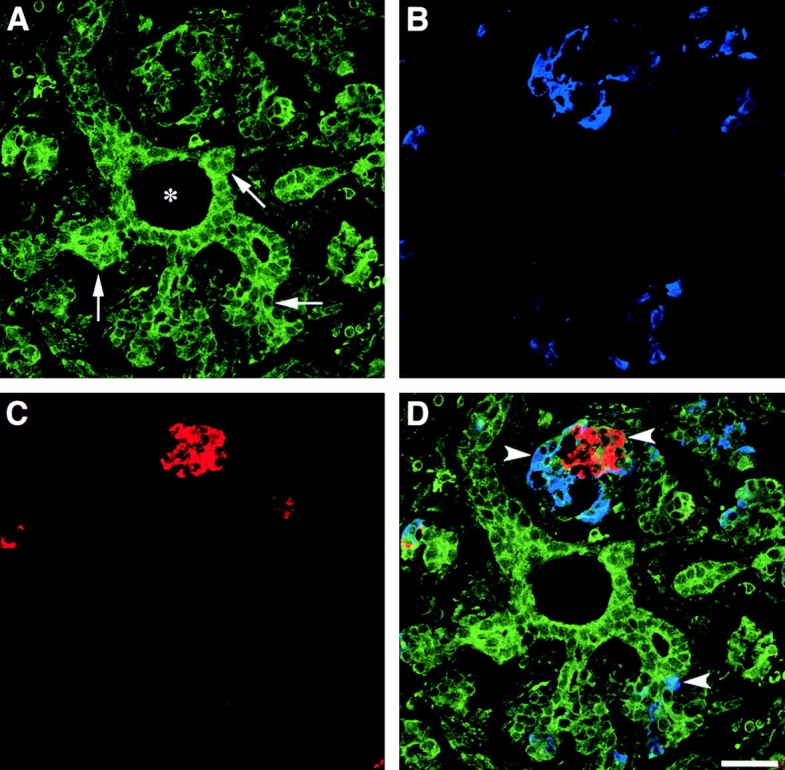
Expression of αvβ3 in the developing human pancreas. αvβ3-specific immune reactivity (A, green) highlights the pancreatic ductal epithelium and clusters of cells branching from the ducts (arrows). Note the αvβ3-specific staining marking the regions of cell–cell and cell–matrix contacts. A number of developing endocrine cells budding from the ductal epithelium are identified by the insulin- (C, red) and glucagon-specific (B, blue) immune reactivities. The three fluorescence patterns are merged in D, demonstrating colocalization of hormone-positive cells with αvβ3 expression (arrowheads). We consistently observed that the brightness of αvβ3-specific immune reactivity is decreased in developing islet clusters when compared with the levels of αvβ3 expression in ductal cells. Bar, 50 μm.
Figure 2.
Decreased transcription of the αv integrin subunit in endocrine differentiated cells. Total RNA isolated from fetal and adult human pancreatic islets was reversed transcribed to generate cDNAs and amplify αv-specific sequences. Products of PCR reactions obtained at 10 and 15 cycles using αv -specific and cyclophilin-specific primers are shown. Lanes 1 and 2, 1-Kb and 100-bp ladder, respectively. Lanes 3, 6, 11, and 14 were loaded with PCR reactions performed using no template DNA (water); lanes 4, 7, 9, and 12 were loaded with reactions performed using cDNA from adult pancreatic islets; and lanes 5, 8, 10, and 13 were loaded with reactions performed using cDNA from fetal pancreatic islets. Significantly higher levels of αv-specific PCR product (824 bp) are detected in samples of fetal pancreatic islets as compared with samples of adult islets. Representative of three independent determinations using three independent tissue donors.
Previous studies on αvβ3 expression in models of angiogenesis occurring during wound healing or tumor formation demonstrated that this integrin is significantly upregulated in newly formed vessels (Friedlander et al. 1995). These observations have suggested that upregulation of αvβ3 is involved in various forms of angiogenesis. In contrast to this prediction, and despite the rich vascular network evident within and around islet clusters (see Fig. 4 and Fig. 5), we did not observe significant expression of αvβ3 in endothelial cells colonizing fetal islets (data not shown). This result suggests that there may be cell-, tissue-, or developmental stage-specific differences in the mechanisms regulating αvβ3 expression during blood vessel formation.
Figure 4.
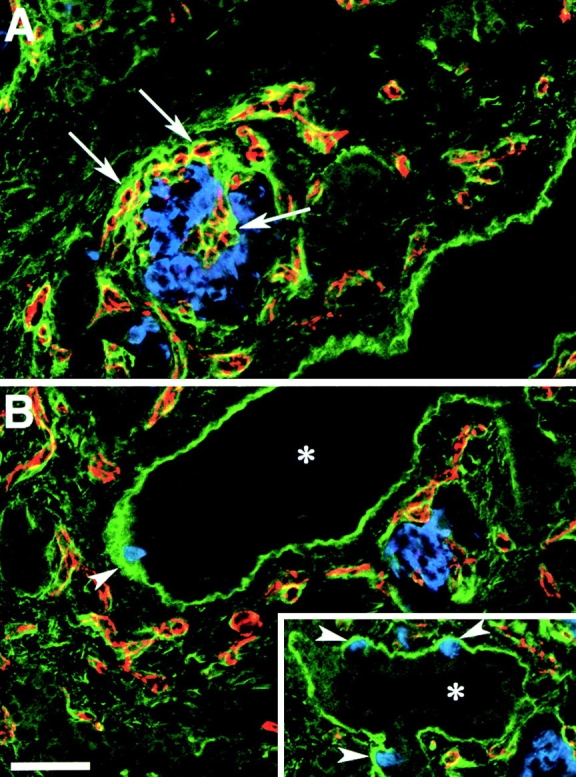
FN expression pattern in the developing human pancreas. Confocal images of two adjacent microscopic fields (A and B) from cryostat sections stained for FN (green), insulin (blue), and PECAM-1 (red). A strong FN-specific immune reactivity (green) highlights the basal membrane of ducts (asterisk) and of insulin-positive cells (blue) emerging from the ductal epithelium (arrowheads, B and inset). The PECAM-1-specific staining (red), identifying blood vessels, reveals that the basal membrane of endothelial cells, infiltrating developing islet clusters, is also marked by a bright FN-specific staining (arrows). Bar, 100 μm.
Figure 5.
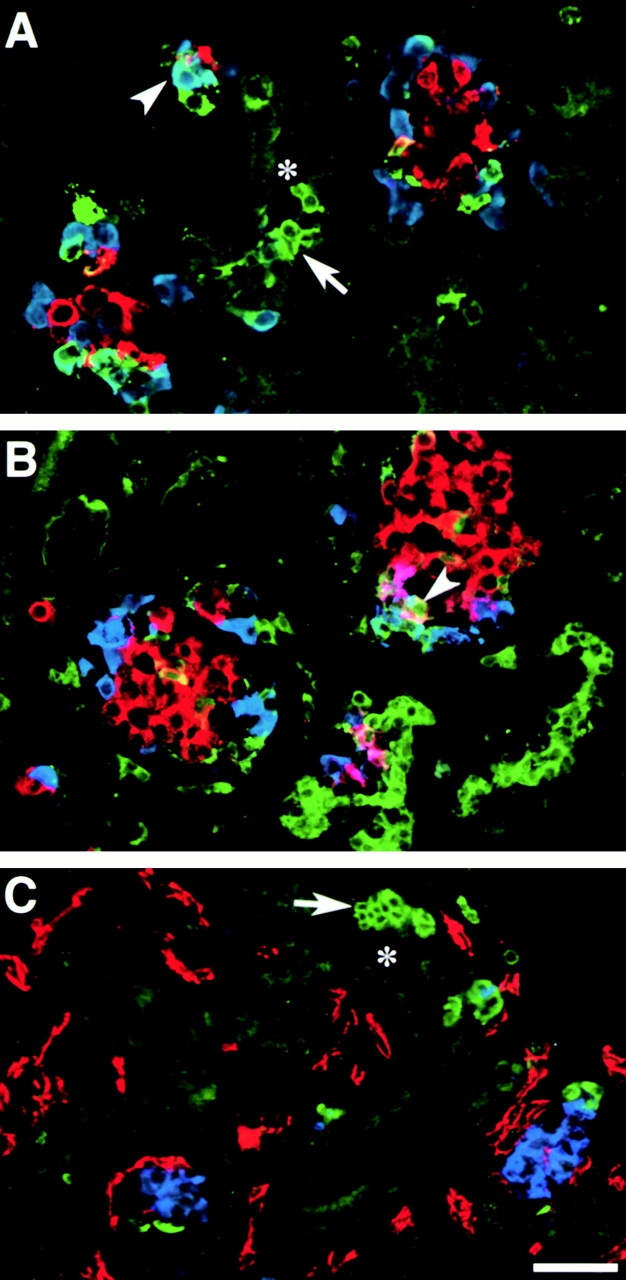
VN expression pattern in the developing human pancreas. A and B show representative fields recorded by confocal microscopy from cryostat sections stained for VN (green), glucagon (blue), and insulin (red). C shows staining for VN (green), PECAM-1 (red), and insulin (blue). Note the strong VN-specific immune reactivity identifying groups of epithelial cells emerging from, or adjacent to, ductal structures (asterisk; A and C, arrows). Some VN-positive cells coexpress glucagon (A, arrowhead) or insulin (B, arrowhead), suggesting a cell lineage relationship of VN-positive cells with putative endocrine progenitors. Staining for PECAM-1 (C, red) shows that none of the VN-positive cells are endothelial in nature. Bar, 100 μm.
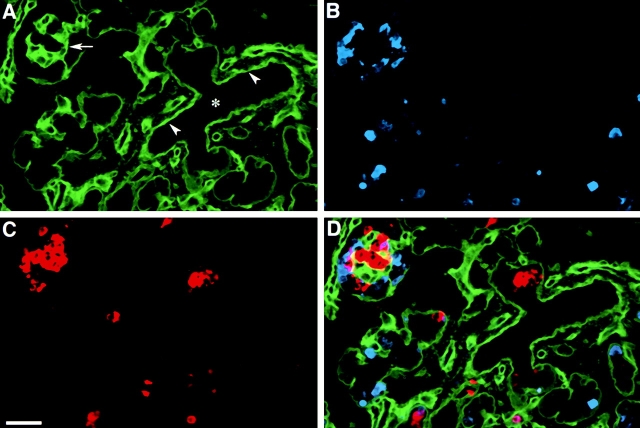
Analysis of αvβ5 expression in similar preparations of human fetal pancreata revealed a pattern similar to that observed for αvβ3, with the stronger immune reactivity in ductal cells, and in islet cells emerging from the ductal epithelium (Fig. 3 D; arrowheads). Similar to αvβ3, the intensity of αvβ5-specific immune reactivity decreased in endocrine cells forming larger and more organized islet-like cell clusters.
Figure 3.
Identification of αvβ5 in the developing human pancreas. Confocal immune fluorescence on cryostat sections stained for αvβ5 (A, green), insulin (C, red), and glucagon (B, blue). The three fluorophore spectra are merged in D. Bright levels of αvβ5-specific immune reactivity are detected in pancreatic ducts (asterisk), whereas significantly lower levels characterize endocrine cells organized in larger islet-like clusters. However, single endocrine cells adjacent to, or emerging from, the ductal epithelium retain significant higher levels of αvβ5 expression (D; arrowheads). Bar, 100 μm.
Quantitative Analysis of αvβ3 and αvβ5 Expression in the Fetal and Adult Human Pancreas
To quantitatively evaluate the levels of αvβ3 and αvβ5 expression in specific cell types, confocal images from human fetal pancreata were studied by computer-aided morphometric analysis. Table shows the pixel intensities of αvβ3- and αvβ5-specific fluorescence recorded in ductal, insulin- and glucagon-positive cells, as well as in undifferentiated epithelial cells (e.g., islet hormone-negative and amylase-negative). Ductal cells, including isolated insulin- and glucagon-positive cells within or immediately adjacent to ducts, displayed the highest mean pixel intensity of αvβ3-specific immune reactivity (173.9 ± 25.1). In contrast, insulin-positive cells associated with developing islet-like cell clusters displayed a significantly lower pixel intensity (93.1 ± 20.5; P < 0.001). Conversely, glucagon-positive cells in these clusters showed pixel intensity closer to that measured in the ductal cells (140.2 ± 25.4). This observation suggests that glucagon cells, recently proposed to derive from a cell lineage independent from that of insulin-producing cells (Herrera 2000; Jensen et al. 2000), may retain differential adhesive and/or migratory properties perhaps closer to that of more immature ductal-like endocrine progenitor cells. The same analysis performed on sections stained for αvβ5 revealed that although ductal cells display the highest values of pixel intensities (i.e., 205.7 ± 33.7), both glucagon- and insulin-positive cells located in developing islets showed statistically significant lower values (100.1 ± 22.9 and 95.3 ± 18.2, respectively). The stromal cells located in the interstitium are essentially negative.
Table 1.
Pixel Intensity of αvβ3- and αvβ5-specific Immune Fluorescence
| Cell type | Ductal | Insulin | Glucagon | Epithelialundifferentiated | Stromal |
|---|---|---|---|---|---|
| αvβ3 | 173.9 ± 25.1 | 93.1 ± 20.5 | 140.2 ± 25.4 | 170.5 ± 29.3 | 29.6 ± 11.3 |
| αvβ5 | 205.7 ± 33.7 | 95.3 ± 18.2 | 100.1 ± 22.9 | 202.6 ± 24.4 | 32.1 ± 9.6 |
The pixel intensity of αvβ3- and αvβ5-specific immunofluorescence was recorded in 250 domains of cell–cell and/or cell–matrix contact for each cell type. No. of donors, 3 (fetal pancreata at gestational age 18, 19, and 20 wk). No. of fields scored in each fetal pancreas, 47.
Identification of ECM Proteins in the Developing Human Pancreas
FN, VN, and Coll-IV are important ECM proteins with known adhesive sites for the integrins αvβ3 and αvβ5. It has also been reported that αvβ3 expressed by endothelial cells can bind and present MMP-2, an enzyme capable of processing collagen (Brooks et al. 1996). To identify the expression of these ECM proteins in the developing human pancreas, cryostat sections from human fetal pancreata were stained by immune fluorescence using a multiple labeling protocol for the simultaneous identification of each ECM protein with insulin- and glucagon-positive cells. Fig. 4 shows the reconstruction of two adjacent microscopic fields, A and B, stained for FN (green), insulin (blue), and PECAM-1 (red). FN-specific immune reactivity highlights the basal membrane of an abundant network of PECAM-1+ blood vessels (red) infiltrating developing islet clusters (A; arrows). The basal membrane of pancreatic ducts (asterisk) is also strongly marked by the FN-specific immune reactivity. Note that all insulin-positive cells (blue) emerging from the pancreatic ductal epithelium are associated with a strongly FN-stained basal membrane (B; arrowheads and inset), whereas little, if any, FN-specific immune reactivity is evident around the insulin-positive cells in the core of the islet clusters. These observations suggest that interactions with FN may be required very early during the emigration of islet cells and/or islet cell progenitors from the ductal epithelium. However, subsequent aggregation and organization of endocrine cells to form the islet of Langerhans may also involve additional adhesion receptors such as cadherins and members of the Ig superfamily (e.g., N-CAM; Rouiller et al. 1991; Cirulli et al. 1994; Dahl et al. 1996).
Fig. 5 shows representative microscopic fields acquired from fetal pancreatic tissue immune stained for (A and B) VN (green), insulin (red), and glucagon (blue), and for (C) VN (green), PECAM-1 (red), and insulin (blue). VN-specific immune fluorescence highlights groups of cells that are adjacent to, or emerging from, the ductal epithelium (A and C; arrows). Some of these VN-positive cells coexpress glucagon and/or insulin (A and B; arrowheads), and appear to cluster with developing pancreatic islets (B; arrowhead). It is interesting that VN-specific staining is largely cellular rather than in basal membranes. Moreover, larger islet-like cell clusters contain few or no VN-positive cells. These observations suggest that endocrine progenitors may arise from a larger pool of VN-positive cells.
Immune staining for Coll-IV (Fig. 6) reveals a distinct pattern marking the basal membranes of the epithelial compartment, which includes both ducts and developing islets. Note the strong fluorescent signal defining the basal membranes of the ducts (Fig. 6 A; arrowheads). A similar strong staining highlights the basal membranes of developing pancreatic islets (A; arrow). This pattern suggests that Coll-IV may be required as a pathfinder matrix for endocrine progenitor cells and as a structural template for newly developing islets.
Figure 6.
Identification of Coll-IV in the developing human pancreas. Three-color confocal immune fluorescence on cryostat sections of human fetal pancreas. A strong Coll-IV-specific immune reactivity (A, green) identifies basal membranes (A, arrowheads) of cells in the ducts (asterisk), and in clusters of developing islet cells, identified by staining for insulin (C, red) and glucagon (B, blue). The three fluorophore spectra are combined in D. Bar, 100 μm.
The immune localization of these ECMs in the basal membrane of ductal structures, together with the identification of their integrin receptors αvβ3 and αvβ5 in ductal cells (see Fig. 1 and Fig. 3) demonstrate identical histological compartmentalization of these two integrins and their ligands, and suggests their functional involvement in the emergence of endocrine progenitor cells from the ductal epithelium.
Fetal and Adult Pancreatic Epithelial Cells Adhere to ECM Proteins
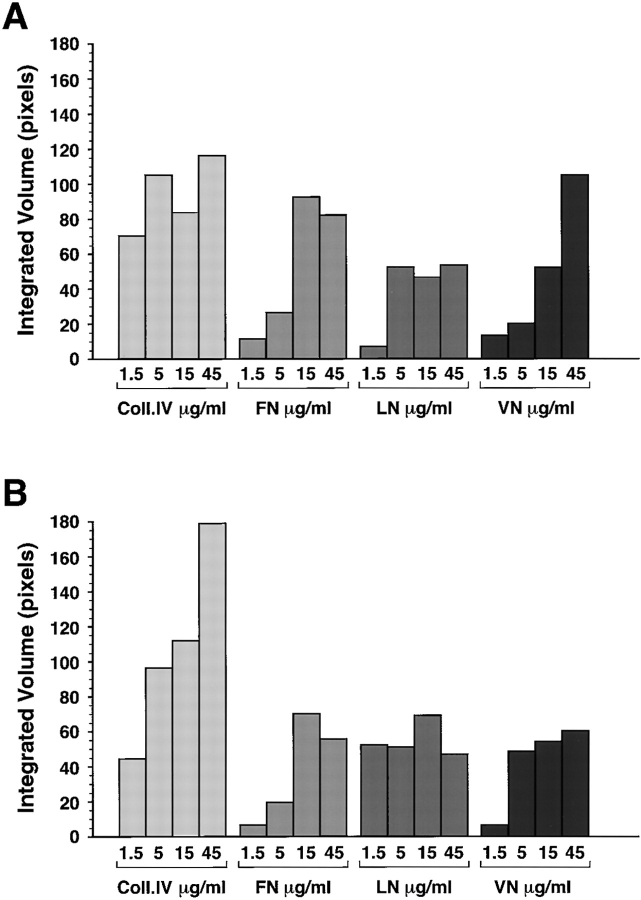
The coexpression of integrins and ECM proteins in the pancreas does not provide evidence of function. Therefore, to test the hypothesis that these putative integrin/ECM interactions are functional, we assessed the adhesive properties of fetal and adult islet cells with different purified ECM proteins. Fig. 7 shows the results of adhesion assays performed on increasing concentrations of Coll-IV, FN, LN, and VN. Both fetal (Fig. 7 A) and adult (Fig. 7 B) islets demonstrate a ligand concentration–dependent adhesion to these purified ECM proteins. Adhesion to purified Coll-IV was similar for fetal and adult cells at the lower concentrations (1.5–5 μg/ml), but increased significantly for adult cells at higher substrate concentrations (15–45 μg/ml). We consistently observed that adhesion to VN at higher ligand concentrations was increased for fetal as compared with adult islet cells, a result consistent with the increased expression levels of αvβ3 and αvβ5 in fetal cells. In separate experiments, to assess the specificity of adhesion of fetal cells mediated by αvβ3 and αvβ5 we used function-blocking monoclonal antibodies LM609 (specific for αvβ3) and 15F11 (specific for αvβ5). We observed that blockade of αvβ3 by the LM609 antibody (50 μg/ml) resulted in a 71.2% inhibition of adhesion to VN and a 69.3% inhibition of adhesion to FN. Combination of LM609 and 15F11 antibodies in these adhesion assays inhibited adhesion of fetal pancreatic cells to VN by 84.9%. Adhesion to Coll-IV, also known to contain some RGD binding sequences for αvβ3, was inhibited by 50.2% in the presence of LM609. Conversely, the αvβ5-specific monoclonal, 15F11, inhibited adhesion of fetal pancreatic cells to VN by 75%, but had no effect on adhesion to FN and Coll-IV.
Figure 7.
Adhesion of fetal and adult pancreatic epithelial cells to purified ECM proteins. Adhesion to Coll-IV is constitutively high in both fetal (A) and adult cells (B), although adult cells consistently demonstrated stronger adhesion. Conversely, adhesion to FN and VN increased progressively at the higher concentrations for fetal as compared with adult cells.
Subcellular Localization of αvβ3 and αvβ5 Integrins: Preferential Targeting to Focal Contacts
In our model system, when pancreatic fetal islet-like cell clusters, mainly composed of undifferentiated putative islet cell progenitors (Beattie et al. 1994; Cirulli et al. 1998), are plated on the complex ECM matrix deposited by the epithelial cell line 804G, they first adhere and then establish monolayers (Beattie et al. 1996). The 804G matrix is composed of several ECM proteins, including FN, VN, LNs, and collagens (Langhofer et al. 1993). Based on these studies, 804G matrix may be considered a surrogate for a natural basal membrane and may be used as a model to assess the ability of epithelial cells to adhere and migrate on a complex substrate. Similar spreading and migration can also be demonstrated on matrices assembled by the HACAT (human keratinocyte cell line; Salomon, D.R., unpublished observations) and HTB-9 cell lines (human gallbladder carcinoma cell line; Beattie et al. 1997). We used this in vitro model system to characterize the subcellular localization of αvβ3 and αvβ5 integrins with special attention to focal adhesion contacts, known as critical regulatory domains for cell adhesion, spreading, and migration on ECM (Felding-Habermann and Cheresh 1993; Schwartz and Ingber 1994).
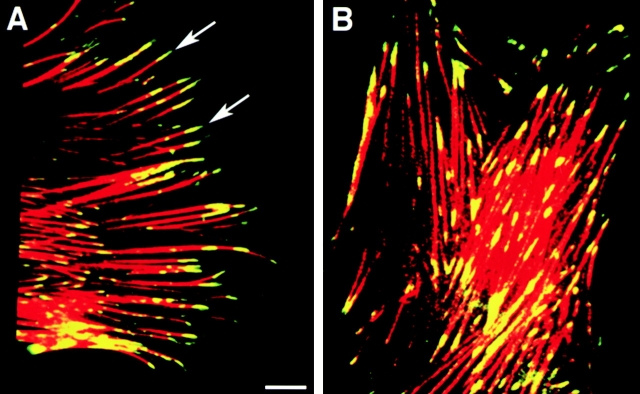
Fig. 8 depicts undifferentiated fetal pancreatic epithelial cells obtained after the plating of islet-like cell clusters on 804G matrix. Representative confocal microscopic fields are shown to demonstrate distinct patterns of co-localization for αvβ3 (Fig. 8 A) and αvβ5 (Fig. 8 B; green) with F-actin filaments (red) in focal adhesion contacts. αvβ3 is confined to discrete sites located at the very tips of the F-actin filaments in the focal adhesion contacts (A; arrows). In contrast, αvβ5 is detected in the focal adhesion contacts but also extends back along the F-actin filaments covering a larger area of the cell/ECM contact surface (B). Similar results were obtained when cells were plated on purified FN and VN (data not shown). These results demonstrate that the spatial organization of these two integrins with respect to the focal contacts is distinct and suggest that these two integrins may have different functions in mediating cell migration and maintaining cell/matrix contact during the formation of a cell monolayer (see below).
Figure 8.
Subcellular localization of αvβ3 and αvβ5 integrins. Representative confocal microscopic fields showing distinctive patterns for αvβ3 (A) and αvβ5 (B) fluorescence (green) colocalizing with F-actin filaments (red fluorescence) in fetal pancreatic epithelial cells. While αvβ3-specific immune reactivity is mainly confined at the very tips of F-actin filaments (A, arrows), fluorescence specific for αvβ5 extends back along the F-actin filaments identifying a larger number of focal adhesion contacts in the basal pole of adherent cells. Bar, 2.5 μm.
Primary Role of αvβ3 and αvβ5 Integrins in the Attachment and Migration of Fetal Pancreatic Epithelial Cells on ECM Proteins
When islet-like cell clusters are plated on 804G matrix–coated surfaces, cells forming the three-dimensional islet-like cell clusters first adhere and then migrate out of the cluster to form a monolayer within 12 h. Therefore, we used this in vitro model system to test the effect of function-blocking antibodies for αvβ3 and αvβ5 integrins during the initial process of adhesion and monolayer formation and at the later stage when the monolayers are already established. We reasoned that by providing ligands for multiple integrin adhesion receptors, this complex ECM would be a stringent test for assessing the functional significance of αvβ3- and αvβ5-mediated adhesion to a basal-like membrane.
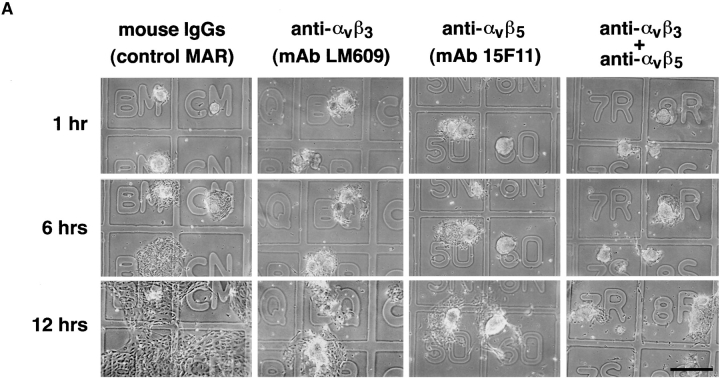
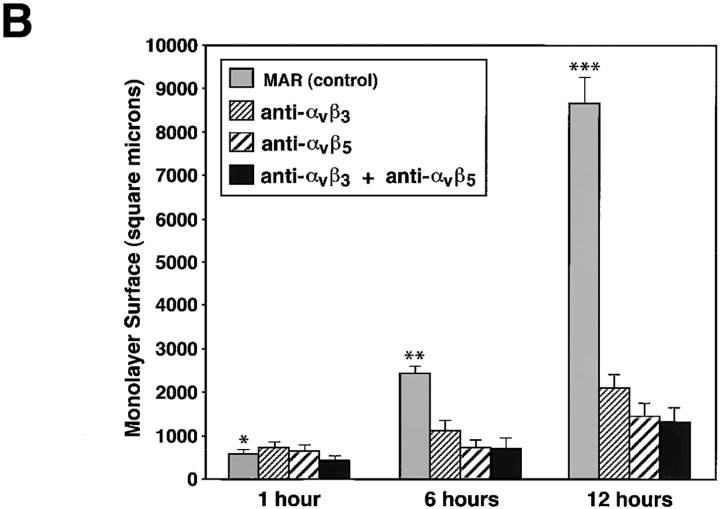
To allow the identification of identical microscopic fields at specific time intervals, we used glass coverslips marked with an alpha-numerical grid and precoated with the 804G matrix. Islet-like cell clusters were plated in the presence of either a control, isotype-matched mouse IgG (MAR), or with function blocking antibodies specific for αvβ3 (LM609) and αvβ5 (15F11). Fig. 9 A shows representative light microscopic fields of cell clusters recorded serially after 1, 6, and 12 h. Most of the cell clusters adhered to the 804G substratum in all conditions after 15 min (data not shown). After 1 h, cell clusters remained attached with no significant evidence of cell spreading on the matrix-coated coverslips. However, after 6 h in culture the cell clusters plated in the presence of the control IgG started to migrate and spread onto the coverslips. This process is almost completely inhibited in conditions containing either the anti-αvβ3 and/or the anti-αvβ5 mAbs (25 μg/ml). After 12 h, while cells in the control condition have all migrated into a semiconfluent monolayer, only few cells in the presence of the anti-αvβ3 and/or the anti-αvβ5 mAbs have migrated, although the clusters remain attached. Thus, in the presence of anti-αvβ3 or the anti-αvβ5 mAbs most of the cells remain in three-dimensional clusters, suggesting that these two integrins are important mediators of cell migration. To quantify this phenomenon and integrate the results from multiple microscopic fields (n = 50/condition), we also measured the surface area of the cell monolayers in all conditions (Fig. 9 B). This analysis reveals that the blocking antibodies produced >75% overall inhibition of monolayer development in these assays.
Figure 9.
Integrins αvβ3 and αvβ5 mediate migration of fetal pancreatic epithelial cells. Time course adhesion and migration of fetal pancreatic epithelial cells on 804G matrix–coated coverslips marked by an alpha-numerical grid to identify identical microscopic fields over time (A). Note that cell migration is inhibited by anti-αvβ3 and anti-αvβ5 function blocking antibodies. Quantitative morphometric analysis performed on multiple microscopic fields (n = 50 fields/condition) from four independent experiments revealed that blocking antibodies produced >75% overall inhibition of monolayer development in these assays (B). Bar, 150 μm. *n.s.; **P < 0.05; ***P < 0.0001.
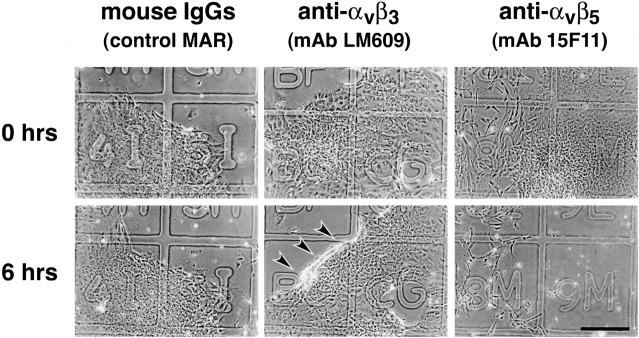
In a separate set of experiments, we first established monolayers on the matrix-coated coverslips by overnight culture (18 h) and then tested whether the anti-αvβ3 and anti-αvβ5 mAbs could disrupt cell adhesion over an additional 6 h. As shown in Fig. 10, addition of anti-αvβ3 and anti-αvβ5 mAbs (25 μg/ml) disrupted the anchorage of cells to the ECM in the established monolayers. After 6 h with anti-αvβ3 mAb, the leading edges of the cell monolayers detached and rolled back (Fig. 10; arrows). In contrast, when anti-αvβ5 mAb was added to the cell monolayers, most of the cells detached completely from the ECM and the integrity of the monolayer was lost. It is important to note that the concentrations of blocking antibodies were identical in the experiments described in Fig. 9 and Fig. 10. Thus, these data suggest that αvβ3 and αvβ5 cooperate to establish spreading and migration on matrix. However, the data also indicate that αvβ5 plays a more critical role in maintaining cell anchorage in the established monolayer. We have already noted that the localization of these two integrins in focal contacts is such that αvβ3 is concentrated at the tips of F-actin filaments in migrating cells, whereas αvβ5 is found in the focal contacts and extending behind αvβ3 (Fig. 8). Thus, in light of the functional data presented here, we propose that the unique spatial distribution of these two integrins with respect to the focal adhesion contacts and F-actin filaments may reflect a distinct role for αvβ3 mainly in islet progenitor cell migration, whereas αvβ5, in addition to cell migration, may be also required to maintain cell anchorage to the ECM.
Figure 10.
Integrins αvβ3 and αvβ5 mediate attachment of fetal pancreatic epithelial cells. Established cell monolayers were challenged with anti-αvβ3 and anti-αvβ5 function blocking antibodies (25 μg/ml) to assess the role of these two integrin receptors in the maintenance of cell monolayer integrity. After 6 h of incubation in the presence of anti-αvβ3, mAb cells from the periphery of monolayers started to detach and roll back into three-dimensional clusters (lower middle panel, arrows). Conversely, incubation with the anti-αvβ5 mAb resulted in the almost complete detachment of cell monolayers from the matrix (lower right panel). Results shown are representative of four independent experiments. Bar, 150 μm.
Cyclic RGD Peptide Analogues Block the Emergence of Developing Endocrine Progenitors from the Ductal Epithelium
The primary RGD-dependent integrins expressed by the putative endocrine progenitors in the developing human pancreas are αvβ3 and αvβ5, as demonstrated by flow cytometry, confocal microscopy, and the functional studies described above. Previous studies have shown that cyclic RGD peptide analogues can be effectively used to inhibit function of these integrins (Pierschbacher and Ruoslahti 1987; Cheng et al. 1994). To determine the role of these integrins in an in vivo model of islet development, fragments of human fetal pancreas (15-wk gestation) were transplanted under the kidney capsules of NODlt/szSCID mice. At this early point in development the human fetal pancreas exhibits only few and very small islet cell clusters (Tuch et al. 1984). 2 wk after transplantation, to allow time for the engraftment and revascularization of these tissue grafts, osmotic pumps loaded with either the integrin-blocking 27O RGD peptide analogue or a control peptide analogue, 39M, were implanted subcutaneously, and changed every week for 12 consecutive weeks. After 12 wk the grafts were removed from the two groups of animals and analyzed by immune fluorescence staining for the four islet hormones.
Fig. 11 A shows two representative adjacent microscopic fields acquired from the pancreatic grafts from animals treated with the control 39M peptide. Several large, well-formed islet clusters are evident with the majority of insulin-positive cells (red) located in the center, surrounded by cells which are positive for glucagon (blue) and somatostatin/pancreatic polypeptide (green). In contrast, analysis of the pancreatic grafts harvested from mice treated with the RGD peptide inhibitor 27O revealed a profoundly perturbed organization and development of islet cell clusters. Thus, as can be observed in Fig. 11 B, most islet cells appear closely associated with ductal structures (asterisk), often completely or nearly surrounding ductal elements. In addition, a dramatic reduction in insulin-positive cells (red) is observed and islet cells are grouped in small clusters containing mainly glucagon-positive cells (blue). These data were validated by morphometric analysis to identify the positional segregation of islet cell types from ductal structures and their architectural organization within developing islets, as well as to assess changes in the relative mass of insulin-, glucagon-, and pancreatic polypeptide/somatostatin–producing cells. To determine the relationship of islet cells with ductal structures, we defined as “ductal-associated” all islet cells which appeared directly adjacent to, and/or clustered with, ductal elements within a range of 30 μm from the pancreatic ductal epithelium. As shown in Fig. 12 A, this analysis reveals that all four islet cell types are much more closely associated with ducts in the mice treated with the 27O blocking peptide than in control animals. Thus, a five to sevenfold increase in the ductal association of endocrine cells can be observed in grafts from mice treated with the 27O peptide analogue. These results strongly suggest that inhibition of αvβ3 and αvβ5 integrins, the two RGD-dependent integrins we have demonstrated in the developing human pancreas, significantly reduces the emergence of islet cells from the ductal epithelium. However, we acknowledge that this approach does not exclude contributions from other RGD-dependent integrins that currently are unidentified. In this context, we note that both fetal and adult islet cells were negative for α5β1 (data not shown). A surprising finding in grafts of mice treated with the RGD peptide analogue was the reduced number of insulin-positive cells associated with islet cell clusters. As shown in Fig. 12 B, we quantified an ∼66% reduction in the number of insulin-positive cells as determined by measurements of cell surface areas. In contrast, the number of glucagon- and pancreatic polypeptide/somatostatin–positive cells is significantly increased. All together, these morphometric data show that despite the significant differences in relative representation of each islet cell type, the total endocrine cell mass is not decreased, but rather increased.
Figure 11.
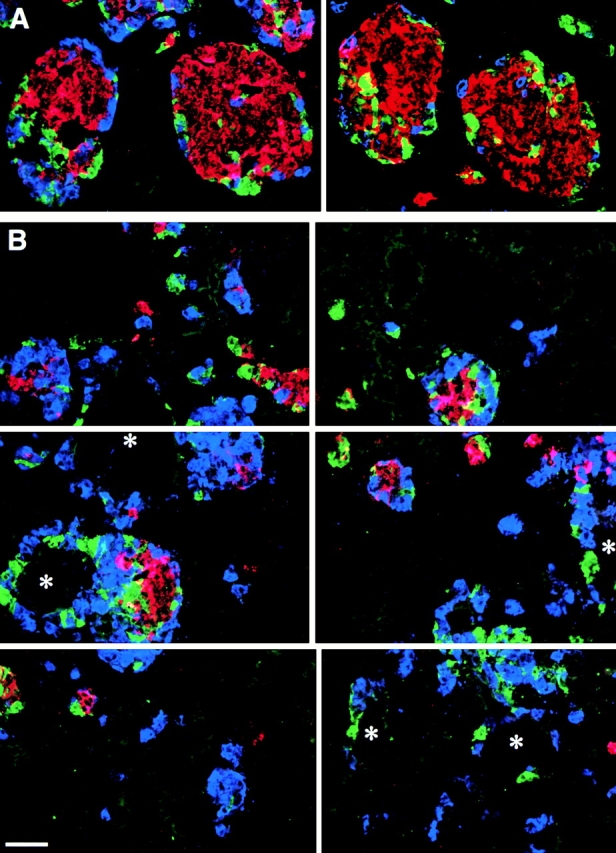
Migration of developing islet cells from the ductal epithelium is perturbed by cyclic RGD peptide analogues. A shows two representative adjacent microscopic fields of pancreatic grafts from animals treated with the control 39M peptide. Large and well organized islet clusters are evident with the majority of insulin-positive cells (red) located in the center, surrounded by cells that are positive for glucagon (blue) and somatostatin/pancreatic polypeptide (green). In contrast, analysis of the pancreatic grafts harvested from mice treated with the RGD peptide inhibitor 27O reveals a profoundly perturbed organization and development of islet cell clusters (B, reconstruction of six adjacent microscopic fields). Most islet cells appear closely associated with ductal structures (asterisk), often completely or nearly surrounding ducts. Bar, 100 μm.
Figure 12.
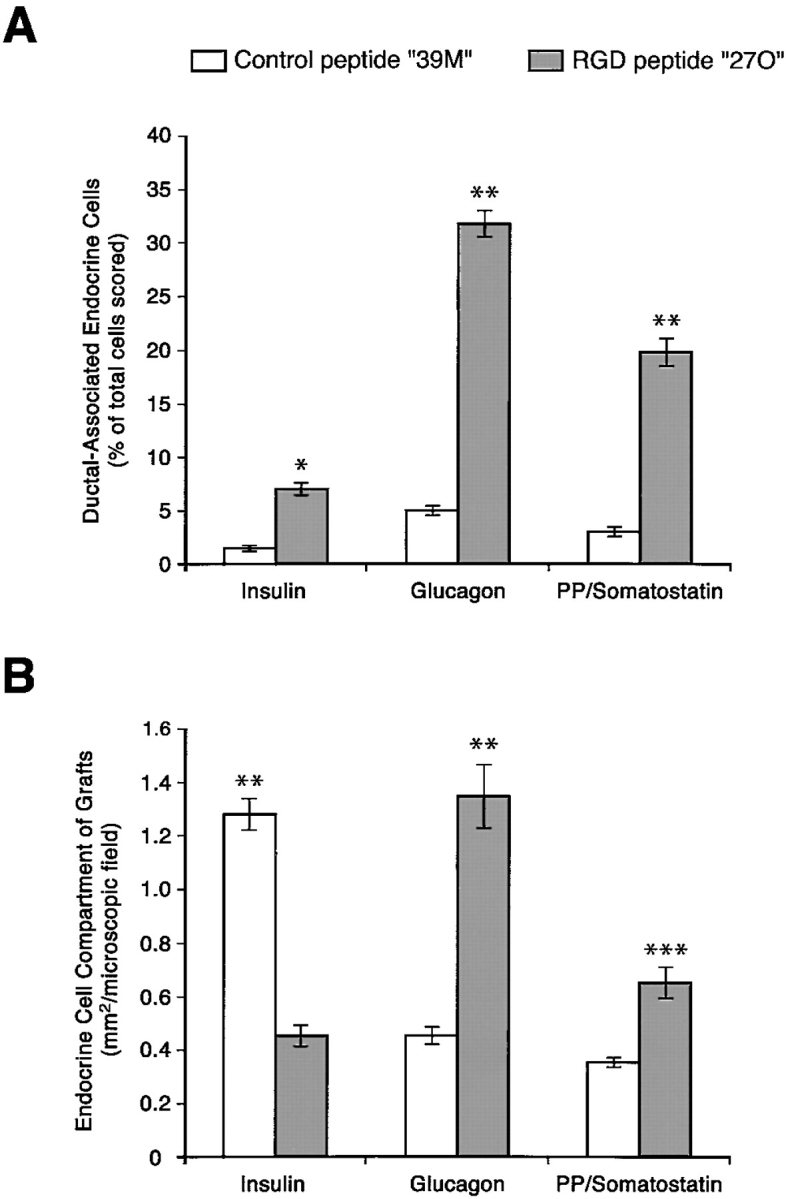
Morphometric changes in islet development by cyclic RGD peptide analogues. Human pancreatic grafts harvested from mice treated with the 27O RGD peptide analogue show an increased frequency of islet cells associated with ductal elements (A). Thus, the frequency of ductal-associated islet cells is increased 4.5-fold for insulin-producing cells, 6.5-fold for glucagon-producing cells, and 6.6-fold for somatostatin/pancreatic polypeptide–producing cells. Measurements of the relative surface area per microscopic field calculated for each islet cell type (B) reveal a 65% decrease of insulin-producing cells, paralleled by a 193% increase of glucagon-producing cells, and an 85% increase of somatostatin/pancreatic polypeptide–producing cells. *P < 0.001; **P < 0.0001; ***P < 0.05.
Discussion
Little is known about the expression and function of integrins during pancreatic islet ontogeny. Thus, although it is axiomatic that the complex three-dimensional structure of the pancreatic islet is important to its endocrine function, the determinants of this structure are unknown. Moreover, the development of the architectural organization of islet clusters during fetal morphogenesis may involve mechanisms distinct from those required to maintain the structure of the mature islet during adult life. In this regard, integrin receptors and their ligands are likely candidates to participate in pancreatic islet morphogenesis. Thus, it has been shown that integrin–ECM interactions regulate a variety of cellular functions, encompassing cell adhesion to specific sequences within ECM components, migration and organization of cells within tissues, initiation and maintenance of cellular differentiation, and sustaining mature cellular functions. Here we describe the developmental expression and function of αvβ3 and αvβ5 integrins in the adhesion and migration of undifferentiated human fetal pancreatic epithelial cells comprising putative progenitors of the endocrine lineage (Beattie et al. 1994; Cirulli et al. 1998). We also demonstrate that the ECM proteins FN, VN, and Coll-IV are components of the three-dimensional architecture of the developing islets.
Our data show that αvβ3 and αvβ5 integrins are expressed at the cell surface of cells obtained from fetal pancreatic islet-like cell clusters. These clusters are harvested from whole pancreata after short-term cultures of collagenase digests and are predominantly composed of undifferentiated epithelial cells as determined by previous morphometric studies (Beattie et al. 1994; Cirulli et al. 1998). When these fetal cells are transplanted into immune-deficient mice they develop into functional islet cells expressing insulin, glucagon, pancreatic polypeptide, and somatostatin (Beattie et al. 1994; Cirulli et al. 1998), demonstrating that these cells comprise endocrine progenitors of the fetal pancreas. In contrast, adult islet cells showed consistently lower levels of αvβ3 and αvβ5 expression, whereas levels of the β1 family of integrins are essentially unchanged (data not shown). Interestingly, it was recently demonstrated that downregulation of αvβ3 and signaling through αvβ5 is critical for the differentiation of oligodendrocyte progenitor cells (Blaschuk et al. 2000). Thus, we propose that αvβ3 and αvβ5 are important receptors regulating the emergence of islet cell progenitors from the pancreatic ductal epithelium during islet development.
Our morphological studies demonstrate that αvβ3 and αvβ5 immune reactivity is highest on ductal cells and clusters of cells budding from the ductal epithelium. As endocrine differentiation and islet organization occurs, αvβ3 and αvβ5 expression in islet cell clusters decreases substantially, consistent with the hypothesis that these integrins are more important for early migration and organization events involved in recruiting endocrine progenitors from the ductal epithelium. Support for this interpretation is provided by evidence that VN and FN, two major ECM ligands for αvβ3 and αvβ5, are associated with cells emerging from, or adjacent to, the ductal epithelium. These cells often coexpress insulin and/or glucagon, a landmark of endocrine differentiation. In contrast, the distribution of Coll-IV and LN (data not shown) in discrete basal membrane structures in developing islet cell clusters as well as mature islets suggests that their receptors, including the β1 family of integrins, are important for creating and maintaining islet architecture. The fact that expression levels of the β1 integrins are similar in fetal and adult islet cells is consistent with the hypothesis that these integrins are important throughout development and in adult life in the maintenance of islet structure. In support of this view, it is known that α3β1 is restricted to islet cells in the adult rat pancreas (Kantengwa et al. 1997). Interestingly, the same investigators have recently reported that α3β1 and α6β1 regulate not only islet cell adhesion but also insulin secretion (Bosco et al. 2000).
To address the functional role of integrin adhesion receptors in the developing human pancreas, we performed a series of in vitro assays to assess integrin function, including adhesion and migration to both purified and cell-deposited matrix proteins. These studies demonstrate that both adult islet and fetal pancreatic cells adhere to purified basal membrane ECMs in a concentration-dependent fashion. We observed by electron microscopy that culture on cell-deposited matrix (i.e., 804G or HACAT) favored the formation of tight intercellular junctions and desmosomes not seen when the same cells are cultured on plastic (results not shown). Confocal microscopy of fetal pancreatic cells migrating on cell-deposited matrix demonstrated the colocalization of αvβ3 and αvβ5 integrins in focal adhesion contacts. However, while αvβ3 was confined to the very tips of the F-actin filaments in the focal contacts, the expression of αvβ5 extended back along the filament at the lower surface of the cell. A specific spatial organization for αvβ3 and αvβ5 with respect to focal contacts in a migrating pancreatic cell suggests a functional cooperation of these two integrins. This interpretation is consistent with previous observations in a model of growth factor–dependent angiogenesis showing that different integrins are engaged in response to distinct sets of factors (Friedlander et al. 1995). To provide additional functional evidence for the roles of αvβ3 and αvβ5, we developed an assay to determine the adhesion and migration of fetal islet-like cell clusters on cell-deposited matrix. First, we demonstrated that blocking antibodies for αvβ3 and αvβ5 inhibited the migration of cells out of intact islet-like cell clusters, and consequently prevented the establishment of cell monolayers. Second, we showed that the integrity of established cell monolayers developed on the same matrix was disrupted by these antibodies. Blocking αvβ3 caused the edge of the intact monolayer to detach and roll back, consistent with the tensile forces exerted by growing monolayers on their leading edges (Choquet et al. 1997). Conversely, blocking αvβ5 resulted in the complete detachment of the monolayer from the matrix. These results demonstrate that both αv integrins are functional in mediating islet cell adhesion and migration onto matrix. They also provide evidence supporting our hypothesis that αvβ3 and αvβ5 play a role in mediating the recruitment of endocrine progenitors from the ductal epithelium during islet ontogeny.
Although there is evidence demonstrating that the ductal epithelium is a reservoir for endocrine progenitors during islet development, nothing is known about the molecular mechanisms regulating the emergence of these progenitors and the creation of islet cell clusters. Conceivably, islet ontogeny requires a series of events that includes early endocrine determination of progenitors, induction of cell migration from the duct into the adjacent interstitium, ECM production and modification, the initial clustering of islet cells, vasculogenesis, and ultimately the three-dimensional organization of the mature islet. In this context, our in vivo transplantation studies with human fetal pancreas and the RGD-blocking peptide 27O demonstrate that with inhibition of integrin function the migration of endocrine progenitors from the ductal epithelium is severely limited, resulting in many small endocrine cell clusters mostly located immediately adjacent to ducts. Secondly, the number of insulin-positive cells in these small clusters is also significantly reduced with a relative increase in glucagon- and somatostatin/pancreatic polypeptide–positive cells. These results support the conclusion that distinct integrin signals may be required for the migration, proliferation, and differentiation of each islet cell type. Thus, it is possible that αvβ3 and αvβ5 signals regulate migration of all islet cell types and of their precursors, yet differentially affect the growth and differentiation of insulin and glucagon cell lineages. This view is consistent with the recent evidence that insulin- and glucagon-producing cells arise from two independent progenitor lineages (Herrera 2000; Jensen et al. 2000). Another important role described for integrins is epithelial cell survival and, thus, it is also possible that our results reflect a preferential loss of insulin-positive cells, or their precursors, during RGD peptide blockade.
In summary, our data provide the first evidence that αvβ3 and αvβ5 integrins are expressed in the developing human pancreas and that undifferentiated putative endocrine progenitor cells can use these adhesion molecules to mediate adhesion and migration on ECM. The data also show that the ECM proteins, FN and Coll-IV, contribute to the complex architecture of the developing islet. In addition, the αvβ3 and αvβ5 ligands, FN and VN, colocalize with cell clusters comprising insulin- and/or glucagon-positive cells emerging from, or adjacent to, the ductal epithelium. Most importantly, functional inhibition of these integrins in an in vivo model of islet development significantly reduced the migration of putative progenitors of islet cells from the ducts and perturbed the architecture and size of developing islet clusters. We conclude that αvβ3 and αvβ5 are critical integrins in the ontogeny of pancreatic islets. We also suggest that integrin/ECM interactions are involved in maintaining the structural integrity of mature islets. To the extent that islet structure is critical to islet function, efforts toward the development of novel strategies to protect and enhance integrin/ECM interactions may prove useful to improve the success of islet transplantation in diabetes.
Acknowledgments
This paper is dedicated to the memory of George Klier, a trusted collaborator and friend.
We are grateful to Dr. L. Crisa (The Scripps Research Institute) for her insightful contribution to the design of the in vivo experiments, and Dr. Anthony Montgomery (University of California, San Diego) for his comments on the manuscript. We are grateful to Drs. R. Ingram and M. Pierschbacher (Integra Life Sciences) for their assistance in the design and conduct of the blocking RGD peptide experiments.
D.R. Salomon was supported by National Institutes of Health (NIH) grant AI42384; V. Quaranta by NIH grant GM46902; and V. Cirulli by NIH grant DK98183 as well as a Career Development Award (296112) and Research Grant (197009) from the Juvenile Diabetes Foundation International (JDFI). A. Hayek was supported by JDFI Research Grant (199952). We also acknowledge support for C. Ricordi by the JDFI Human Islet Distribution Program at the Diabetes Research Institute of the University of Miami. The National Center for Microscopy and Imaging Research is supported by NIH grants RR04050 and DK54441 to M.H. Ellisman. This is manuscript No. 12239-MEM from The Scripps Research Institute.
Footnotes
Abbreviations used in this paper: Coll-IV, collagen IV; ECM, extracellular matrix; FN, fibronectin; LN, laminin; PECAM, platelet endothelial cell adhesion molecule; RGD, arginine, glycine, aspartic acid; VN, vitronectin.
References
- Alpert S., Hanahan D., Tietelman G. Hybrid insulin gene reveal a developmental lineage for pancreatic endocrine cell and imply a relationship with neurons. Cell. 1988;53:295–308. doi: 10.1016/0092-8674(88)90391-1. [DOI] [PubMed] [Google Scholar]
- Bauer G.E., Balsamo J., Lilien J. Cadherin-mediated adhesion in pancreatic islet cells is modulated by a cell surface N-acetylgalactosaminylphosphotransferase. J. Cell Sci. 1992;103:1235–1241. doi: 10.1242/jcs.103.4.1235. [DOI] [PubMed] [Google Scholar]
- Beattie G.M., Levine F., Mally M.I., Otonkoski T., O'Brien J.S., Salomon D.R., Hayek A. Acid β-galactosidasea developmentally regulated marker of endocrine cell precursors in the human fetal pancreas. J. Clin. Endocrinol. Metab. 1994;78:1232–1240. doi: 10.1210/jcem.78.5.8175983. [DOI] [PubMed] [Google Scholar]
- Beattie G.M., Rubin J.S., Mally M.I., Otonkoski T., Hayek A. Regulation of proliferation and differentiation of human fetal pancreatic islet cells by extracellular matrix, hepatocyte growth factor and cell-cell contact. Diabetes. 1996;45:1223–1228. doi: 10.2337/diab.45.9.1223. [DOI] [PubMed] [Google Scholar]
- Beattie G.M., Cirulli V., Lopez A.D., Hayek A. Ex vivo expansion of human pancreatic endocrine cells. J. Clin. Endocrinol. Metab. 1997;82:1852–1856. doi: 10.1210/jcem.82.6.4009. [DOI] [PubMed] [Google Scholar]
- Blaschuk K.L., Frost E.E., ffrench-Constant C. The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by αv integrins. Development. 2000;127:1961–1969. doi: 10.1242/dev.127.9.1961. [DOI] [PubMed] [Google Scholar]
- Bosco D., Meda P., Halban P.A., Rouiller D.G. Importance of cell-matrix interactions in rat islet β-cell secretion in vitrorole of α6β1 integrin. Diabetes. 2000;49:233–243. doi: 10.2337/diabetes.49.2.233. [DOI] [PubMed] [Google Scholar]
- Brooks P.C., Clark R.A., Cheresh D.A. Requirement of vascular integrin alphaVbeta3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks P.C., Stromblad S., Sanders L.C, Schalscha T.L, Aimes R.T., Stetler-Stevenson W.G., Quigley J.P., Cheresh D.A. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alphaVbeta3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Calof A.L., Lander A.D. Relationship between neuronal migration and cell-substratum adhesionlaminin and merosin promote olfactory neuronal migration but are anti-adhesive. J. Cell Biol. 1991;115:779–794. doi: 10.1083/jcb.115.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Craig W.S., Mullen D., Tschopp J.F., Dixon D., Pierschbacher M.D. Design and synthesis of novel cyclic RGD-containing peptides as highly potent and selective integrin alpha IIb beta 3 antagonists. J. Med. Chem. 1994;37:1–8. doi: 10.1021/jm00027a001. [DOI] [PubMed] [Google Scholar]
- Choquet D., Felsenfeld D.P., Sheetz M.P. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Cirulli V., Baetens D., Rutishauser U., Halban P.A., Orci L., Rouiller D.G. Expression of neural cell adhesion molecule (NCAM) in rat islets and its role in islet cell type segregation. J. Cell Sci. 1994;107:1429–1436. doi: 10.1242/jcs.107.6.1429. [DOI] [PubMed] [Google Scholar]
- Cirulli V., Ricordi C., Hayek A. E-cadherin, NCAM, and Ep-CAM expression in human fetal pancreata. Transplant. Proc. 1995;27:3335. [PubMed] [Google Scholar]
- Cirulli V., Crisa L., Beattie G.M., Mally M.I., Lopez A.D., Fannon A., Ptasznik A., Inverardi L., Ricordi C., Deerinck T. KSA antigen (Ep-CAM) mediates cell–cell adhesion of pancreatic epithelial cellsmorphoregulatory roles in pancreatic islet development. J. Cell Biol. 1998;140:1519–1534. doi: 10.1083/jcb.140.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A., Grant A.M. Quantitative morphology of endocrine cells in human fetal pancreas. Diabetologia. 1983;25:31–35. doi: 10.1007/BF00251893. [DOI] [PubMed] [Google Scholar]
- Clark R.A., Tonnesen M.G., Gailit J., Cheresh D.A. Transient functional expression of alphaVbeta 3 on vascular cells during wound repair. Am. J. Pathol. 1996;148:1407–1421. [PMC free article] [PubMed] [Google Scholar]
- Dahl U., Sjödin A., Semb H. Cadherins regulate aggregation of pancreatic β-cells in vivo . Development. 1996;122:2895–2902. doi: 10.1242/dev.122.9.2895. [DOI] [PubMed] [Google Scholar]
- DiPersio C.M., Hodivala-Dilke K.M., Jaenisch R., Kreidberg J.A., Hynes R.O. Alpha3beta1 integrin is required for normal development of the epidermal basement membrane. J. Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C.J., Cheresh D.A., Little C.D. An antagonist of integrin α3β1 prevents maturation of blood vessels during embryonic neovascularization. J. Cell Sci. 1995;108:2655–2661. doi: 10.1242/jcs.108.7.2655. [DOI] [PubMed] [Google Scholar]
- Dudek R.W., Lawrence I.E., Hill R.S., Johnson R.C. Induction of islet cytodifferentiation by fetal mesenchyme in adult pancreatic ductal epithelium. Diabetes. 1991;40:1041–1048. doi: 10.2337/diab.40.8.1041. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Vestweber D., Kemler R. Cell-matrix interactions and cell adhesion during development. Annu. Rev. Cell Biol. 1986;2:27–47. doi: 10.1146/annurev.cb.02.110186.000331. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B., Cheresh D.A. Vitronectin and its receptors. Curr. Opin. Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Friedlander M., Brooks P.C., Shaffer R.W., Kincaid C.M., Varner J.A., Cheresh D.A. Definition of two angiogenic pathways by distinct αv integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- George E.L., Baldwin H.S., Hynes R.O. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- Giancotti F.G. Integrin signalingspecificity and control of cell survival and cell cycle progression. Curr. Opin. Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Gu D., Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-γ transgenic mice. Development. 1993;118:33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- Gu D., Lee M.S., Krahl T., Sarvetnick N. Transitional cells in the regenerating pancreas. Development. 1994;120:1873–1881. doi: 10.1242/dev.120.7.1873. [DOI] [PubMed] [Google Scholar]
- Gullberg D., Ekblom P. Extracellular matrix and its receptors during development. Int. J. Dev. Biol. 1995;39:845–854. [PubMed] [Google Scholar]
- Herrera P.L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Herrera P.L., Huarte J., Sanvito F., Meda P., Orci L., Vassalli J.-D. Embryogenesis of the murine endocrine pancreasearly expression of pancreatic polypeptide gene. Development. 1991;113:1257–1265. doi: 10.1242/dev.113.4.1257. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. Integrinsa family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. Oncogenic transformation. In: Rich A., editor. Fibronectins. Springer-Verlag; New York: 1989. pp. 301–334. [Google Scholar]
- Jaffe R. The pancreas. In: Wigglesworth J.S., Singer D.B., editors. Textbook of Fetal and Perinatal Pathology. Blackwell Scientific Publications; Boston: 1991. pp. 1021–1054. [Google Scholar]
- Jansson L. The regulation of pancreatic islet blood flow. Diabetes Metab. Rev. 1995;10:407–416. doi: 10.1002/dmr.5610100405. [DOI] [PubMed] [Google Scholar]
- Jensen J., Heller R.S., Funder-Nielsen T., Pedersen E.E., Lindsell C., Weinmaster G., Madsen O.D., Serup P. Independent development of pancreatic α- and β-cells from neurogenin3-expressing precursors. Diabetes. 2000;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- Jones P.H., Watt F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Kantengwa S., Baetens D., Sadoull K., Buck C.A., Halban P.A., Rouiller D.G. Identification and characterization of α3β1 integrin on primary and transformed rat islet cells. Exp. Cell Res. 1997;237:394–402. doi: 10.1006/excr.1997.3803. [DOI] [PubMed] [Google Scholar]
- Langerhans P. Contribution to the microscopic anatomy of the pancreas. Doctoral dissertation 1869. Friedrich-Wilhelms Universität; Berlin: pp. 39 [Google Scholar]
- Langhofer M., Hopkinson S.B., Jones J.C.R. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro . J. Cell Sci. 1993;105:753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Like A., Orci L. Embryogenesis of the human pancreatic isletsa light and electron microscopic study Diabetes. 21Suppl. 21972. 511 534 [DOI] [PubMed] [Google Scholar]
- Lin C.Q., Bissell M.J. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB (Fed. Am. Soc. Exp. Biol.) J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- Lombardi T., Montesano R., Wohlwend A., Amherdt M., Vassalli J.D., Orci L. Evidence for polarization of plasma membrane domains in pancreatic endocrine cells. Nature. 1985;313:694–696. doi: 10.1038/313694a0. [DOI] [PubMed] [Google Scholar]
- Marchisio P.C., Bondanza S., Cremona O., Cancedda R., De Luca M. Polarized expression of integrin receptors (α6β4, α2β1, α3β1, and αvβ5) and their relationship with the cytoskeleton and basement membrane matrix in cultured human keratinocytes. J. Cell Biol. 1991;112:761–773. doi: 10.1083/jcb.112.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982;31:538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Orci L., Unger R.H. Functional subdivision of islets of Langerhans and possible role of D-cells. Lancet. 1975;20:1243–1244. doi: 10.1016/s0140-6736(75)92078-4. [DOI] [PubMed] [Google Scholar]
- Orci L., Thorens B., Ravazzola M., Lodish H.F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989;245:295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- Otonkoski T., Beattie G.M., Mally M.I., Ricordi C., Hayek A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J. Clin. Invest. 1993;92:1459–1466. doi: 10.1172/JCI116723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otonkoski T., Beattie G.M., Rubin J.S., Lopez A.D., Baird A., Hayek A. Hepatocyte growth factor/scatter factor has insulinotropic activity in human fetal pancreatic cells. Diabetes. 1994;43:947–953. doi: 10.2337/diab.43.7.947. [DOI] [PubMed] [Google Scholar]
- Otonkoski T., Cirulli V., Beattie G.M., Mally M., Soto G., Rubin J.S., Hayek A. A role for hepatocyte growth factor/scatter factor in fetal mesenchyme-induced pancreatic β-cell growth. Endocrinology. 1996;137:3131–3139. doi: 10.1210/endo.137.7.8770939. [DOI] [PubMed] [Google Scholar]
- Pelletier A.J., Kunicki T., Quaranta V. Activation of the integrin alpha v beta 3 involves a discrete cation-binding site that regulates conformation. J. Biol. Chem. 1996;271:1364–1370. doi: 10.1074/jbc.271.3.1364. [DOI] [PubMed] [Google Scholar]
- Pictet R.L., Rutter W.J. Development of the embryonic endocrine pancreas. In: Steiner D., Freinkel N., editors. Handbook of Physiology. Section 8, Vol. 1, The Endocrine Pancreas. Williams and Wilkins Company; Baltimore: 1972. pp. 25–66. [Google Scholar]
- Pictet R.L., Clark W.R., Williams R.H., Rutter W.J. An ultrastructural analysis of the developing embryonic pancreas. Dev. Biol. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M.D., Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J. Biol. Chem. 1987;262:17294–17298. [PubMed] [Google Scholar]
- Ricordi C., Lacy P.E., Finke E.H., Olack B.J., Sharp D.W. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- Rouiller G.D., Cirulli V., Halban P.A. Uvomorulin mediates Ca++-dependent aggregation of islet cells, whereas Ca++-independent cell adhesion molecules distinguish between islet cell types. Dev. Biol. 1991;148:233–242. doi: 10.1016/0012-1606(91)90332-w. [DOI] [PubMed] [Google Scholar]
- Sandler S., Andersson A., Schnell A., Mellgren A., Tollemar J., Borg H., Petersson B., Groth C.G., Hellerstrom C. Tissue culture of human fetal pancreas. Development and function of B cells in vitro and transplantation of explants to nude mice. Diabetes. 1985;34:1113–1119. doi: 10.2337/diab.34.11.1113. [DOI] [PubMed] [Google Scholar]
- Schwartz M.A., Ingber D.E. Integrating with integrins. Mol. Biol. Cell. 1994;5:389–393. doi: 10.1091/mbc.5.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J.M.W. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A. Integrin and their ligands. Curr. Top. Microbiol. Immun. 1993;184:7–35. doi: 10.1007/978-3-642-78253-4_2. [DOI] [PubMed] [Google Scholar]
- Stefan Y., Grasso S., Perrelet A., Orci L. A quantitative immune fluorescent study of the endocrine cell populations in the developing human pancreas. Diabetes. 1983;32:293–301. doi: 10.2337/diab.32.4.293. [DOI] [PubMed] [Google Scholar]
- Strange R., Li F., Saurer S., Burkhardt A., Friis R.R. Apoptotic cell death and tissue remodeling during mouse mammary gland involution. Development. 1992;115:49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Talhouk R.S., Bissell M.J., Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J. Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelman G., Lee J.K. Cell lineage analysis of pancreatic islet cell developmentglucagon and insulin cells arise from catecholaminergic precursors present in the pancreatic duct. Dev. Biol. 1987;121:454–466. doi: 10.1016/0012-1606(87)90182-5. [DOI] [PubMed] [Google Scholar]
- Tuch B.E., Ng A.B.P., Jones A., Turtle J.R. Histologic differentiation of human fetal pancreatic explants transplanted into nude mice. Diabetes. 1984;33:1180–1187. doi: 10.2337/diab.33.12.1180. [DOI] [PubMed] [Google Scholar]
- Von Dorsche H.H., Falt K., Titlbach M., Reiher H., Hahn H.-J., Falkmer S. Immune histochemical, morphometric and ultrastructural investigations of the early development of insulin, somatostatin, glucagon and PP cells in foetal human pancreas. Diabetes Res. 1989;12:551–556. [PubMed] [Google Scholar]
- Walker N.I., Bennett R.E., Kerr J.F. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am. J. Anat. 1989;185:19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- Watt F.M., Jones P.H. Expression and function of keratinocyte integrins. Development Suppl. 1993;106:185–192. [PubMed] [Google Scholar]