Abstract
FE65 binds to the Alzheimer amyloid precursor protein (APP), but the function of this interaction has not been identified. Here, we report that APP and FE65 are involved in regulation of cell movement. APP and FE65 colocalize with actin and Mena, an Abl-associated signaling protein thought to regulate actin dynamics, in lamellipodia. APP and FE65 specifically concentrate with β1-integrin in dynamic adhesion sites known as focal complexes, but not in more static adhesion sites known as focal adhesions. Overexpression of APP accelerates cell migration in an MDCK cell wound–healing assay. Coexpression of APP and FE65 dramatically enhances the effect of APP on cell movement, probably by regulating the amount of APP at the cell surface. These data are consistent with a role for FE65 and APP, possibly in a Mena-containing macromolecular complex, in regulation of actin-based motility.
Keywords: amyloid precursor protein, FE65, Mena, cell movement, adhesion
Introduction
Altered proteolytic processing of the Alzheimer amyloid precursor protein (APP) is thought to be at least one cause of Alzheimer disease. Despite extensive efforts to pinpoint the normal function of APP, its function in the cell remains elusive. Roles in cell adhesion (Schubert et al. 1989), cell proliferation (Saitoh et al. 1989), neuroprotection (Mattson et al. 1993) and neurite outgrowth (Milward et al. 1992; Jin et al. 1994; Small et al. 1994; Perez et al. 1997) have been proposed. It has also been suggested that APP might have a role in signal transduction since APP structurally resembles a receptor (Kang et al. 1987) and is targeted to the cell surface.
FE65 interacts with the YENPTY motif in the cytoplasmic domain of APP (Fiore et al. 1995; Borg et al. 1996; Bressler et al. 1996; Guenette et al. 1996; Zambrano et al. 1997; Duilio et al. 1998; Tanahashi and Tabira 1999). Aside from its interaction with APP, very little is known about FE65. It has no known enzymatic activity and contains three protein–protein interaction domains, with a WW domain in its NH2 terminus and tandem phosphotyrosine/protein interaction domains (PIDs) in its COOH terminus. FE65 binds to APP through its more COOH-terminal PID. Overexpression of FE65 increases the proteolytic processing of APP (Guenette et al. 1999; Sabo et al. 1999). It also causes a translocation of APP to the cell surface (Sabo et al. 1999), suggesting that binding of FE65 to the YENPTY motif might regulate the function of APP by regulating the amount of APP at the cell surface.
We do not yet know whether, and if so which, other proteins interact with FE65 when FE65 interacts with APP. It has been shown previously that Mena coprecipitates with the WW domain of FE65 (Ermekova et al. 1997). Mena is genetically linked to the Abl tyrosine phosphorylation signaling cascade and is required for normal neural development (Gertler et al. 1990, Gertler et al. 1995; Lanier et al. 1999). It localizes to cell-substrate adhesion sites and sites of dynamic actin assembly and disassembly. Interestingly, Mena also binds to profilin, placing it in a position to regulate actin dynamics. It has been proposed that Mena, FE65, and APP may be components of a tripartite complex (Ermekova et al. 1997). However, it has not been shown that this tripartite complex exists.
At the neuronal cell surface, APP colocalizes with patches of integrins (Yamazaki et al. 1997). Integrins are a family of heterodimeric cell adhesion receptors that mediate cell–matrix interactions required for cell proliferation, differentiation, and migration (Howland et al. 1995). This raises the question of whether an APP–FE65 complex might have some role in integrin-based adhesion or signaling.
Integrins and Mena are both found in two types of cell-substrate adhesion sites, known as focal adhesions and focal complexes (Hotchin and Hall 1995; Gertler et al. 1996; Rottner et al. 1999a; Lanier and Gertler 2000). Focal adhesions are the widely studied adhesion sites found at the tips of actin stress fibers. They are typically very stable and strong and are found in relatively static regions of the cell. Focal complexes are less studied, more dynamic adhesion sites typically found at a migrating cell's leading edge (Nobes and Hall 1995). Although focal adhesions and focal complexes are functionally and morphologically distinct, there has been, to our knowledge, no known biochemical distinction between them. It seemed possible that APP and FE65 might function together in focal adhesions, focal complexes, or both.
Here we show that APP and FE65 selectively localize with Mena in integrin-based focal complexes in mobile membrane compartments. Furthermore, we provide evidence that APP modulates cell movement, and that APP-dependent changes in cell motility are regulated by FE65. We propose a link between FE65-dependent changes in APP trafficking and metabolism and actin-based membrane motility.
Materials and Methods
Tissue Culture
Standard tissue culture techniques were used. H4 neuroglioma cells and MDCK cells were maintained in DME supplemented with 10% FBS and antibiotics. MDCK cells that overexpress the 695–amino acid isoform of APP (+APP/−FE65) were a gift of Dr. C. Haass (Ludwig Maximilians University, Munich, Germany) (Haass et al. 1994). These cells were stably transfected with FE65 cDNA to yield cells that overexpress both APP and FE65 (+APP/+FE65; Sabo et al. 1999). FE65 overexpression does not alter steady-state APP expression levels.
Antibodies
Polyclonal FE65 antibodies 170/173, which recognize the WW domain of FE65, were affinity purified as described previously (Sabo et al. 1999). Polyclonal APP antibody 369, which recognizes the cytoplasmic domain of APP, was affinity purified with a peptide corresponding to the last 50 amino acids of APP. The APP monoclonal antibodies 5A3/1G7 (a gift from Dr. E.H. Koo, University of California at San Diego, CA) recognize epitopes in the extracellular domain of APP. Polyclonal antibodies against Mena were a gift of Dr. F. Gertler (MIT, Cambridge, MA) and have been used previously for immunoblotting and immunofluorescence (Ermekova et al. 1997; Lanier et al. 1999). Integrin β1 and phosphotyrosine monoclonal antibodies were purchased from Upstate Biotechnology.
Immunofluorescence
H4 cells were plated at 3.5 × 104 cells/cm−2 in Labtek chamberslides. Cells were fixed in cold 4% paraformaldehyde in PBS with 0.12 M sucrose for 10 min at room temperature. After permeabilization for 5 min with 0.1% Triton X-100 in PBS, the cultures were blocked with 10% normal goat serum (NGS) in PBS for 30 min at room temperature. Cells were incubated overnight at 4°C with primary antibodies diluted in 5% NGS in PBS, then incubated with secondary antibodies, also diluted in 5% NGS in PBS, for 1 h at room temperature. Coverslips were mounted with DABCO in polyvinyl alcohol.
Secondary antibodies were used at dilutions of 1:400 for Oregon green goat anti–mouse and Texas red goat anti–rabbit (Molecular Probes), 1:200 for Cy5 goat anti–mouse, and 1:100 for rhodamine red X goat anti–rabbit (Jackson ImmunoResearch Laboratories). Oregon green phalloidin (Molecular Probes) was used at 1:40 dilution and was added with the secondary antibody.
In all cases, immunofluorescence was eliminated by omission of the primary antibody. For FE65 and APP labeling, the signals were also eliminated by competition with excess soluble antigen (Fig. 3d and Fig. h). In double and triple label experiments, labeling patterns were identical to those seen with single labeling. In addition, each labeling pattern was identical when an alternative set of secondary antibodies was used. Immunofluorescence was examined by confocal laser scanning microscopy (LSM510; Carl Zeiss, Inc.) using a 63× water immersion lens; Ar 488-, HeNe 543-, and HeNe 633-nm lasers; and BP505-550, BP560-615, and LP650 filters. For colocalization experiments, optical sections were no thicker than 1.5 μm and each channel was imaged separately, eliminating the possibility of “bleed-through.”
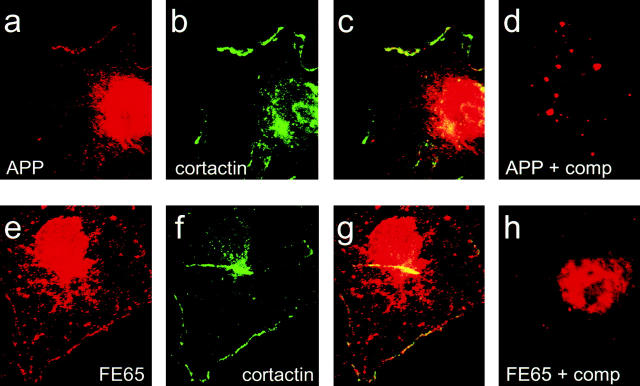
Figure 3.
The membranes enriched in APP and FE65 are also enriched in cortactin, a marker for lamellipodia. (a–c) H4 cells immunolabeled with a combination of APP polyclonal antibody (a) and cortactin monoclonal antibody (b). Overlap is indicated by yellow in the overlay (c). (d) Lamellipodial staining of APP is eliminated by preincubation of APP antibody with excess soluble antigen. (e–g) H4 cells immunolabeled with a combination of FE65 polyclonal antibodies (e) and cortactin monoclonal antibody (f). Overlap is indicated by yellow in the overlay (g). (h) FE65 immunofluorescence is eliminated by preincubation of FE65 antibodies with excess soluble antigen.
Coprecipitation
GST fusion proteins were purified from bacterial lysates by incubation with glutathione-Sepharose beads (Amersham Pharmacia Biotech) followed by extensive washing with HBS. Purified fusion proteins were quantified both by SDS-PAGE followed by Coomassie staining and elution with glutathione, dialysis, and BCA assay (Pierce Chemical Co.). H4 cells were homogenized in 10 mM Tris HCl, pH 7.5; 150 mM NaCl; 0.1 mM sodium vanadate; 50 mM NaF; 0.5% NP-40 and protease inhibitors. The beads were blocked with 2% BSA. Extracts were incubated with equal amounts of various GST fusion proteins for 2–3 h. Complexes precipitated on glutathione-Sepharose beads were washed several times with lysis buffer. Beads were then boiled in Laemmli buffer, and the proteins separated by SDS-PAGE and transferred to nitrocellulose. Blots were probed with polyclonal primary antibodies and anti–rabbit HRP-conjugated IgG (Amersham Pharmacia Biotech), then examined by ECL (Pierce Chemical Co.).
Coimmunoprecipitation
Confluent monolayers of MDCK cells were split 1:3 and plated. 24 h later, cells were homogenized in 0.25% NP-40 in PBS containing a protease inhibitor cocktail (Amersham Pharmacia Biotech) and 2% BSA at 4°C. After centrifugation for 10 min at 10,000 g, the supernatant was incubated for 1.5 h at 4°C with Mena antibody precoupled to protein A–Sepharose beads. The beads were then pelleted and washed several times with lysis buffer. Immunoprecipitates were boiled in Laemmli buffer and separated by SDS-PAGE. Immunoblotting was performed in 5% nonfat milk and 0.05% Tween-20 in TBS with 6E10, a monoclonal antibody against APP, and anti–Flag-M2 antibody to recognize a Flag epitope tag on expressed FE65. Detection was with anti–mouse HRP-conjugated secondary antibody (Amersham Pharmacia Biotech) and ECL (Pierce Chemical Co.).
Wounding Assay
Wounding assays using MDCK cells have been published previously (Rosen and Misfeldt 1980; Fenteany et al. 2000). Confluent monolayers of MDCK cells were wounded by scraping with a 200-μl pipette tip. Unattached cells were removed by washing and agitation. For immunofluorescence, wounded monolayers were allowed to recover for 2 h and were then fixed and immunostained. For measurements of wound healing, the cells were grown on grids. Photographs of the wounds were taken at the same points on the grid at various time points. The distances the wound edges had traveled were determined by measuring, at each time point, the distance between the two edges at regular intervals from the grid marker. In addition, wounds were examined for degree of closure after ∼1 d of wound healing and scored as fully closed, partially closed or fully open.
Cross-Correlation Analysis
Images were collected for cells labeled as described above. The detector gain was adjusted such that none of the fluorescence levels reached saturation. A series of ∼5–10-μm lines were drawn perpendicular to the lamellipodial leading edge at intervals of ∼3–5 μm. Line length was constant for an individual lamellipodium. An intensity profile was generated for all three labels along each line. The resulting intensities were compared by cross-correlation analysis. A more detailed description of cross-correlation analysis can be found elsewhere (Bendat and Piersol 1971; Oppenheim and Schafer 1975; Brody 1999a,Brody 1999b). To avoid artifacts due to differences in the overall intensity of each label, the “shuffle-corrected” cross-correlogram, also referred to as a cross-covariogram, was calculated. The cross-correlogram and cross-covariogram are essentially equivalent to correlation and covariance commonly calculated for scalars and given by the following equations:
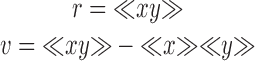 |
where r is the correlation and v is the covariance of two scalars, x and y
 〈 〉 indicates the expected value operation. The cross-correlogram and cross-covariogram are given by the following equations:
〈 〉 indicates the expected value operation. The cross-correlogram and cross-covariogram are given by the following equations:
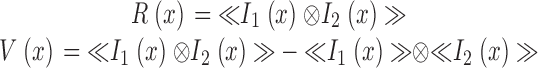 |
where R(x) is the cross-correlogram and V(x) is the shuffle-corrected correlogram. I(x) is the intensity along one of the lines. Subscripts denote the label being examined. The covariograms were calculated using the “xcov” function in Matlab (signal processing toolbox, Mathworks). The cross-covariograms were normalized such that the shuffle-corrected autocorrelation equals 1.0 at zero displacement. The autocorrelation is computed by correlating a vector with itself and thus gives the highest correlation possible. This normalization results in a cross-covariogram in which the values on the y-axis correspond to the correlation coefficient at each displacement.
Results
Colocalization of APP and FE65 with Mena and Lamellipodial Actin
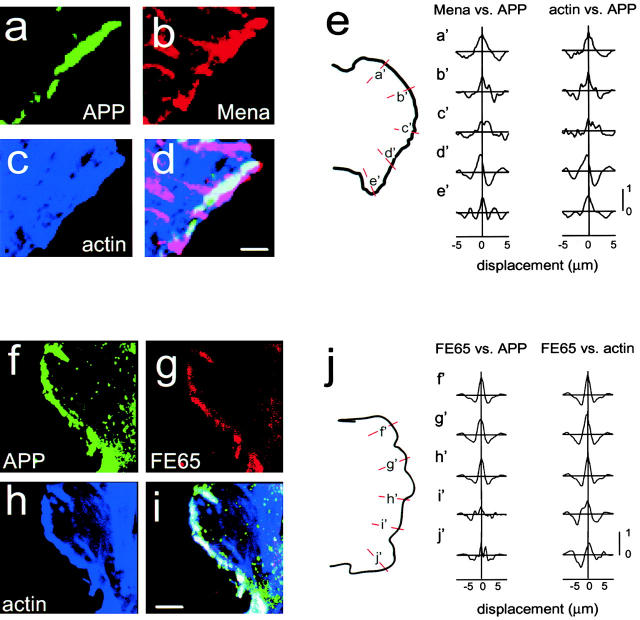
FE65 interacts with Mena in vitro, but the relevance of this interaction to APP and FE65 function was unknown. Furthermore, it was not known if FE65 interacts with APP and Mena simultaneously. To test if a tripartite complex between APP, FE65, and Mena is possible, we triple labeled H4 human neuroglioma cells either with APP monoclonal antibody, Mena polyclonal antibody, and Oregon green phalloidin or with APP monoclonal antibody, FE65 polyclonal antibody, and Oregon green phalloidin. The phalloidin labeling allowed us to identify the membrane domains in which APP, FE65, and Mena localized. APP and Mena colocalized at ruffled edges of cells that contained a characteristic lamellipodial actin structure (Fig. 1, a–d). In fact, edges that contained APP and Mena could be identified based solely on the presence of a dense meshwork of short actin filaments. APP also colocalized with FE65 in lamellipodia (Fig. 1, f–i). To avoid over- or underestimation of the colocalization, we performed a novel, quantitative, objective analysis of the colocalization (described in Materials and Methods). Quantification of the intensity of the immunofluorescence signals and cross-correlation analysis showed that APP and Mena (Fig. 1 e) and APP and FE65 (Fig. 1 j) indeed colocalize, since cross-covariograms derived from lamellipodial line intensity profiles displayed significant correlation with no shift in the peak.
Figure 1.
APP colocalizes with FE65, Mena, and actin in lamellipodia. Lamellipodia are characterized by aggregates of actin and a dense meshwork of short actin filaments and could therefore be chosen for imaging based on their actin structure while blind to immunolabeling. Images are presented in pseudocolor. (a–d) High magnification image of H4 cells triple labeled with APP monoclonal antibodies (a), Mena polyclonal antibody (b), and Oregon green–conjugated phalloidin (c), then examined by confocal microscopy. Overlap of APP, Mena, and actin is indicated by white in the overlay (d). (f–i) High magnification image of H4 cells triple labeled with APP monoclonal antibodies (f), FE65 polyclonal antibodies (g), and Oregon green–conjugated phalloidin (h), then examined by confocal microscopy. Overlap of APP, FE65, and actin is indicated by white in the overlay (i). (e and j) Cross-covariograms from cross-correlation analysis of APP, Mena, and actin (e) and of APP, FE65, and actin (j) in lamellipodia. Lines were drawn perpendicular to the lamellipodial edge (shown in orange and labeled a′–e′ for APP, Mena, and actin and f′–j′ for APP, FE65, and actin). The intensities were determined for each line and the cross-covariograms calculated as described in Materials and Methods. All of the cross-covariograms calculated here, with the exception of c′ for both Mena and actin, have peaks >0.5, indicating significant correlation. None have a displacement greater than the half-width at half-height, indicating colocalization. Bars, 5 μm.
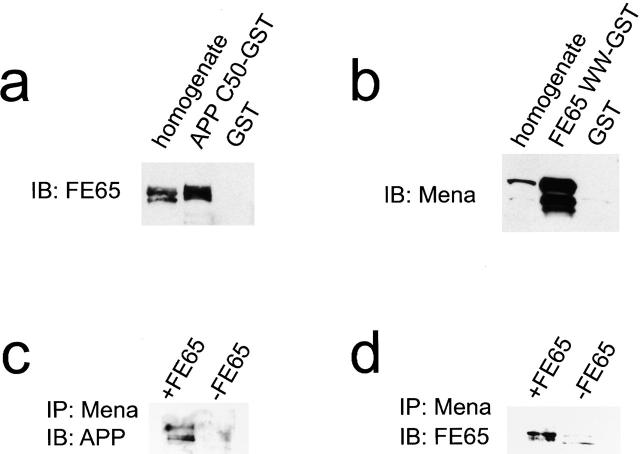
To determine if the APP–FE65–Mena tripartite complex exists, coprecipitations and immunoprecipitations were performed. When H4 cell lysates were incubated with a GST fusion protein containing the cytoplasmic domain of APP, FE65 was precipitated on glutathione-Sepharose beads through an interaction with the APP fusion protein (Fig. 2 a). Similarly, when the same lysates were incubated with a GST fusion protein containing the WW domain of FE65, Mena bound to the FE65 fusion protein was precipitated on glutathione-Sepharose beads (Fig. 2 b). Finally, when lysates from MDCK cells stably expressing both APP and FE65 were subjected to immunoprecipitation with antibodies raised against Mena, FE65 and APP were found in the immunoprecipitates by immunoblotting (Fig. 2c and Fig. d). When lysates from MDCK cells stably expressing only APP were used side-by-side in the same experiment, APP was not coimmunoprecipitated with Mena, indicating that the APP found in the immunoprecipitates associated with Mena indirectly through FE65. These data lend strong support to the idea that a macromolecular complex containing APP, FE65, and Mena exists in vivo. Since Mena is known to bind to profilin and is thought to regulate actin dynamics (Gertler et al. 1996; Lanier and Gertler 2000), the colocalization and interaction of APP and FE65 with Mena provides evidence for an indirect link between an APP–FE65 complex and the lamellipodial actin cytoskeleton.
Figure 2.
APP and Mena interact with FE65 simultaneously. (a and b) H4 cell lysates were incubated with GST fusion proteins corresponding to either the cytoplasmic domain of APP (APP C50-GST) or the WW domain of FE65 (FE65 WW-GST) for precipitation with glutathione-Sepaharose. Precipitated complexes were immunoblotted with FE65 (a) and Mena (b) antibodies, respectively. As a control for nonspecific coprecipitation, experiments were performed in parallel with GST alone. (c and d) Homogenates from MDCK cells that overexpressed both APP and FE65 (+FE65) and MDCK cells that overexpressed APP, but not FE65 (−FE65), were immunoprecipitated with Mena antibodies. The immunoprecipitates were then immunoblotted with APP (c) and FE65 (d) antibodies. APP coimmunoprecipitated with Mena only when FE65 was present.
Dynamic, but Not Static, Compartments Contain APP and FE65
To confirm that the membrane domains that contained APP and FE65 were the dynamic lamellipodial membranes, H4 cells were double labeled with antibodies against APP and cortactin or FE65 and cortactin. Cortactin is an actin-binding protein highly enriched within lamellipodia (Wu and Parsons 1993). Both APP (Fig. 3, a–c) and FE65 (Fig. 3, e–g) localized specifically to cortactin-rich lamellipodia. The FE65 and APP labeling in lamellipodia was specific, since competition with excess soluble antigen eliminated lamellipodial staining (Fig. 3d and Fig. h). The membranes that contained APP and FE65 also contained another marker of dynamic membranes, Rac1 (data not shown). Rac1 is a member of the Rho family of small GTP-binding proteins, which, upon activation, is targeted to membranes where it induces lamellipodia formation (Bokoch et al. 1994; Nobes and Hall 1995). This confirmed that the labeled edges were indeed the mobile lamellipodia of the cells.
APP and Mena colocalized in lamellipodia (Fig. 4b and f–h) but not in focal adhesions (Fig. 4b and c–e). Occasionally APP and Mena were seen in fine lines toward the edges of the lamellipodia, which most likely corresponded to focal complexes. Focal complexes are integrin-based adhesion sites found at the base of protruding and ruffling lamellipodia (Hotchin and Hall 1995; Nobes and Hall 1995; Rottner et al. 1999b). The lamellipodial actin meshwork terminates at the focal complexes, the role of which is unclear. They are not required for lamellipodial formation, but might be important for cell movement or signaling.
Figure 4.
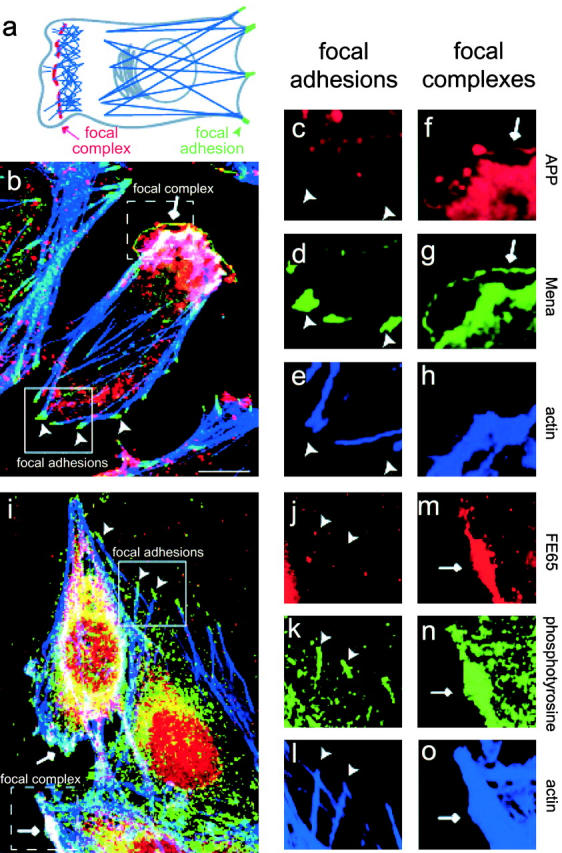
APP and FE65 accumulate in mobile lamellipodia, but not in focal adhesions. Arrows indicate the location of focal complexes and arrowheads indicate the location of focal adhesions. (a) Schematic diagram. Focal adhesions are found at the ends of stress fibers in stable membranes, whereas focal complexes anchor a meshwork of short actin filaments to the substrate under the cell's mobile lamellipodia. (b–h) H4 cells triple labeled with a combination of APP monoclonal antibodies, Mena polyclonal antibody, and Oregon green phalloidin. Images of labeled cells were collected by confocal microscopy. (b) Overlay of APP (red), Mena (green), and actin (blue). Overlap of staining for all three is indicated by white. (c–e) Individual signals from the solid box in panel b for APP (c), Mena (d), and actin (e). Notice the absence of APP immunoreactivity from focal adhesions. (f–h) Individual signals from dashed box in panel b for APP (f), Mena (g), and actin (h). Notice that APP colocalizes with Mena in a fine line at the edge of the lamellipodia. (i–o) H4 cells triple labeled with a combination of FE65 polyclonal antibodies, phosphotyrosine monoclonal antibody, and Oregon green phalloidin. Phosphotyrosine antibodies were used here as a marker for adhesion sites since the FE65 antibody and the Mena antibody were both raised in rabbits. (i) Overlay of FE65 (red), phosphotyrosine (green), and actin (blue). Overlap of staining for all three is indicated by white. (j–l) Individual signals from solid box in panel i for FE65 (j), phosphotyrosine (k), and actin (l). Notice the absence of FE65 immunoreactivity from focal adhesions. (m–o) Individual signals from dashed box in panel i for FE65 (m), phosphotyrosine (n), and actin (o). Notice that lamellipodia are labeled by both FE65 and phosphotyrosine antibodies. Bar, 10 μm.
In the absence of a monoclonal antibody to either FE65 or Mena for double labeling of FE65 and Mena, we tested whether FE65 was also selectively found in mobile membranes by labeling adhesion sites with antiphosphotyrosine antibodies. Tyrosine-phosphorylated proteins accumulate in adhesion sites in both dynamic and static membranes, making phosphotyrosine a good marker for both focal adhesions and focal complexes. FE65 colocalized with phosphotyrosine in membrane regions that displayed characteristics of lamellipodia, such as actin aggregates and ruffled edges (Fig. 4i and m–o), but not in focal adhesions (Fig. 4i and j–l). Thus, both FE65 and APP selectively localize to mobile membrane compartments containing weak sites of cell–substrate attachment.
APP and FE65 in Integrin-based Focal Complexes
Several studies have suggested a role for APP in cell adhesion (Mattson 1997). Given the localization of APP and FE65 within lamellipodia, it seemed that they might play some role in regulation of adhesion or signaling in integrin-dependent lamellipodial focal complexes. Since integrins containing the β1 integrin subunit are found in focal complexes (Hotchin and Hall 1995; Rottner et al. 1999b), we used β1-integrin as a marker. To determine if FE65 and APP colocalize with β1 integrin at adhesion sites in lamellipodia, H4 cells were triple labeled with β1-integrin monoclonal antibody, Oregon green phalloidin, and either FE65 or APP polyclonal antibodies. Lamellipodia were identified and chosen for imaging by their characteristic ruffled structure and actin meshwork. APP and β1-integrin colocalized in lamellipodia (Fig. 5, a–d). A similar pattern of lamellipodial staining was seen when FE65 antibody was used (Fig. 5, f–i), as expected from the APP/FE65 colocalization data presented above. Quantitative analysis by cross-correlation showed that for the labeling of APP and β1-integrin, as well as for APP and actin, the signals were correlated with no significant shift in the peak, indicating colocalization (Fig. 5 e). As expected from the colocalization of FE65 and APP, the FE65 signal also correlated well with the β1-integrin and actin signals (Fig. 5 j). The correlation tended to be better in the middle of the lamellipodia. The focal complexes may not extend to the flanks of the lamellipodia or there may be preferential colocalization in the direction of motion. These data support the idea that FE65 and APP interact in mobile lamellipodia and possibly at weak adhesion sites in those membranes.
Figure 5.
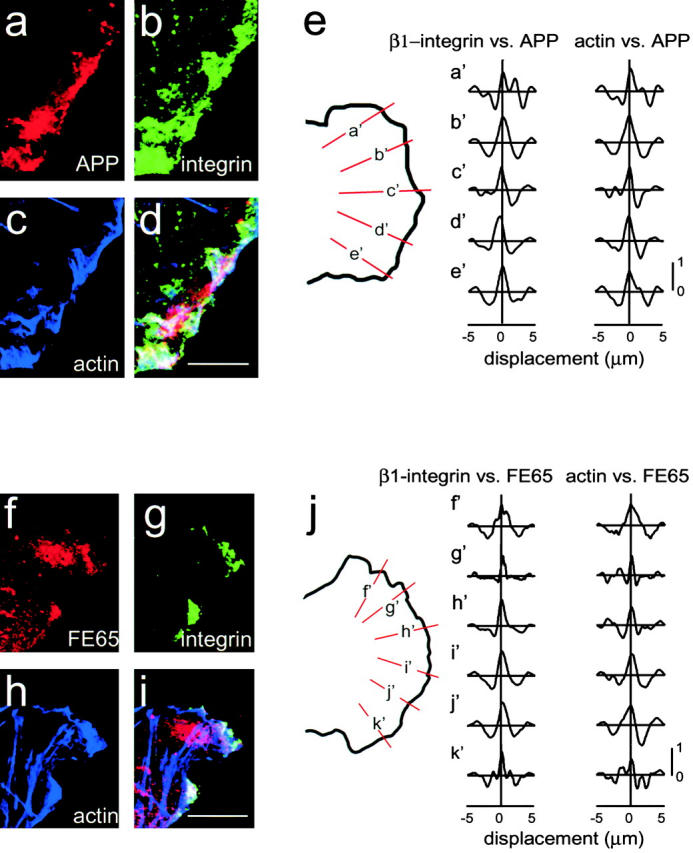
APP and FE65 colocalize with β1-integrins and actin in lamellipodia. (a–e) H4 cells triple labeled with APP polyclonal antibodies (a), β1-integrin monoclonal antibody (b), and Oregon green–conjugated phalloidin (c). Overlap of all three proteins is indicated by white in the overlay (d). (f–j) H4 cells triple labeled with FE65 polyclonal antibody (f), β1-integrin monoclonal antibody (g), and Oregon green–conjugated phalloidin (h). Overlap of all three proteins is indicated by white in the overlay (i). (e and j) Cross-correlation analysis of APP and FE65 colocalization with β1-integrins and actin in focal complexes. Lines were drawn perpendicular to the lamellipodial edge (shown in orange and labeled a′–e′ for APP and f′–k′ for FE65). The intensities were determined along each line and the cross-covariograms calculated as described in Materials and Methods. All of the cross-covariograms have peaks >0.5, indicating significant correlation. None have a displacement greater than the half-width at half-height, indicating colocalization. Bars, 10 μm.
Often when cells are torn from their substrates, the adhesion sites that had anchored the cell to the substrate are left behind. These adhesion sites contain many proteins associated with adhesion sites in intact cells. APP can be found in such sites (Yamazaki et al. 1997). To test if FE65 is also present in “cell-free” adhesion sites, we looked at H4 cells labeled with FE65 and β1-integrin antibodies. When the lamellipodia were peeled back from the surface on which the cells were grown, remaining adhesion sites were labeled with both β1-integrin antibody and FE65 antibody (Fig. 6, a and b). This is striking since FE65 is expected to be a soluble protein. To confirm previous reports about APP, remaining adhesion sites were also labeled with APP antibody (Fig. 6 c). To confirm the specificity of the association of FE65 with the APP-containing adhesion sites, an antibody against X11/Mint2, another cytoplasmic protein that can interact with the cytoplasmic tail of APP, was used in parallel. Adhesion sites that contained APP were not labeled by X11/Mint2 antibody (Fig. 6 d). These results further support the idea that an FE65–APP complex colocalizes with β1-integrins at focal complexes.
Figure 6.
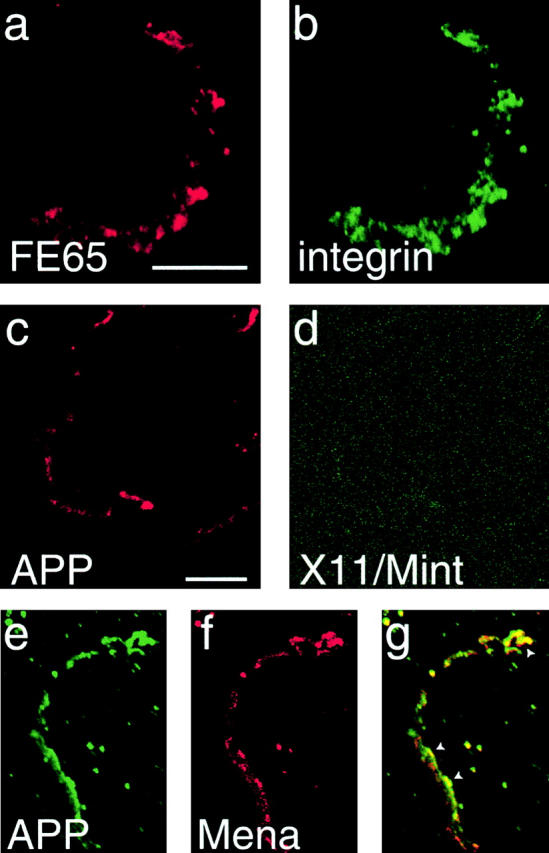
FE65 and APP remain associated with focal complexes left behind after detachment of lamellipodia. (a and b) H4 cells were labeled with polyclonal FE65 antibodies and monoclonal β1-integrin antibody. Where a lamellipodium was detached from the substrate, the integrin-dependent focal complex was left behind and labeled with both FE65 antibodies (a) and integrin antibody (b). (c and d) H4 cells were labeled with APP polyclonal antibody and X11/Mint monoclonal antibody. Where lamellipodia were detached from the substrate, adhesion sites left behind were labeled with APP antibody (c), but not with X11/Mint antibody (d). (e–g) Similar adhesion sites that are double labeled with APP monoclonal antibodies (e) and Mena polyclonal antibody (f). Overlap between APP and Mena in adhesion sites is indicated by yellow in the overlay (g). Arrowheads point to areas that contain high levels of both APP and Mena. Bars, 10 μm.
Finally, when cells were double labeled with monoclonal APP antibodies and polyclonal Mena antibody, APP and Mena could be found associated with the same cell-free adhesion sites (Fig. 6, e–g), supporting the hypothesis that FE65 and APP function in focal complexes as part of a Mena-containing macromolecular complex. The specific localization of APP and FE65 in focal complexes, but not focal adhesions, is especially interesting since no difference in the biochemical composition of these two structurally and functionally different adhesion structures has been reported previously (Rottner et al. 1999b).
FE65 and APP Accumulate at Edges of Lamellipodia Induced by Wounding
It was recently shown that Mena localizes to focal complexes in protruding lamellipodia (Rottner et al. 1999a). This suggests that FE65 and APP, possibly in a complex with Mena, might have a role in membrane extension. To test further if FE65 and APP could be involved in lamellipodial extension, we forced cells into a state in which the membranes were actively extending and examined APP and FE65 localization in the induced lamellipodia. To induce motility, confluent monolayers of MDCK cells stably transfected with both APP and FE65 were wounded with a pipette tip. In response to wounding, the cells along the edge of the wound first sent out lamellipodia then eventually migrated into the wound. We examined APP and FE65 localization in the wounded monolayers by immunofluorescence labeling with monoclonal APP antibodies and polyclonal FE65 antibodies. Confocal microscopy of monolayers fixed and labeled 2 h after wounding revealed that both proteins localize to the extending lamellipodia (Fig. 7, a–c). Within the lamellipodia, FE65 and APP colocalized in a thin line near the lamellipodial edge. Interestingly, not all of the lamellipodia induced by wounding were labeled with APP or FE65 antibodies, suggesting that these proteins are specifically found in lamellipodia in a particular functional state. This is in agreement with the published observations for Mena, which is found in protruding, but not retracting, lamellipodia (Rottner et al. 1999a). Importantly, immunofluorescent labeling of wounded MDCK cell monolayers with both APP and Mena antibodies suggested that induced lamellipodia that contained APP tended to also contain Mena (Fig. 7, g–i). This supports the idea that an FE65–APP complex localizes to mobile membranes and might function in regulation of membrane motility.
Figure 7.
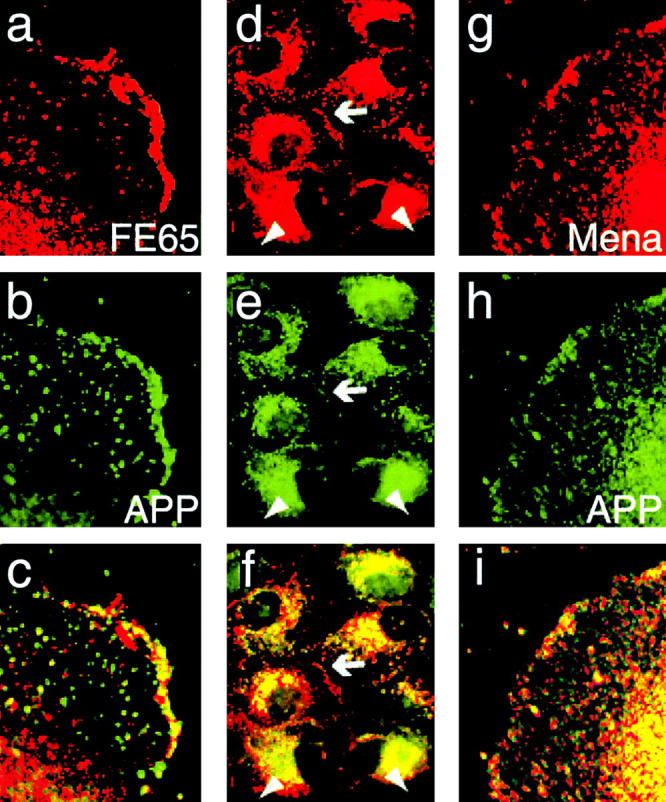
FE65 and APP colocalize in protruding lamellipodia induced by wounding. (a–c) Lamellipodial protrusion was induced by wounding a confluent monolayer of MDCK cells stably transfected with both APP and FE65. The monolayer was allowed to recover for 2 h, enough time to allow lamellipodial extension into the wound, then fixed and labeled with FE65 and APP antibodies. (a) High magnification image of an extending lamellipodium labeled with FE65 polyclonal antibodies. (b) The same lamellipodium labeled with APP monoclonal antibodies. (c) Overlay of the images in a and b. Colocalization of FE65 and APP is indicated by yellow in the overlay. APP and FE65 colocalize in a fine line at the edge of the extending lamellipodium, consistent with localization in focal complexes. (d–f) Lower magnification image of clusters of “resting” MDCK cells labeled with FE65 (d) and APP (e) antibodies. (f) Overlay of the images in panels d and e. APP and FE65 colocalize at cell–cell contacts (arrows) but not at the edges of the clusters (arrowheads). (g–i) High magnification image of an extending lamellipodium induced by wounding and labeled with Mena polyclonal antibodies (g) and APP monoclonal antibodies (h). Colocalization is indicated by yellow in the overlay (i).
To ensure that the protruding edges that contained APP and FE65 were lamellipodia induced by wounding and not just a general property common to all edges of MDCK cells, we labeled clusters of MDCK cells with APP and FE65 antibodies without induction of motility by wounding. Under these conditions, MDCK cells are “resting” and do not typically extend lamellipodia (Fenteany et al. 2000). APP and FE65 sometimes localized to cell–cell contact sites, but did not localize to the edges of the stationary clusters where there was no contact with other cells (Fig. 7, d–f).
Acceleration of Wound Healing by APP and FE65
Wound healing has been used previously as a measure of the rate of cell migration or movement (Rosen and Misfeldt 1980; Fenteany et al. 2000). In this context, the terms “cell migration” and “cell movement” are meant to encompass any of the mechanisms involved in such processes, including but not limited to actin dynamics, membrane dynamics, and adhesion. As an assay for the possible role of the APP–FE65 complex in cell movement, we measured the rate of closure of wounds formed as described above. We compared wound closure 1 d after wounding of confluent monolayers of wild-type MDCK cells, MDCK cells stably transfected with APP or FE65, and MDCK cells stably transfected with both APP and FE65. As a measure of wound closure, we scored wounds as fully closed, partially closed, or fully open (Table ). None of the wild-type wounds had closed after 1 d of wound healing (n = 25). With APP overexpression, 17% of the wounds were completely closed and 74% were partially closed after 1 d (n = 23). During the same period, 91% of the wounds were completely closed and 9% were partially closed when cells expressed both APP and FE65 (n = 57). Similar results were seen with additional stably overexpressing clonal cell lines. The effects of FE65 and APP together could not be accounted for by FE65 alone since overexpression of FE65, but not APP, yielded complete closure of only 20% of the wounds (n = 20). Thus, APP and FE65 accelerate wound closure, and the effects of either protein are greatly enhanced by additional overexpression of the other, consistent with a role for an APP–FE65 complex in regulation of cell movement.
Table 1.
Wound Closure after 1 d
| Fully closed | Partially closed | Fully open | Totals | |
|---|---|---|---|---|
| −APP/−FE65 | 0 | 0 | 25 | 25 |
| −APP/+FE65 | 4 | 6 | 10 | 20 |
| +APP/−FE65 | 4 | 17 | 2 | 23 |
| +APP/+FE65 | 52 | 5 | 0 | 57 |
| Totals | 60 | 28 | 37 | 125 |
Confluent monolayers of wild-type MDCK cells (−APP/−FE65), MDCK cells stably overexpressing APP (+APP/−FE65) or FE65 (−APP/+FE65), and MDCK cells stably overexpressing both APP and FE65 (+APP/+FE65), grown on grids, were wounded with a pipette tip. The wounds were allowed to heal for 1 d and then examined at grid intersections and scored as fully closed, partially closed, or fully open.
We then determined the rate of migration of cells along the wound edge. FE65 and APP overexpression increased the rate at which the wound edge cells traveled when compared with cells transfected with only APP (Fig. 8). By 15 h of recovery, the differences in the distance that the FE65 and APP overexpressing cells had traveled, compared with the APP overexpressing cells, reached significance (n = 18, P < 0.05 by ANOVA and Fisher's PLSD post hoc test). The morphology of the confluent cells and the cells at the wound edge was not noticeably changed upon transfection with APP or FE65 (data not shown). These data show that FE65 and APP increase the rate of migration of cells at the wound edge. Although we cannot distinguish between changes in the rate of movement due to altered adhesion, altered actin dynamics, or both, these data strongly support a role for an FE65–APP complex in regulation of cell movement.
Figure 8.
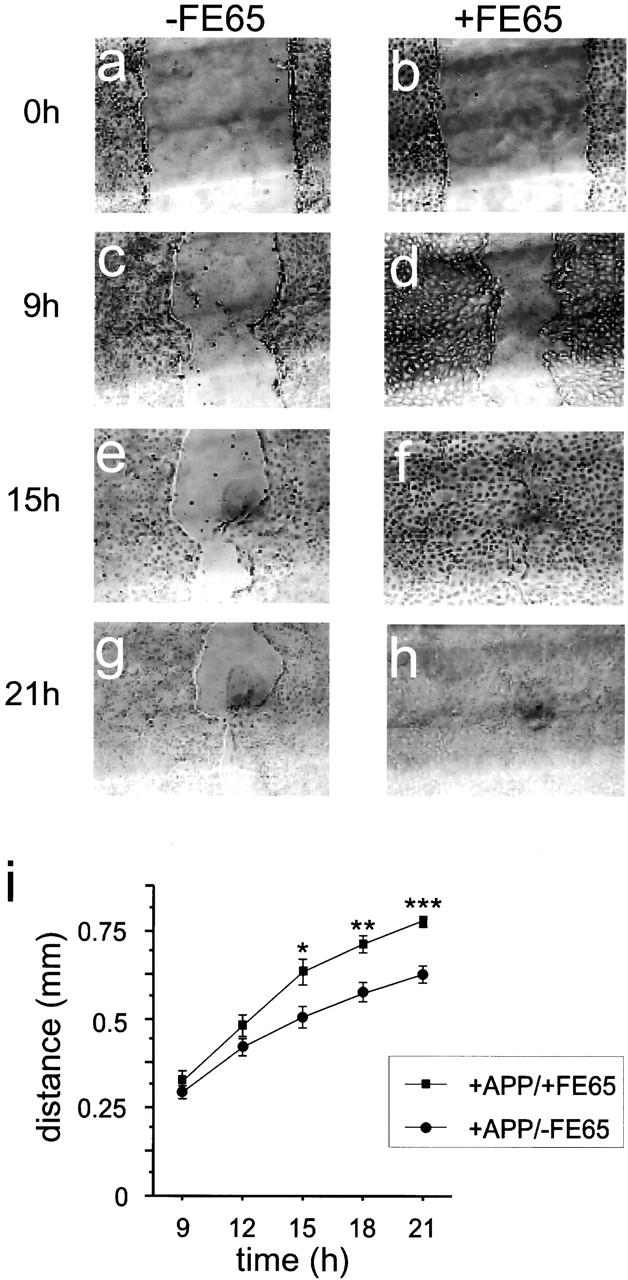
FE65 enhances the ability of APP to increase the rate of wound healing. Confluent monolayers of MDCK cells overexpressing APP (+APP/−FE65; a, c, e, and g) or both APP and FE65 (+APP/+FE65, b, d, f, and h) were grown on grids then wounded with a pipette tip. The wounds were photographed immediately after wounding (a and b). The same points on the grids were again photographed 9 (c and d), 12 (not shown), 15 (e and f), 18 (not shown), and 21 h (g and h) after wounding. (i) The distance the wound edges had traveled was determined at the same three points on each of six wounds. The data represent means ± SE. *P < 0.05, **P < 0.001, and **P < 0.0001 by ANOVA. Similar results were seen with two additional FE65 overexpressing clones.
Discussion
Here we provide compelling evidence that APP is involved in cell motility and that this function of APP is modulated by FE65. The APP-dependent increase in the rate of cell migration was enhanced upon FE65 overexpression. Moreover, APP and FE65 colocalized in adhesion sites of dynamic but not static membranes. Lending additional support for a role for an APP–FE65 complex in regulation of cell movement, we have recently found that APP and FE65 colocalize in another type of mobile membrane, the neuronal growth cone, in vivo (Sabo, S.L., A.F. Ikin, J.D. Buxbaum, P. Greengard, unpublished observations).
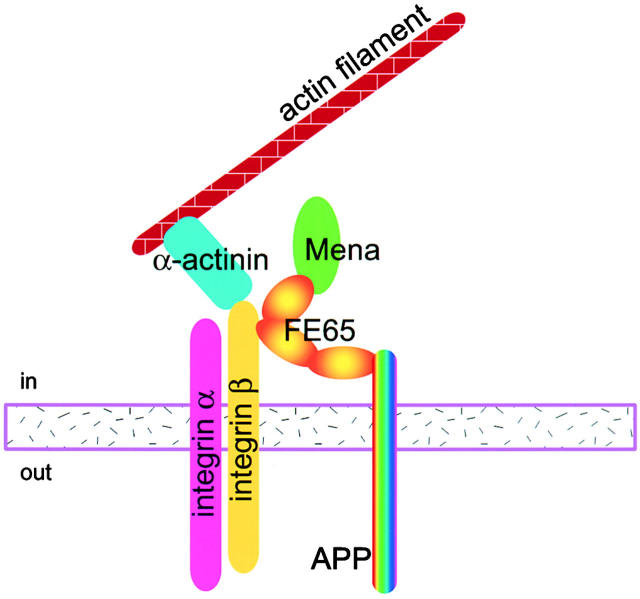
Based on the data presented here and on previously published results, we envision that APP and FE65 regulate lamellipodial motility as part of a larger macromolecular complex (Fig. 9). It has been shown previously that FE65 and Mena interact in cell lysates (Ermekova et al. 1998). We have shown here that FE65 and APP colocalize with Mena in lamellipodia and that both APP and Mena interact with FE65 simultaneously. In turn, Mena is thought to regulate the actin cytoskeleton through its interaction with profilin (Gertler et al. 1996; Lanier et al. 1999; Lanier and Gertler 2000). Interestingly, the FE65 and profilin binding sites in Mena overlap, suggesting that the APP–FE65 complex could negatively regulate the association of Mena with profilin. Integrins concentrate in lamellipodial adhesion sites (Hotchin and Hall 1995; Rottner et al. 1999b), where, as demonstrated here, they colocalize with APP and FE65. There are two NPXY motifs in the cytoplasmic domain of β1-integrins (Howland et al. 1995). It is possible that the more NH2-terminal PID of FE65 interacts with one of them. We do not yet know whether the various proteins of this macromolecular complex might interact simultaneously or sequentially to regulate cell movement.
Figure 9.
APP and FE65 probably regulate membrane motility as part of a larger macromolecular complex. The schematic depicts the possible components of the macromolecular complex and their putative interactions. FE65 might interact with APP through its COOH-terminal PID and with Mena through its WW domain. Mena modulates actin polymerization, possibly through its interaction with profilin, whereas integrins are indirectly linked to actin through a variety of integrin- and actin-binding proteins, such as α-actinin. Regulation of the various interactions shown could connect environmental cues to actin dynamics at the lamellipodial focal complex.
We propose that at least some of the effects of this APP- and FE65-containing macromolecular complex on cell movement are mediated by Mena. It has recently been shown that lamellipodial Mena regulates cell motility in fibroblasts (Bear et al. 2000). In contrast to the increase in cell movement caused by FE65 and APP, Mena decreases cell motility. In addition, Ena, the Drosophila homologue of Mena, negatively regulates growth cone migration through an interaction with Robo, a transmembrane receptor that mediates repulsion of axons at the midline (Bashaw et al. 2000). It is possible that Mena acts as a switch, either binding to Robo or to the FE65–APP complex: when Mena binds to Robo, motility is inhibited, but when conditions are shifted so that Mena associates with the FE65–APP complex rather than with Robo, the inhibition is released and motility increases. Because the negative regulation of motility by Mena was seen by Bear et al. 2000 with both overexpression and underexpression in lamellipodia, it is likely that in an “unstimulated” state Mena associates with a Robo-like receptor to negatively regulate motility, then, in response to some signal, Mena is recruited to the FE65–APP complex and away from the Robo-like receptor. Overexpression of FE65 and APP might increase the local concentration of the FE65–APP complex sufficiently to recruit some Mena from a Robo-like receptor to FE65–APP. We do not yet know which signals regulate the interaction between FE65 and Mena or FE65 and APP. If Mena only binds to FE65 when FE65 is complexed with APP, either interaction could serve as a source of regulation.
To move, cells need to anchor their motile membranes to the surface or matrix over which they crawl, but these anchors need to be more dynamic than the adhesion sites found in stationary membranes. We show that FE65 and APP colocalize with β1-integrins in relatively unstable focal complexes, but are not found in more stable focal adhesions. This is especially interesting since focal complexes can be precursors to focal adhesions (Rottner et al. 1999b), and until now there was, to our knowledge, no known difference in the biochemical composition of these two kinds of adhesion sites. The APP–FE65 complex may help destabilize adhesion sites. The effects of FE65 and APP on cell migration in the wound-healing assay might be due to changes in adhesion.
Since we have shown previously that FE65 increases the trafficking of APP to the cell surface (Sabo et al. 1999), we propose that FE65 regulates the role of APP in cell movement by targeting APP to the cell surface. APP might function there as a transmembrane molecule or as a secreted fragment. Conditioned medium from cells overexpressing APP did not significantly increase the rate of migration of the wound edges in the wound-healing assay (data not shown). Unfortunately, these experiments did not allow us to distinguish between these two mechanisms, since the local concentration of secreted APP fragments at the lamellipodia might not be as high during bathing in conditioned medium as it is when APP fragments are secreted directly from the wound edge cells.
There is some evidence to support the idea that the role of APP and FE65 in regulation of cell movement may be directly related to the proteolytic processing of APP. We have shown previously that FE65 increases the proteolytic processing of APP (Sabo et al. 1999). In addition, TACE, an ADAM family protease, cleaves APP at the cell surface in response to PKC activity (Buxbaum et al. 1998), which is also known to stimulate cell motility. Integrin activity is similarly regulated by proteolytic processing of its α-subunits (Delwel et al. 1996), probably by ADAM family proteases (Chen et al. 1999). Secreted APP fragments could compete with extracellular matrix ligands for binding to integrins, or to an APP ligand, decreasing the strength of an adhesion site and promoting motility. It has been shown that exogenous application of APP fragments can increase neurite outgrowth (Koo et al. 1993; Jin et al. 1994; Perez et al. 1997). Moreover, the most abundant secreted APP fragments contain a sequence, RHDS, that resembles the integrin binding site on the β1-integrin extracellular matrix ligand, fibronectin (Sabo et al. 1995), and, therefore, can potentially interact with integrins through this sequence. It is interesting to note that presenilins, which also regulate the proteolytic processing of APP (Haass and De Strooper 1999), have recently been found at the surface of lamellipodia (Schwarzman et al. 1999).
Recently, there has been great interest in proteins that serve dual functions in regulation of actin-based membrane motility and in transport of proteins to or from the plasma membrane. For example, members of the Rho family of small GTP-binding proteins regulate cell motility and are involved in regulation of the endocytic pathway (Lamaze et al. 1996; Kroschewski et al. 1999; Merrifield et al. 1999). In addition, both myosin II, an actin-based motor protein, and cdc42, activation of which induces formation of filopodial actin spikes, have been shown to regulate secretory trafficking (Musch et al. 1997; Kroschewski et al. 1999). In addition to its role in actin-based membrane motility, FE65 regulates the transport of APP to the plasma membrane (Sabo et al. 1999). This places FE65 in the growing family of dual function proteins.
Acknowledgments
We thank Dr. Michael Sceniak for inspiring discussions on cross-correlation. We also thank Drs. Edward H. Koo and Frank Gertler for antibodies.
This work was supported by United States Public Health Service grant AG09464 (to P. Greengard), National Institutes of Health training grant GM07524 (to S.L. Sabo) and a Rockefeller University fellowship (to S.L. Sabo).
Footnotes
Abbreviations used in this paper: APP, amyloid precursor protein; NGS, normal goat serum; PID, protein interaction domain.
References
- Bashaw G.J., Kidd T., Murray D., Pawson T., Goodman C.S. Repulsive axon guidanceabelson and enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. doi: 10.1016/s0092-8674(00)80883-1. [DOI] [PubMed] [Google Scholar]
- Bear J.E., Loureiro J.J., Libova I., Fassler R., Wehland J., Gertler F.B. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101:717–728. doi: 10.1016/s0092-8674(00)80884-3. [DOI] [PubMed] [Google Scholar]
- Bendat J.S., Piersol A.G. Random DataAnalysis and Measurement Procedures 1971. John Wiley and Sons; New York: pp. 332 [Google Scholar]
- Bokoch G., Bohl B., Chuang T.-H. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J. Biol. Chem. 1994;269:31674–31679. [PubMed] [Google Scholar]
- Borg J.P., Ooi J., Levy E., Margolis B. The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol. Cell. Biol. 1996;16:6229–6241. doi: 10.1128/mcb.16.11.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler S.L., Gray M.D., Sopher B.L., Hu Q., Hearn M.G., Pham D.G., Dinulos M.B., Fukuchi K., Sisodia S.S., Miller M.A., Disteche C.M., Martin G.M. cDNA cloning and chromosome mapping of the human Fe65 geneinteraction of the conserved cytoplasmic domains of the human β-amyloid precursor protein and its homologues with the mouse Fe65 protein. Hum. Mol. Genet. 1996;5:1589–1598. doi: 10.1093/hmg/5.10.1589. [DOI] [PubMed] [Google Scholar]
- Brody C. Correlations without synchrony Neural Comput. 11 1999. 1537 1551a [DOI] [PubMed] [Google Scholar]
- Brody C. Disambiguating different covariation types Neural Comput. 11 1999. 1527 1535b [DOI] [PubMed] [Google Scholar]
- Buxbaum J.D., Liu K.N., Luo Y., Slack J.L., Stocking K.L., Peschon J.J., Johnson R.S., Castner B.J., Cerretti D.P., Black R.A. Evidence that tumor necrosis factor α converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- Chen M.S., Almeida E.A., Huovila A.P., Takahashi Y., Shaw L.M., Mercurio A.M., White J.M. Evidence that distinct states of the integrin α6β1 interact with laminin and an ADAM. J. Cell Biol. 1999;144:549–561. doi: 10.1083/jcb.144.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel G.O., Hogervorst F., Sonnenberg A. Cleavage of the α6A subunit is essential for activation of the α6Aβ1 integrin by phorbol 12-myristate 13-acetate. J. Biol. Chem. 1996;271:7293–7296. doi: 10.1074/jbc.271.13.7293. [DOI] [PubMed] [Google Scholar]
- Duilio A., Faraonio R., Minopoli G., Zambrano N., Russo T. Fe65L2a new member of the Fe65 protein family interacting with the intracellular domain of the Alzheimer's β-amyloid precursor protein. Biochem. J. 1998;330:513–519. doi: 10.1042/bj3300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermekova K.S., Zambrano N., Linn H., Minopoli G., Gertler F., Russo T., Sudol M. The WW domain of neural protein FE65 interacts with proline-rich motifs in Mena, the mammalian homolog of Drosophila enabled. J. Biol. Chem. 1997;272:32869–32877. doi: 10.1074/jbc.272.52.32869. [DOI] [PubMed] [Google Scholar]
- Ermekova K.S., Chang A., Zambrano N., de Candia P., Russo T., Sudol M. Proteins implicated in Alzheimer disease. The role of FE65, a new adapter which binds to β-amyloid precursor protein. Adv. Exp. Med. Biol. 1998;446:161–180. [PubMed] [Google Scholar]
- Fenteany G., Janmey P.A., Stossel T.P. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr. Biol. 2000;10:831–838. doi: 10.1016/s0960-9822(00)00579-0. [DOI] [PubMed] [Google Scholar]
- Fiore F., Zambrano N., Minopoli G., Donini V., Duilio A., Russo T. The regions of the Fe65 protein homologous to the phosphotyrosine interaction/phosphotyrosine binding domain of Shc bind the intracellular domain of the Alzheimer's amyloid precursor protein. J. Biol. Chem. 1995;270:30853–30856. doi: 10.1074/jbc.270.52.30853. [DOI] [PubMed] [Google Scholar]
- Gertler F.B., Doctor J.S., Hoffmann F.M. Genetic suppression of mutations in the Drosophila abl proto-oncogene homolog. Science. 1990;248:857–860. doi: 10.1126/science.2188361. [DOI] [PubMed] [Google Scholar]
- Gertler F.B., Comer A.R., Juang J.L., Ahern S.M., Clark M.J., Liebl E.C., Hoffmann F.M. Enabled, a dosage-sensitive suppressor of mutations in the Drosophila Abl tyrosine kinase, encodes an Abl substrate with SH3 domain-binding properties. Genes Dev. 1995;9:521–533. doi: 10.1101/gad.9.5.521. [DOI] [PubMed] [Google Scholar]
- Gertler F.B., Niebuhr K., Reinhard M., Wehland J., Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- Guenette S.Y., Chen J., Jondro P.D., Tanzi R.E. Association of a novel human FE65-like protein with the cytoplasmic domain of the β-amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 1996;93:10832–10837. doi: 10.1073/pnas.93.20.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenette S.Y., Chen J., Ferland A., Haass C., Capell A., Tanzi R.E. hFE65L influences amyloid precursor protein maturation and secretion. J. Neurochem. 1999;73:985–993. doi: 10.1046/j.1471-4159.1999.0730985.x. [DOI] [PubMed] [Google Scholar]
- Haass C., De Strooper B. The presenilins in Alzheimer's disease - proteolysis holds the key. Science. 1999;286:916–919. doi: 10.1126/science.286.5441.916. [DOI] [PubMed] [Google Scholar]
- Haass C., Koo E.H., Teplow D.B., Selkoe D.J. Polarized secretion of β-amyloid precursor protein and amyloid β-peptide in MDCK cells. Proc. Natl. Acad. Sci. USA. 1994;91:1564–1568. doi: 10.1073/pnas.91.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin N.A., Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J. Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland D.S., Savage M.J., Huntress F.A., Wallace R.E., Schwartz D.A., Loh T., Melloni R.H.J., DeGennaro L.J., Greenberg B.D., Siman R. Mutant and native human β-amyloid precursor proteins in transgenic mouse brain. Neurobiol. Aging. 1995;16:685–699. doi: 10.1016/0197-4580(95)00078-s. [DOI] [PubMed] [Google Scholar]
- Jin L.W., Ninomiya H., Roch J.M., Schubert D., Masliah E., Otero D.A., Saitoh T. Peptides containing the RERMS sequence of amyloid β/A4 protein precursor bind cell surface and promote neurite extension. J. Neurosci. 1994;14:5461–5470. doi: 10.1523/JNEUROSCI.14-09-05461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H.G., Unterbeck A., Salbaum J.M., Masters C.L., Grzeschik K.H., Multhaup G., Beyreuther K., Muller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Koo E.H., Park L., Selkoe D.J. Amyloid β-protein as a substrate interacts with extracellular matrix to promote neurite outgrowth. Proc. Natl. Acad. Sci. USA. 1993;90:4748–4752. doi: 10.1073/pnas.90.10.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R., Hall A., Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat. Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- Lamaze C., Chuang T.H., Terlecky L.J., Bokoch G.M., Schmid S.L. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Lanier L.M., Gertler F.B. From Abl to actinAbl tyrosine kinase and associated proteins in growth cone motility. Curr. Opin. Neurobiol. 2000;10:80–87. doi: 10.1016/s0959-4388(99)00058-6. [DOI] [PubMed] [Google Scholar]
- Lanier L.M., Gates M.A., Witke W., Menzies A.S., Wehman A.M., Macklis J.D., Kwiatkowski D., Soriano P., Gertler F.B. Mena is required for neurulation and commissure formation. Neuron. 1999;22:313–325. doi: 10.1016/s0896-6273(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Mattson M.P. Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- Mattson M.P., Cheng B., Culwell A.R., Esch F.S., Lieberburg I., Rydel R.E. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the β-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- Merrifield C.J., Moss S.E., Ballestrem C., Imhof B.A., Giese G., Wunderlich I., Almers W. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat. Cell Biol. 1999;1:72–74. doi: 10.1038/9048. [DOI] [PubMed] [Google Scholar]
- Milward E.A., Papadopoulos R., Fuller S.J., Moir R.D., Small D., Beyreuther K., Masters C.L. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- Musch A., Cohen D., Rodriguez-Boulan E. Myosin II is involved in the production of constitutive transport vesicles from the TGN. J. Cell Biol. 1997;138:291–306. doi: 10.1083/jcb.138.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Oppenheim A.V., Schafer R.W. Digital Signal Processing 1975. Prentice Hall, ; Englewood Cliffs, NJ: pp. 585 [Google Scholar]
- Perez R.G., Zheng H., Van der Ploeg L.H., Koo E.H. The β-amyloid precursor protein of Alzheimer's disease enhances neuron viability and modulates neuronal polarity. J. Neurosci. 1997;17:9407–9414. doi: 10.1523/JNEUROSCI.17-24-09407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen P., Misfeldt D.S. Cell density determines epithelial migration in culture. Proc. Natl. Acad. Sci. USA. 1980;77:4760–4763. doi: 10.1073/pnas.77.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K., Behrendt B., Small J.V., Wehland J. VASP dynamics during lamellipodia protrusion Nat. Cell Biol 1 1999. 321 322a [DOI] [PubMed] [Google Scholar]
- Rottner K., Hall A., Small J.V. Interplay between Rac and Rho in the control of substrate contact dynamics Curr. Biol. 9 1999. 640 648b [DOI] [PubMed] [Google Scholar]
- Sabo S., Lambert M.P., Kessey K., Wade W., Krafft G., Klein W.L. Interaction of β-amyloid peptides with integrins in a human nerve cell line. Neurosci. Lett. 1995;184:25–28. doi: 10.1016/0304-3940(94)11159-g. [DOI] [PubMed] [Google Scholar]
- Sabo S.L., Lanier L.M., Ikin A.F., Khorkova O., Sahasrabudhe S., Greengard P., Buxbaum J.D. Regulation of β-amyloid secretion by FE65, an amyloid protein precursor-binding protein. J. Biol. Chem. 1999;274:7952–7957. doi: 10.1074/jbc.274.12.7952. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Sundsmo M., Roch J.M., Kimura N., Cole G., Schubert D., Oltersdorf T., Schenk D.B. Secreted form of amyloid β protein precursor is involved in the growth regulation of fibroblasts. Cell. 1989;58:615–622. doi: 10.1016/0092-8674(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Schubert D., Jin L.W., Saitoh T., Cole G. The regulation of amyloid β protein precursor secretion and its modulatory role in cell adhesion. Neuron. 1989;3:689–694. doi: 10.1016/0896-6273(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Schwarzman A.L., Singh N., Tsiper M., Gregori L., Dranovsky A., Vitek M.P., Glabe C.G., St P.H., George-Hyslop, Goldgaber D. Endogenous presenilin 1 redistributes to the surface of lamellipodia upon adhesion of Jurkat cells to a collagen matrix. Proc. Natl. Acad. Sci. USA. 1999;96:7932–7937. doi: 10.1073/pnas.96.14.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D.H., Nurcombe V., Reed G., Clarris H., Moir R., Beyreuther K., Masters C.L. A heparin-binding domain in the amyloid protein precursor of Alzheimer's disease is involved in the regulation of neurite outgrowth. J. Neurosci. 1994;14:2117–2127. doi: 10.1523/JNEUROSCI.14-04-02117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi H., Tabira T. Molecular cloning of human Fe65L2 and its interaction with the Alzheimer's β-amyloid precursor protein. Neurosci. Lett. 1999;261:143–146. doi: 10.1016/s0304-3940(98)00995-1. [DOI] [PubMed] [Google Scholar]
- Wu H., Parsons J.T. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J. Cell. Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T., Koo E.H., Selkoe D.J. Cell surface amyloid β-protein precursor colocalizes with β 1 integrins at substrate contact sites in neural cells. J. Neurosci. 1997;17:1004–1010. doi: 10.1523/JNEUROSCI.17-03-01004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano N., Buxbaum J.D., Minopoli G., Fiore F., de Candia P., De Renzis S., Faraonio R., Sabo S., Cheetham J., Sudol M., Russo T. Interaction of the phosphotyrosine interaction/phosphotyrosine binding-related domains of Fe65 with wild-type and mutant Alzheimer's β-amyloid precursor proteins. J. Biol. Chem. 1997;272:6399–6405. doi: 10.1074/jbc.272.10.6399. [DOI] [PubMed] [Google Scholar]