Abstract
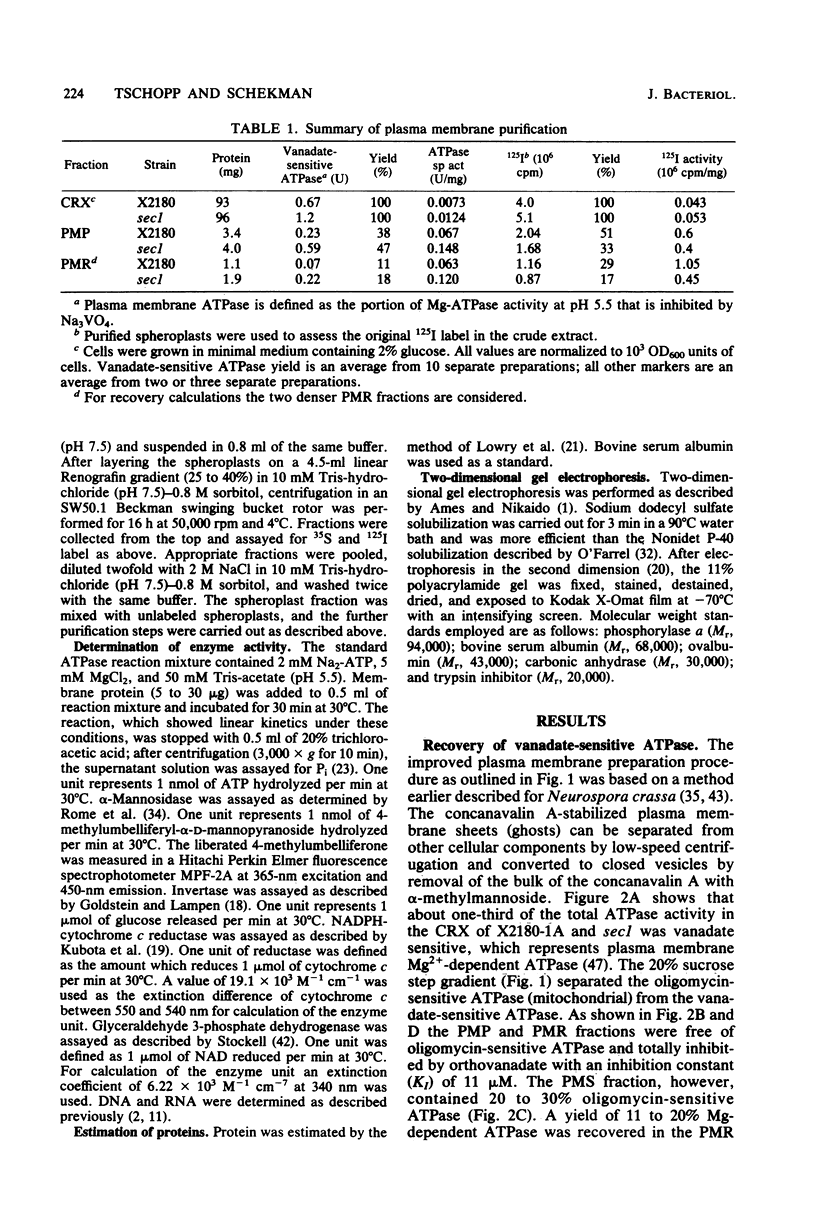
The plasma membrane from Saccharomyces cerevisiae X2180-1A and a secretion-blocked mutant, secl (P. Novick and R. Schekman, Proc. Natl. Acad. Sci. U.S.A. 76:1858-1862, 1979) has been purified. Cell walls were digested by treatment with lyticase followed by concanavalin A coating of spheroplasts. alpha-Methylmannoside treatment after lysis, sonication at high salt concentration, and fractionation on a Renografin gradient resulted in two highly purified membrane fractions sedimenting at densities of 1.15 and 1.17 g/cm3. Yields determined by recovery of vanadate-sensitive ATPase activity were 11 to 18%, and those determined by recovery of the spheroplast surface label 125I were 17 to 29%. Iodinated cells have most of their label in sedimentable, nonspheroplast material. However, both membrane populations contain some 125I surface label and show ATPase activity with pH optima only at 5.5. The apparent Vmax of the plasma membrane ATPase equals 360 to 560 nmol of ATP hydrolyzed per min per mg of protein, with a Km for ATP of 0.7 mM. ATPase specific activity is not decreased in mutant plasma membrane. Analysis of 125I-labeled plasma membrane proteins by two-dimensional gel electrophoresis revealed seven major proteins on the plasma membrane surface.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14(11):93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. Characterization of plasma membrane adenosine triphosphatase of Neurospora crassa. J Biol Chem. 1977 May 25;252(10):3357–3363. [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Bussey H., Saville D., Chevallier M. R., Rank G. H. Yeast plasma membrane ghosts. An analysis of proteins by two-dimensional gel electrophoresis. Biochim Biophys Acta. 1979 May 17;553(2):185–196. doi: 10.1016/0005-2736(79)90224-4. [DOI] [PubMed] [Google Scholar]
- Cahn F. H., Fox M. S. Fractionation of transformable bacteria from ocompetent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968 Mar;95(3):867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Cantley L. G., Josephson L. A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications. J Biol Chem. 1978 Oct 25;253(20):7361–7368. [PubMed] [Google Scholar]
- Christensen M. S., Cirillo V. P. Yeast membrane vesicles: isolation and general characteristics. J Bacteriol. 1972 Jun;110(3):1190–1205. doi: 10.1128/jb.110.3.1190-1205.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour J. P., Boutry M., Goffeau A. Plasma membrane ATPase of yeast. Comparative inhibition studies of the purified and membrane-bound enzymes. J Biol Chem. 1980 Jun 25;255(12):5735–5741. [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Franzusoff A. J., Cirillo V. P. Glucose transport activity in isolated plasma membrane vesicles from Saccharomyces cerevisiae. J Biol Chem. 1983 Mar 25;258(6):3608–3614. [PubMed] [Google Scholar]
- Fuhrmann G. F., Boehm C., Theuvenet A. P. Sugar transport and potassium permeability in yeast plasma membrane vesicles. Biochim Biophys Acta. 1976 May 21;433(3):583–596. doi: 10.1016/0005-2736(76)90283-2. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G. F., Wehrli E., Boehm C. Preparation and identification of yeast plasma membrane vesicles. Biochim Biophys Acta. 1974 Sep 23;363(3):295–310. doi: 10.1016/0005-2736(74)90070-4. [DOI] [PubMed] [Google Scholar]
- Goldstein A., Lampen J. O. Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Kubota S., Yoshida Y., Kumaoka H., Furumichi A. Studies on the microsomal electron-transport system of anaerobically grown yeast. V. Purification and characterization of NADPH-cytochrome c reductase. J Biochem. 1977 Jan;81(1):197–205. doi: 10.1093/oxfordjournals.jbchem.a131436. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARSH B. B. The estimation of inorganic phosphate in the presence of adenosine triphosphate. Biochim Biophys Acta. 1959 Apr;32:357–361. doi: 10.1016/0006-3002(59)90607-9. [DOI] [PubMed] [Google Scholar]
- Matile P., Moor H., Mühlethaler K. Isolation and properties of the plasmalemma in yeast. Arch Mikrobiol. 1967;58(3):201–211. doi: 10.1007/BF00408804. [DOI] [PubMed] [Google Scholar]
- McDonough J. P., Jaynes P. K., Mahler H. R. Partial characterization of the plasma membrane ATPase from a rho0 petite strain of Saccharomyces cerevisiae. J Bioenerg Biomembr. 1980 Aug;12(3-4):249–264. doi: 10.1007/BF00744687. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Export of major cell surface proteins is blocked in yeast secretory mutants. J Cell Biol. 1983 Feb;96(2):541–547. doi: 10.1083/jcb.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen T., Oura E., Suomalainen H. The enzymic composition of the isolated cell wall and plasma membrane of baker's yeast. Biochem J. 1970 Jan;116(1):61–69. doi: 10.1042/bj1160061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen T., Taskinen L., Suomalainen H. Distribution of membranes, especially of plasma-membrane fragments, during zonal centrifugations of homogenates from glucose-repressed Saccharomyces Cerevisiae. Biochem J. 1976 Mar 15;154(3):751–763. doi: 10.1042/bj1540751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen T., Taskinen L., Suomalainen H. Distribution of plasma-membrane fragments during zonal centrifugations of homogenates from aerobic Saccharomyces cerevisiae. J Gen Microbiol. 1977 Jan;98(1):301–304. doi: 10.1099/00221287-98-1-301. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Robertson A. J., Gerlach J. H., Rank G. H., Fowke L. C. Yeast cell wall, membrane, and soluble marker polypeptides identified by comparative two-dimensional electrophoresis. Can J Biochem. 1980 Jul;58(7):565–572. doi: 10.1139/o80-077. [DOI] [PubMed] [Google Scholar]
- STOCKELL A. The binding of diphosphopyridine nucleotide by yeast glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1959 May;234(5):1286–1292. [PubMed] [Google Scholar]
- Scarborough G. A. Isolation and characterization of Neurospora crassa plasma membranes. J Biol Chem. 1975 Feb 10;250(3):1106–1111. [PubMed] [Google Scholar]
- Scarborough G. A. Properties of Neurospora crassa plasma membrane ATPase. Arch Biochem Biophys. 1977 Apr 30;180(2):384–393. doi: 10.1016/0003-9861(77)90052-2. [DOI] [PubMed] [Google Scholar]
- Scheele G. A., Palade G. E., Tartakoff A. M. Cell fractionation studies on the guinea pig pancreas. Redistribution of exocrine proteins during tissue homogenization. J Cell Biol. 1978 Jul;78(1):110–130. doi: 10.1083/jcb.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibeci A., Rattray J. B., Kidby D. K. Isolation and identification of yeast plasma membrane. Biochim Biophys Acta. 1973 Jun 7;311(1):15–25. doi: 10.1016/0005-2736(73)90250-2. [DOI] [PubMed] [Google Scholar]
- Scott J. H., Schekman R. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J Bacteriol. 1980 May;142(2):414–423. doi: 10.1128/jb.142.2.414-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons T. J. Vanadate--a new tool for biologists. Nature. 1979 Oct 4;281(5730):337–338. doi: 10.1038/281337a0. [DOI] [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Stroobant P., Scarborough G. A. Large scale isolation and storage of Neurospora plasma membranes. Anal Biochem. 1979 Jun;95(2):554–558. doi: 10.1016/0003-2697(79)90771-1. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Hybridization of membranes by sonic irradiation. Biochemistry. 1971 Aug 17;10(17):3309–3313. doi: 10.1021/bi00793a023. [DOI] [PubMed] [Google Scholar]
- Welten-Verstegen G. W., Boer P., Steyn-Parvé E. P. Lipid-mediated glycosylation of endogenous proteins in isolated plasma membrane of Saccharomyces cerevisiae. J Bacteriol. 1980 Jan;141(1):342–349. doi: 10.1128/jb.141.1.342-349.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R. Characterization of the plasma membrane Mg2+-ATPase from the yeast, Saccharomyces cerevisiae. J Biol Chem. 1979 May 10;254(9):3326–3332. [PubMed] [Google Scholar]



