Abstract
Cell adhesion to the extracellular matrix (ECM) is a requirement for proliferation that is typically lost in malignant cells. In the absence of adhesion, nontransformed cells arrest in G1 with increased levels of the cyclin-dependent kinase inhibitor p27. We have reported previously that the degradation of p27 requires its phosphorylation on Thr-187 and is mediated by Skp2, an F-box protein that associates with Skp1, Cul1, and Roc1/Rbx1 to form the SCFSkp2 ubiquitin ligase complex. Here, we show that the accumulation of Skp2 protein is dependent on both cell adhesion and growth factors but that the induction of Skp2 mRNA is exclusively dependent on cell adhesion to the ECM. Conversely, the expression of the other three subunits of the SCFSkp2 complex is independent of cell anchorage. Phosphorylation of p27 on Thr-187 is also not affected significantly by the loss of cell adhesion, demonstrating that increased p27 stability is not dependent on p27 dephosphorylation. Significantly, ectopic expression of Skp2 in nonadherent G1 cells resulted in p27 downregulation, entry into S phase, and cell division. The ability to induce adhesion-independent cell cycle progression was potentiated by coexpressing Skp2 with cyclin D1 but not with cyclin E, indicating that Skp2 and cyclin D1 cooperate to rescue proliferation in suspension cells. Our study shows that Skp2 is a key target of ECM signaling that controls cell proliferation.
Keywords: F-box protein, SCF, Skp2, p27, cell adhesion control
Introduction
Protein degradation by the ubiquitin-proteasome pathway plays a fundamental role in the regulation of the eukaryotic cell cycle (for reviews see Deshaies 1999; Koepp et al. 1999). Proteolysis of many G1 regulatory proteins is mediated by SCF ubiquitin ligases, each composed of four major subunits, Skp1, Cul1, Rbx1/Roc1, and one of many F-box proteins (Fbps). There are 11 Fbps in budding yeast, 22 in Drosophila (Rubin et al. 2000), and ≥38 in humans (Cenciarelli et al. 1999; Winston et al. 1999). Fbps contain an F-box, an ∼40–amino acid motif that is necessary for the interaction of Fbps with Skp1 (for review see Kipreos and Pagano 2000). Fbps also contain additional protein–protein interaction domains such as leucine-rich repeats and WD-40 domains that are involved in the recognition of phosphorylated substrates. Thus, the substrate specificity of SCFs is dictated by distinct Fbps that target proteins for ubiquitin-mediated degradation.
The cyclin-dependent kinase (Cdk) inhibitor p27 negatively regulates cyclin E–Cdk2 and cyclin A–Cdk2 complexes, two kinases necessary for DNA replication. We have reported previously that upon mitogenic stimulation p27 is degraded through the ubiquitin-proteasome pathway (Pagano et al. 1995) and that the ubiquitinylation of p27 is dependent on its Cdk2-mediated phosphorylation and is stimulated by its association to cyclin E–Cdk2 or cyclin A–Cdk2 complexes (Montagnoli et al. 1999). Accordingly, in vivo and in vitro degradation of p27 requires its phosphorylation on Thr-187 by Cdk2 (Sheaff et al. 1997; Vlach et al. 1997; Nguyen et al. 1999). Skp2 is an Fbp first identified together with Skp1 as interactors of the cyclin A–Cdk2 complex, hence the name S phase kinase-associated proteins (Skps). Stimulation of quiescent cells with growth factors induces the expression of Skp2 in late G1 (Carrano et al. 1999; Marti et al. 1999). This induction occurs through a stabilization of Skp2 protein but without activating SKP2 gene transcription (Wirbelauer et al. 2000). We and others have demonstrated that Skp2 is necessary for the ubiquitinylation and subsequent degradation of p27 both in vivo (Carrano et al. 1999; Sutterluty et al. 1999) and in vitro (Carrano et al. 1999; Tsvetkov et al. 1999). In agreement with these findings, interference of Skp2 function by microinjection of an anti-Skp2 antibody into living adherent cells resulted in an inhibition of the entry into S phase (Zhang et al. 1995). Skp2-deficient mice are smaller than littermate controls, and Skp2−/− cells exhibit high levels of p27 and free cyclin E (not bound to Cdk2), polyploidy, and multiple centrosomes (Nakayama et al. 2000). In addition to studies demonstrating that Skp2 is a major player in the degradation of p27 bound to Cdk complexes, there is also evidence of a p27 translational control (Hengst and Reed 1996) and a regulation of p27 subcellular localization (Tomoda et al. 1999).
Cell adhesion to the extracellular matrix (ECM) is required for cell cycle progression through the G1 phase. The cooperative action of cell adhesion and growth factors results in the upregulation of cyclins and the downregulation of Cdk inhibitors, permitting cells to pass through the G1 restriction point and complete the cell cycle (for reviews see Assoian 1997; Giancotti and Ruoslahti 1999). It has been reported that the loss of substratum attachment in fibroblasts induces a stabilization of p27 and an increased association of p27 to Cdk2 with a subsequent decrease in its kinase activity (Fang et al. 1996; Schulze et al. 1996; Zhu et al. 1996; Kawada et al. 1997; Resnitzky 1997). Ultimately, the inhibition of Cdk2 activity results in a G1 arrest. Similar findings were reported for epithelial, arterial smooth muscle, and endothelial cells that were prevented to bind the ECM (Croix et al. 1996; Koyama et al. 1996; Huang et al. 1998). Thus, p27 accumulation plays an important role in inducing a G1 block in response to loss of adhesion. Yet, the mechanism through which p27 accumulates in nonadherent cells is not understood.
The relationship between cell adhesion and cell cycle, particularly the regulation of p27 abundance by ECM–dependent signals, prompted us to test the hypothesis that Skp2 plays a role in this process. The results of these studies are herein presented.
Materials and Methods
Cell Cultures, Cell Synchronization, and Cell Cycle Analysis
IMR-90 cells are nontransformed nonimmortalized human diploid lung fibroblasts. After culturing for 2–3 d in the presence of 0.2% serum, 95% of the cells showed a 2n DNA content by flow cytometry, and <3% of the IMR-90 cell population incorporated BrdU during a 24-h incubation period. IMR-90 cells were obtained from American Type Culture Collection at PDL (population doubling) 20 and grown in DME plus 10% FBS for ≤40 total PDLs. Cells were counted at each passage, and the increase in PDL was calculated as the log2-fold increase in cell number. Rat-6 cells are a clonal line of rat embryo fibroblasts (Krauss et al. 1992). These cells are adhesion-dependent nontumorigenic in nude mice and compared with the widely used Rat-1 cells produce fewer colonies in soft agar and when transfected with activated Ras. Rat-6 cells were cultured in DME plus 5% calf serum. After culturing for 2–3 d in the presence of 0.2% serum, 90% of the cells showed a 2n DNA content by flow cytometry, and ∼10% of the Rat-6 cell population incorporated BrdU during a 24-h incubation period. This 10% background was subtracted in all analyses of BrdU incorporation to highlight the differences observed under different adhesion conditions (see below). Umbilical vein endothelial cells were obtained from Clonetics Corp. and cultured as described (Tam et al. 1994) for four passages or less (passaged at a 1:4 ratio). Hs913T, 293T, HeLa, and U2-OS cells were obtained from American Type Culture Collection. Rat-6, IMR-90, and endothelial cells were synchronized in G0/G1 by serum starvation for 48 h. To test the effects of lack of adhesion to the ECM, quiescent cells were trypsinized and reseeded on tissue culture–coated Petri dishes (adherent) or Petri dishes coated with 1% agarose (suspension) using 0.5–1 × 106 cells per 10-cm dish. To study the relative effects of fibronectin and growth factors, trypsinized quiescent cells were replated in the presence of a cocktail of purified growth factors (10 ng/ml EGF, 10 ng/ml PDGF, and 10 mg/μl insulin) on tissue culture–coated dishes coated with fibronectin or poly-l-lysine as described (Zhu et al. 1999). Colony formation in soft agar was done as described (Guadagno et al. 1993). For cell cycle analyses, adherent cells were grown on glass coverslips, labeled for 3 h with 10 μm BrdU, rinsed with PBS, and fixed for 10 min in −20°C methanol–acetone (1:1). Fixed cells were rehydrated in PBS at room temperature and processed for cell staining. Suspended cells were also labeled for 3 h with 10 μm BrdU, but during the last 30 min of incubation cells were collected and pulled through a syringe to break up cell clumps. Cells were then plated on glass coverslips that had been coated (4 h at room temperature) with poly-l-lysine (5 μg/ml in PBS; Sigma-Aldrich) for the remaining 20 min and finally fixed as described above. Cells were stained for BrdU as described previously (Ohtsubo et al. 1995) and counterstained with Hoechst to identify all nuclei. The percentage of BrdU-labeled cells (BrdU-positive cells/Hoechst-positive cells × 100) was quantified using a fluorescence microscope. At least 300 cells were counted for each sample, and each experiment was performed at least four times.
Retroviral-mediated Gene Transfer
Packaging GP-293 cells (CLONTECH Laboratories, Inc.) were transfected with retroviral plasmids according to the manufacturer's instructions. 48 h after transfection, the virus-containing medium was collected and supplemented with 8 μg/ml polybrene (Sigma-Aldrich). Then, the culture medium of the target cells was replaced with this viral supernatant for 24 h. This infection process was repeated a second time after a 12-h recovery in normal medium. The percentage of infected cells was quantified by flow cytometry or immunofluorescence. In all cases, >85% of the cells were infected. Multiple genes were introduced sequentially.
Cycloheximide Treatment and Pulse–Chase Analysis
To measure protein half-lives, cells were incubated in the presence of 100 μg/ml cycloheximide (Chx) (Sigma-Aldrich) diluted in DME. Pulse–chase analysis was performed as described (Pagano et al. 1995) with the only difference being that after a 30-min starvation cells were labeled for 30 min with 200 μCi/ml of [35S]methionine and [35S]cysteine (51006; ICN Biomedicals) and chased with medium for the indicated times.
Immunoreagents, Extract Preparation, Immunoblot Analysis, and Kinase Assay
Mouse monoclonal antibodies (Mabs) to Skp2 were produced using bacterial Skp2 produced and purified as described (Schulman et al. 2000). Mabs to Skp2 (Latres et al. 2001), cyclin D1 (Lukas et al. 1994), Ubc3 (Latres et al. 1999), cyclin E (Faha et al. 1993), and rabbit polyclonal antibodies to Skp2 (Carrano et al. 1999), Cul1 (Latres et al. 1999), cyclin A (Pagano et al. 1992), cyclin B (Pagano et al. 1992), cyclin D1 (Lukas et al. 1994), Roc1 (Ohta et al. 1999), and Thr-187 phospho-site p27-specific antibody (Carrano et al. 1999; Montagnoli et al. 1999) were described previously. Mabs to p21 (C24420), p27 (K25020), and Skp1 (P46020) were from Transduction Labs. Mab to Rb (14001A) was from Pharmingen, and Mab to E2F-1 was from Santa Cruz Biotechnology, Inc. (sc-251). Rabbit antibody to cyclin E (sc-481) was from Santa Cruz Biotechnology, Inc. Protein extraction and immunoblot analysis were performed as described (Carrano et al. 1999). Histone H1 kinase reactions were performed as described (Pagano et al. 1992).
Northern Blot Analysis
Total RNA was extracted using Trizol reagent (GIBCO BRL) according to the manufacturer's instructions. For Northern blots, 15 μg of total RNA was loaded per lane and fractionated in a 1% agarose/formaldehyde gel. After transfer onto Hybond N+ membrane (Amersham Pharmacia Biotech), blots were fixed by UV cross-linking and hybridized with a 32P probe specific for human Skp2. A probe specific for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to confirm equal loading. To measure mRNA half-lives, cells were incubated in the presence of 1 μg/ml actinomycin D (Act D) diluted in DME.
Results
Adhesion-dependent Expression of Skp2
We determined the requirement of cell adhesion for the expression of SCFSkp2 subunits and Ubc3, the ubiquitin conjugating enzyme working in concert with the SCFSkp2 complex, in nontransformed nonimmortalized human diploid IMR-90 fibroblasts and in immortalized Rat-6 fibroblasts (Fig. 1). Monolayer cultures of these adhesion-dependent fibroblasts were arrested in G0/G1 by serum deprivation, trypsinized, and replated in the presence of serum either in suspension or in monolayer. Both in Rat-6 fibroblasts and human diploid IMR-90 fibroblasts, Skp2 but not Cul1, Skp1, Roc1, and Ubc3 failed to accumulate in nonadherent conditions. As reported previously, nonadherent cells failed to degrade p27 and to express cyclin A, cyclin D1, and p21, whereas cyclin E expression was not dependent on adhesion (Guadagno et al. 1993; Kang and Krauss 1996; Zhu et al. 1996; Bottazzi et al. 2000). Similarly, quiescent IMR-90 fibroblasts and quiescent normal human endothelial cells stimulated with a cocktail of purified growth factors expressed Skp2 when plated on fibronectin-coated dishes but not on dishes coated with poly-l-lysine, a substratum that does not allow cell adhesion-dependent signaling (data not shown). These results indicate that Skp2 is a general target of ECM signals that control the G1–S transition in nontransformed cells.
Figure 1.
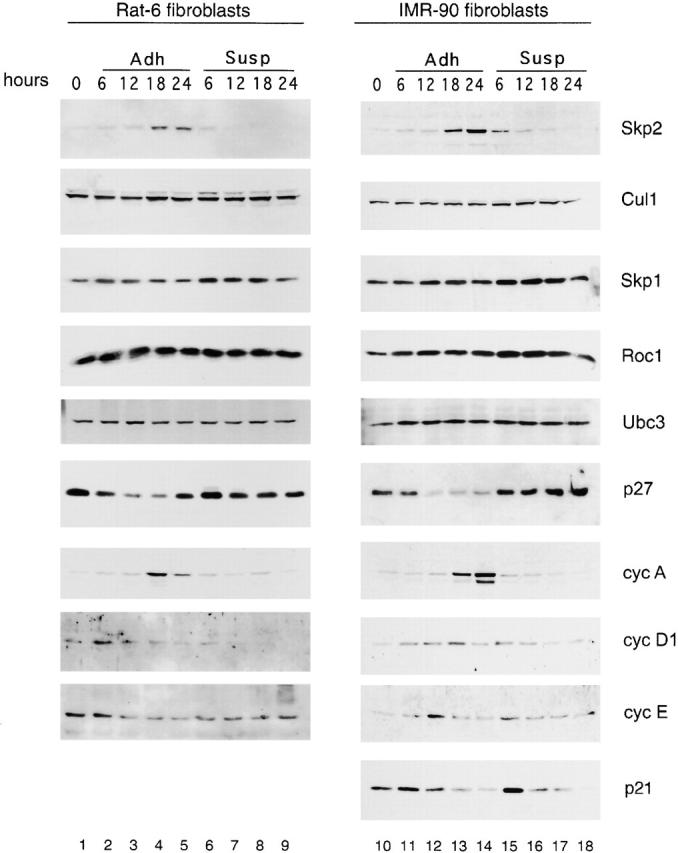
Skp2 expression is cell adhesion dependent. Rat-6 fibroblasts (lanes 1–9) and IMR-90 (lanes 10–18) fibroblasts were synchronized in G0/G1 by serum deprivation (lanes 1 and 10; indicated as 0 hours), trypsinized, and then replated in the presence of serum either in monolayer (adherent [Adh]; lanes 2–5 and 11–14) or in suspension (Susp; lanes 6–9 and 15–18). Cells were collected at the indicated times. Protein extracts were analyzed by immunoblot with antibodies to the indicated proteins. Rat-6 and IMR-90 showed similar profiles of protein expression under both adhesion and suspension conditions with the exception that p21 was not expressed in Rat-6 (bottom).
Enforced Expression of Skp2 Allows Entry into S Phase, p27 Downregulation, and Cell Division in the Absence of Adhesion
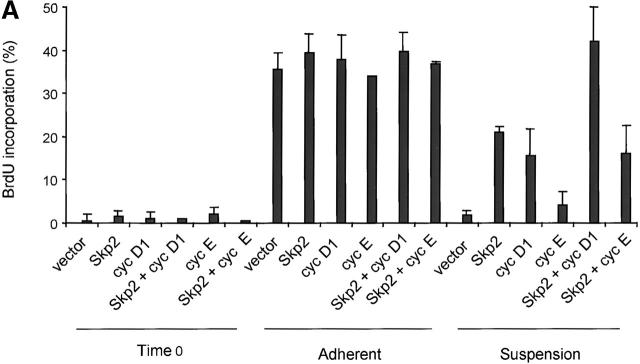
To determine if the regulation of Skp2 expression was causally linked to the block in G1 observed in nonadherent cells, we studied the phenotype of fibroblasts constitutively expressing Skp2 and/or G1 cyclins from exogenous cDNAs. To express these proteins in Rat-6 fibroblasts, we used retroviral expression vectors and found that Skp2, cyclin D1, and cyclin E were all expressed in an adhesion-independent manner at levels two- to fourfold higher than those of the respective endogenous proteins (data not shown). Infected fibroblasts were synchronized in G0/G1 and then replated for 12 h in the presence of serum either in suspension or in monolayer. The percentage of cells in S phase was then analyzed by BrdU incorporation (Fig. 2 A). Adherent cells infected with either Skp2 or G1 cyclins were still dependent on serum growth factors for BrdU incorporation similarly to noninfected cells, indicating that enforced expression of these proteins did not produce a general alteration of cell cycle progression. In contrast, significant differences were observed in nonadherent cultures: compared with control cells, a significant increase in BrdU incorporation was observed in Skp2-expressing cells. Similar results were obtained with cyclin D1-expressing cells, in agreement with previously published results (Zhu et al. 1996; Resnitzky 1997). Interestingly, coexpression of Skp2 and cyclin D1 induced a further increase in cells entering S phase to a percentage comparable to that of cells plated in adhesion. In contrast, cyclin E expression was unable to increase the number of BrdU-positive cells or to enhance the effect of Skp2 when the two proteins were coexpressed. These results show that the enforced expression of Skp2 allows growth in the absence of substratum adhesion and that Skp2 and cyclin D1 cooperate to fully rescue entry into S phase in nonadherent Rat-6 cells.
Figure 2.
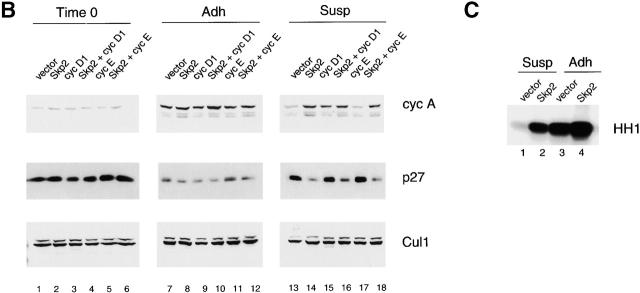
Ectopic expression of Skp2 in nonadherent fibroblasts allows DNA replication, p27 degradation, and Cdk2 activation. (A–C) Rat-6 cells infected with retroviruses expressing the indicated proteins were synchronized in G0/G1 by serum deprivation, trypsinized, and then replated in the presence of serum either in monolayer (Adh) or in Susp. Cells were collected 12 h later. (A) The percentage of Rat-6 cells in S phase analyzed by BrdU incorporation. The results shown are the mean percentage obtained from three representative experiments. (B) Immunoblotting of Rat-6 cell extracts with antibodies to the indicated proteins. (C) Cdk2 kinase activity assayed using histone H1 (HH1) as a substrate after immunoprecipitation of Cdk2 from Rat-6 cellular extracts.
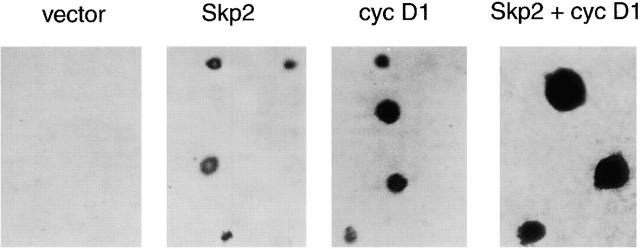
Infected nonadherent cells also differed from each other at the level of p27 and cyclin A expression (Fig. 2 B, lanes 13–18). As expected based on cyclin A function, all of the cells that were able to enter into S phase also expressed cyclin A. However, only those expressing Skp2 showed a decrease in p27 levels, whereas cyclin D1–expressing cells showed no decrease (Fig. 2 B, lanes 15 and 17). The ability of cyclin D1 to induce entry into S phase in the presence of high p27 levels is likely due to cyclin D1 ability to sequester p27 from Cdk2 complexes (for review see Sherr and Roberts 1999). In agreement with the effects on cyclin A and p27, Skp2 expression induced Cdk2 kinase activity as assayed using histone H1 as a substrate (Fig. 2 C). Finally, Skp2 expression allowed Rat-6 cells to enter mitosis and complete multiple rounds of cell cycle division as shown by their ability to form colonies in soft agar (Fig. 3 and Table ). Rat-6 cells coinfected with Skp2 and cyclin D1 formed considerably larger colonies than in the presence of either one of the two. In sum, these results show that ectopic expression of Skp2 can induce adhesion-independent cell proliferation and that Skp2 and cyclin D1 cooperate in this process.
Figure 3.
Expression of Skp2 allows cell division in nonadherent fibroblasts. Rat-6 cells were infected with retroviruses expressing the indicated proteins and grown in semisolid agarose for 3–4 wk. See Table for details and quantification of results.
Table 1.
Soft Agar Colony Formation in Rat-6 Fibroblasts Infected with Various Retroviruses
| Vector(s) | Growth in soft agar (number of colonies) | Size of colonies | |||
|---|---|---|---|---|---|
| Exp 1 | Exp 2 | Exp 3 | Exp 4 | mm | |
| Empty | 1 | 0 | 5 | 3 | |
| Skp2 | 304 | 180 | 265 | 107 | 0.06–0.12 |
| Cyclin D1 | 398 | 357 | 526 | 162 | 0.09–0.26 |
| Skp2 + cyclin D1 | 228 | 388 | 114 | 0.09–0.44 | |
p27 Stabilization Is Not Dependent on Its Dephosphorylation in Proliferating Cells Transferred to Suspension
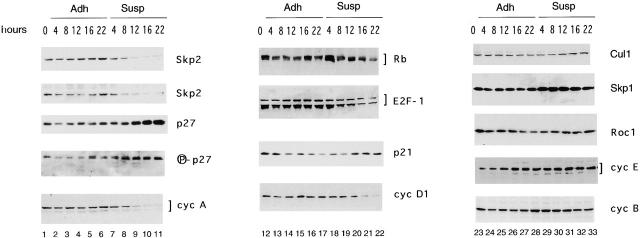
In contrast to Rat-6 cells, p27 is phosphorylated on Thr-187 only in adherent IMR-90 cells but not in suspension cells restimulated with serum (data not shown). Therefore, it is possible that p27 accumulates not only because of the absence of Skp2 but also because of its unphosphorylated state. To distinguish whether p27 stabilization correlates to Skp2 downregulation, p27 dephosphorylation, or both, we used IMR-90–proliferating cells in which p27 is phosphorylated on Thr-187 as detected with a well-established p27 phospho-Thr-187–specific antibody (Carrano et al. 1999; Montagnoli et al. 1999) (Fig. 4, lane 1, fourth panel from the top). When proliferating cells are transferred to suspension, they accumulate p27 because of an increase in its protein stability (p27 half-life; Fig. 5 D, bottom panel). Fig. 4 shows that proliferating IMR-90 fibroblasts plated in suspension exited the cell cycle as demonstrated by (a) the disappearance of cyclin A, (b) the increase in p27, (c) the decrease in the levels of cyclin D1 and E2F-1, (d) the dephosphorylation of Rb, and (e) a decrease in BrdU incorporation overtime (data not shown). Under these conditions, the overall amount of Skp2 decreased dramatically by 12 h as detected using two different antibodies against Skp2. The levels of all other proteins analyzed (Cul1, Skp1, Roc1, cyclin E, and cyclin B) remained unchanged in nonadherent cultures. For reasons that are not currently clear, p21 accumulated in proliferating cells transferred to suspension (Fig. 4, lanes 12–22, third panel from the top) but not in quiescent cells stimulated with serum in suspension (Fig. 1). Interestingly, p27 phosphorylation on Thr-187 could still be detected in nonadherent cells using a p27 phospho-Thr-187–specific antibody (Fig. 4, lanes 7–11, fourth panel from the top). Indistinguishable results were obtained with proliferating Rat-6 fibroblasts transferred to suspension (data not shown). Altogether, these results indicate that p27 dephosphorylation does not contribute to an increase in p27 stability in cells withdrawing the cell cycle in response to loss of substratum adhesion.
Figure 4.
p27 stabilization is not dependent on its dephosphorylation in proliferating fibroblasts transferred to suspension. Proliferating IMR-90 fibroblasts (lanes 1, 12, and 23; indicated as 0 hours) were trypsinized and then either reseeded in monolayer (Adh; lanes 2–6, 13–17, and 24–28) or transferred to Susp (lanes 7–11, 18–22, and 29–33) for the indicated intervals. Cells were collected and protein extracts were analyzed by immunoblot with antibodies to the indicated proteins. Lanes 1–11 were immunoblotted with a monoclonal antibody to Skp2 (top) or a polyclonal antibody to Skp2 (second panel from the top). Lanes 1–11 in the fourth panel from the top were immunoblotted with a phospho-site p27-specific antibody (Carrano et al. 1999; Montagnoli et al. 1999) to detect p27 phosphorylated on Thr-187 (indicated as P-p27).
Figure 5.
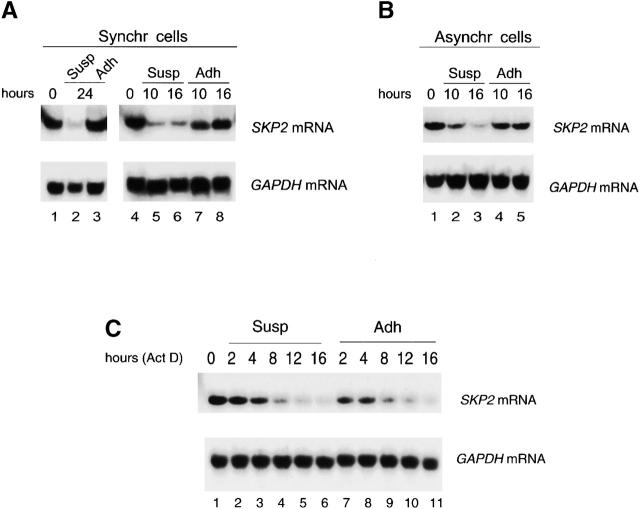
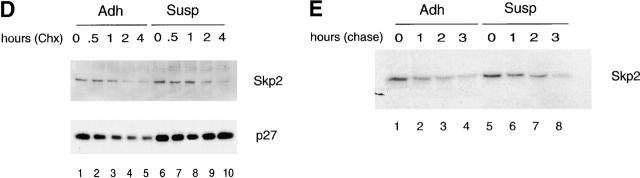
Cell adhesion-dependent serum-independent accumulation of Skp2 transcripts is responsible for Skp2 accumulation. (A) IMR-90 cells were synchronized (Synchr cells) by serum deprivation for 72 h (lanes 1 and 4; indicated as 0 hours), trypsinized, replated in the presence of serum either to Susp (lanes 2, 5, and 6) or in monolayer (Adh; lanes 3, 7, and 8) for the indicated hours. Total RNAs were prepared and processed for Northern blotting using 32P-labeled SKP2 cDNA (top) or GAPDH cDNA (bottom). (B) Proliferating asynchronous IMR-90 cells (Asynchr cells) were trypsinized (lane 1; indicated as 0 hours) and then either reseeded in Susp (lanes 2 and 3) or in monolayer (Adh; lanes 4 and 5) for the indicated hours. Total RNAs were prepared and processed for Northern blotting as in A. (C) Proliferating IMR-90 cells (lane 1; indicated as 0 hours) were trypsinized and then replated in the presence of Act D in monolayer (Adh; lanes 2–6) or Susp (lanes 7–11). Decay of Skp2 mRNA was determined by Northern blotting. No differences were observed if Act D was added 2 h after replating rather than from the beginning (not shown). (D) Proliferating IMR-90 cells were trypsinized and then replated in monolayer (Adh; lanes 1–5) or Susp (lanes 6–10). 8 h after replating, cells were treated with Chx for the indicated intervals. Decay of Skp2 and p27 was determined by immunoblotting. (E) Proliferating IMR-90 cells engineered to express Skp2 by retroviral infection were replated in monolayer (Adh; lanes 1–4) or Susp (lanes 5–8). 8–9 h after replating, cells were labeled with [35S]methionine for 30 min and chased with medium for the indicated times. Extracts were then subjected to immunoprecipitation with an anti-Skp2 monoclonal antibody.
Accumulation of Skp2 Is Due to an Adhesion-dependent Serum-independent Induction of Skp2 mRNA
Skp2 expression in late G1 is regulated by growth factors that block its ubiquitin-proteasome–mediated degradation (Wirbelauer et al. 2000). To determine the mechanism regulating the loss of Skp2 in nonadherent cells, we first analyzed the levels of Skp2 mRNA. Northern blot analysis showed that over time loss of cell adhesion induces a significant decrease in the amount of Skp2 message both in quiescent IMR-90 cells restimulated with serum (Fig. 5 A) and in proliferating IMR-90 cells (Fig. 5 B). To determine whether this decrease in Skp2 mRNA was dependent on an acceleration of Skp2 mRNA processing and/or degradation, we inhibited transcription with Act D and collected cells at different times after treatment. Northern blot analysis from these cells revealed that the half-life of Skp2 mRNA is similar in adherent and nonadherent cells (Fig. 5 C), suggesting that SKP2 gene transcription and not mRNA stability is controlled by adhesion. Finally, to test whether protein degradation contributes to control Skp2 levels in response to loss of cell adhesion as it does in response to serum (Wirbelauer et al. 2000) we compared Skp2 half-life in adherent and suspended IMR-90 using Chx, an inhibitor of protein synthesis. Skp2 stability did not change, whereas p27 half-life was prolonged in nonadherent cells (Fig. 5 D). Similar results were obtained in pulse–chase experiments (Fig. 5 E). Thus, although growth factors control Skp2 expression by increasing its protein stability without influencing the accumulation of its transcript, the ECM controls the induction of Skp2 mRNA without influencing Skp2 protein degradation rate.
Discussion
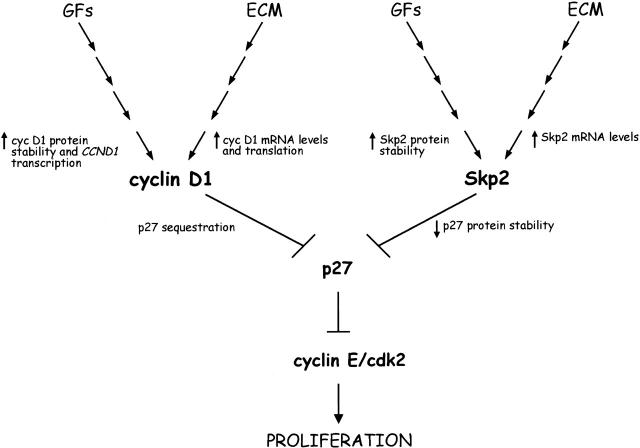
Our results show that cell adhesion to the ECM is required for the expression of the Fbp Skp2. In the absence of cell adhesion, both the Skp2 message and protein disappear, but the enforced expression of Skp2 in nonadherent immortalized fibroblasts allows p27 degradation and cell cycle progression. In agreement with previous reports (Zhu et al. 1996; Resnitzky 1997), cyclin D1 expression induced similar cell cycle effects due to the ability of cyclin D1 to sequester p27 and to be activated by its association with p27 (for review see Sherr and Roberts 1999). Significantly, we observed a cooperative effect between Skp2 and cyclin D1 that induced S phase entry and cell division in nonadherent cells comparable to that of adherent cells. Thus, the cell adhesion-dependent entry into S phase is subject to dual control by the accumulation of both Skp2 and cyclin D1 (Fig. 6). Ultimately, this results in the inactivation of p27 (through degradation and sequestration) and subsequent activation of Cdk2. The elimination and/or inactivation of p27 appears to be a necessary step in allowing adhesion-independent cell growth, yet it is not sufficient. In fact, p27-deficient mouse embryonic fibroblasts do not enter S phase if transferred to suspension and do not form colonies in soft agar (our unpublished results). This is in agreement with the notion that several pathways must be disrupted for adhesion-independent growth to occur. Accordingly, although Skp2 expression induces strong effects on the proliferation of immortalized fibroblasts (Rat-6 and NIH 3T3), very modest effects are observed in nonimmortalized diploid fibroblasts such as IMR-90 and mouse embryonic fibroblasts (data not shown). This difference may reflect the fact that immortalized cells have lost some adhesion-dependent control that is still present in normal cells.
Figure 6.
Model for the function of Skp2 in adhesion-dependent cell cycle progression. The cooperation between ECM–dependent signals and growth factor signaling pathways (GFs) induces by different mechanism the expression of Skp2 and cyclin D1, which in turn induce p27 degradation and p27 sequestration, respectively. The inhibition of p27 contributes to the activation of cyclin E–Cdk2 to promote entry into the S phase of the cell division cycle. See text for details.
Interestingly, the ECM and growth factors increase the levels of Skp2 and cyclin D1 by different mechanisms (Fig. 6). Adhesion-dependent signals induce the expression of Skp2 mRNA, whereas growth factors increase Skp2 protein stability (Wirbelauer et al. 2000). The ECM induces the expression of cyclin D1 mRNA and its translation, whereas growth factors activate the transcription of the CCND1 gene and increase the stability of its product, cyclin D1 (for reviews see Assoian 1997; Assoian and Schwartz 2001). These different mechanisms are complementary and underlie the cooperativity between cell adhesion and growth factors in controlling cell proliferation. Although Skp2 is the only subunit of the SCFSkp2 complex to be regulated by the ECM and by growth factors, some mitogenic and antimitogenic stimuli may control the expression of other subunits. For example, in response to cell–cell contact inhibition, fibroblasts downregulate Cul1 expression, which in turn results in the stabilization of p27 (O'Hagan et al. 2000). It will be interesting to investigate if the cellular abundance of Cks1, a Skp2-interacting protein shown recently by us and others to be necessary for a high affinity binding between phosphorylated p27 and Skp2 (Ganoth et al. 2001; Spruck et al. 2001) is regulated by growth factors, the ECM, and other stimuli.
The yeast phosphatase Cdc14 is able to dephosphorylate the Cdk inhibitor Sic1 causing its accumulation because dephosphorylated Sic1 cannot be recognized by the SCF ubiquitin ligase (for review see Zachariae and Nasmyth 1999). Although a human Cdc14 homologue exists (Li et al. 1997), no phosphatase appears to play a role in the stabilization of p27 observed in proliferating cells transferred to suspension. In fact, phosphorylation of p27 on Thr-187 is still detectable in nonadherent cells (Fig. 4). Likely, p27 is still phosphorylated by Cdk2 during the first 4–8 h after replating the cells in suspension, since Cdk2 activity was totally inhibited only between 12 and 16 h as indicated by the state of Rb phosphorylation (Fig. 4, lanes 18–22) and by measuring Cdk2 activity in vitro (data not shown). Despite the fact that p27 is present in its phosphorylated form, p27 accumulates, since it cannot be degraded due to the absence of Skp2. Thus, during cell cycle withdrawal in response to loss of cell adhesion p27 stabilization is not dependent on its dephosphorylation. Interesting, a recent paper shows that p27 accumulates in its phosphorylated form in response to ectopic PTEN expression in PTEN-deficient cells (Mamillapalli et al. 2001). In agreement with our data, these results indicate that p27 accumulation can be regulated by mechanisms other than dephosphorylation.
It has been shown that infecting cells with a recombinant adenovirus expressing Skp2 in serum starved fibroblasts (Sutterluty et al. 1999) or arrested hepatocytes (Nelsen et al. 2001) induces degradation of p27, Cdk activation, and S phase entry. This is in apparent contrast with our results in both fibroblasts (Fig. 2) and thymocytes (Latres et al. 2001) where the presence of Skp2 alone is unable to produce such effects in resting cells. This discrepancy is probably due to the difference in overexpression levels obtained using different viral systems.
Adhesion independence, one of the fundamental properties of cancer cells, is associated with invasiveness and metastasis (Folkman and Moscona 1978). Changes in the expression of cyclins and Cdk inhibitors have been implicated in the adhesion-independent growth of tumor cells (Fang et al. 1996; Orend et al. 1998). Destabilization of p27 that we and others have documented in human epithelial cancers and lymphomas correlates with tumor aggressiveness, poor prognosis (Esposito et al. 1997; Loda et al. 1997; Steeg and Abrams 1997; Piva et al. 1999; Chiarle et al. 2000), and with the presence of metastases (Thomas et al. 1998). We and others have shown recently that Skp2 levels inversely correlate with p27 expression in human lymphomas (Latres et al. 2001), oral squamous cell carcinomas (Gstaiger et al. 2001), colorectal cancers (Hershko et al. 2001), and prostate carcinomas (our unpublished results). Significantly, Skp2 cooperates with activated N-Ras in an in vivo model of lymphomagenesis (Latres et al. 2001). In contrast to all nontransformed cells tested (human, mouse, and rat fibroblasts and human endothelial cells), transformed cells (such as HeLa, 293T, Hs913T, and U2-OS cells) express the same levels of Skp2 regardless of their adhesion conditions (our unpublished results). The data presented herein support and extend previous studies demonstrating that Skp2 is necessary for entering S phase (Zhang et al. 1995; Sutterluty et al. 1999). Furthermore, our findings show that Skp2 is a target of cell adhesion-dependent signaling and together with the high Skp2 levels observed in human cancers suggests a role for Skp2 in the adhesion-independent ability of tumor cells to grow.
Acknowledgments
We thank M. Roussel, A. Hershko, G. Draetta, F. Giancotti, J. Bloom, and T. Guadagno for helpful discussions and for critical reading of the paper; M. Roussel, B. Amati, F. Giancotti, and R. Krauss for reagents; and J. Bloom, R. Piva, M.S. Nadal, R. Pine, and S. Chang for suggestions. We are grateful to L. Yamasaki, M. Roussel, T.E. Amora, and A.F. Odin for continuous support.
This work was supported by an Irma T. Hirschl scholarship, a Human Frontier Science Program Organization grant (RG0229), National Institutes of Health grants (R01-CA76584 and R01-GM57587), and the Kaplan Comprehensive Cancer Center National Institutes of Health grants (P30-CA16087 and R21-CA66229).
Footnotes
Abbreviations used in this paper: Act D, actinomycin D; Adh, adherent; Cdk, cyclin-dependent kinase; Chx, cycloheximide; ECM, extracellular matrix; Fbp, F-box protein; Mab, mouse monoclonal antibody; PDL, population doubling; SCF, Skp1, Cul1, and Fbp complex; Skp, S phase kinase-associated protein; Susp, suspension.
References
- Assoian R. Anchorage-dependent cell cycle progression. J. Cell Biol. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R., Schwartz M. Coordinate signaling by integrins and receptor kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 2001;11:48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- Bottazzi M., Zhu X., Bohmer R., Assoian R. Regulation of p21(cip1) expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J. Cell Biol. 2000;146:1255–1264. doi: 10.1083/jcb.146.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano A.C., Eytan E., Hershko A., Pagano M. Skp2 is required for the ubiquitin-mediated degradation of the Cdk-inhibitor p27. Nat. Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C., Chiaur D.S., Guardavaccaro D., Parks W., Vidal M., Pagano M. Identification of a human family of F-box proteins. Curr. Biol. 1999;9:1177–1179. doi: 10.1016/S0960-9822(00)80020-2. [DOI] [PubMed] [Google Scholar]
- Chiarle R., Budel L.M., Skolnik J., Frizzera G., Chilosi M., Corato A., Pizzolo G., Magidson J., Montagnoli A., Pagano M. Increased proteasome degradation of cyclin-dependent kinase inhibitor p27 is associated with a decreased overall survival in mantle cell lymphoma. Blood. 2000;95:619–626. [PubMed] [Google Scholar]
- Croix B., Florenes V., Rak J., Flanagan M., Bhattacharya N., Slingerland J., Kerbel R. Impact of the cyclin-dependent kinase inhibitor p27 on adhesion-dependent resistance of tumor cells to anticancer agents. Nat. Med. 1996;2:1204–1210. doi: 10.1038/nm1196-1204. [DOI] [PubMed] [Google Scholar]
- Deshaies R.J. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 1999;15:435–467. doi: 10.1146/annurev.cellbio.15.1.435. [DOI] [PubMed] [Google Scholar]
- Esposito V., Baldi A., DeLuca A., Sgaramella G., Giordano G.G., Caputi M., Baldi F., Pagano M., Giordano G. Prognostic role of the cell cycle inhibitor p27 in non small cell lung cancer. Cancer Res. 1997;57:3381–3385. [PubMed] [Google Scholar]
- Faha B., Harlow E., Lees E. The adenovirus E1A-associated kinase consists of cyclin E-p33cdk2 and cyclin A-p33cdk2. J. Virol. 1993;67:2456–2465. doi: 10.1128/jvi.67.5.2456-2465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Orend G., Watanabe N., Hunter T., Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Ganoth D., Bornstein G., Ko T., Larsen B., Tyers M., Pagano M., Hershko A. The cell cycle regulatory protein Cks1 is required for the SCFSkp2-mediated ubiquitinylation of p27. Nat. Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G., Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Gstaiger M., Jordan R., Lim M., Catzavelos C., Mestan J., Slingerland J., Krek W. Function of human Skp2 as an oncogene. Proc. Natl. Acad. Sci. USA. 2001;98:5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno T., Ohtsubo M., Roberts J., Assoian R. A link between cyclin A expression and adhesion-dependent cell cycle progression. Science. 1993;262:1572–1575. doi: 10.1126/science.8248807. [DOI] [PubMed] [Google Scholar]
- Hengst L., Reed S. Translation control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- Hershko D., Bornstein G., Ben-Izhak O., Carrano A., Pagano M., Krausz M., Hershko A. Inverse relationship between levels of p27 and its ubiquitin ligase subunit Skp2 in colorectal cancers. Cancer. 2001;91:1745–1751. doi: 10.1002/1097-0142(20010501)91:9<1745::aid-cncr1193>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Huang S., Chen C.S., Ingber D.E. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol. Biol. Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Krauss R. Ras induces anchorage-independent growth by subverting multiple adhesion-regulated cell cycle events. Mol. Cell. Biol. 1996;16:3370–3380. doi: 10.1128/mcb.16.7.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada M., Yamagoe S., Murakami Y., Suzuki K., Mizuno S., Uehara Y. Induction of p27Kip1 degradation and anchorage independence by Ras through the MAP kinase signaling pathway. Oncogene. 1997;15:629–637. doi: 10.1038/sj.onc.1201228. [DOI] [PubMed] [Google Scholar]
- Kipreos, E., and M. Pagano. 2000. The F-box protein family. Genome Biol. 1:3002.1–3002.7. [DOI] [PMC free article] [PubMed]
- Koepp D., Harper J.W., Elledge S.J. How the cyclin became a cyclinregulated proteolysis in the cell cycle. Cell. 1999;97:431–433. doi: 10.1016/s0092-8674(00)80753-9. [DOI] [PubMed] [Google Scholar]
- Koyama H., Raines E., Bornfeldt K., Roberts J., Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- Krauss R.S., Guadagno S.N., Weinstein I.B. Novel revertants of H-ras oncogene-transformed R6-PKC3 cells. Mol. Cell. Biol. 1992;12:3117–3129. doi: 10.1128/mcb.12.7.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latres E., Chiaur D.S., Pagano M. The human F-box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene. 1999;18:849–855. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- Latres E., Chiarle R., Schulman B., Pellicer A., Inghirani G., Pagano M. Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl. Acad. Sci. USA. 2001;98:2515–2520. doi: 10.1073/pnas.041475098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Ernsting B.R., Wishart M.J., Lohse D.L., Dixon J.E. A family of putative tumor suppressors is structurally and functionally conserved in humans and yeast. J. Biol. Chem. 1997;272:29403–29406. doi: 10.1074/jbc.272.47.29403. [DOI] [PubMed] [Google Scholar]
- Loda M., Cukor B., Tam S., Lavin P., Fiorentino M., Draetta G., Jessup J., Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- Lukas J., Pagano M., Staskova Z., Draetta G., Bartek J. Cyclin D1 protein oscillates and is essential for cell cycle progression in human tumor cell lines. Oncogene. 1994;9:707–718. [PubMed] [Google Scholar]
- Mamillapalli R., Gavrilova N., Mihaylova V.T., Tsvetkov L.M., Wu H., Zhang H., Sun H. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2) Curr. Biol. 2001;11:263–267. doi: 10.1016/s0960-9822(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Marti A., Wirbelauer C., Scheffner M., Krek W. Interaction between ubiquitin-protein ligase SCFSkp2 and E2F-1 underlies the regulation of E2F-1 degradation. Nat. Cell Biol. 1999;1:14–19. doi: 10.1038/8984. [DOI] [PubMed] [Google Scholar]
- Montagnoli A., Fiore F., Eytan E., Carrano A.C., Draetta G., Hershko A., Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Nagahama H., Minamishima Y., Matsumoto M., Nakamichi I., Kitagawa K., Shirane M., Tsunematsu R., Tsukiyama T., Ishida N. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen C., Hansen L., Rickheim D., Chen C., Stanley M., Krek W., Albrecht J. Induction of hepatocyte proliferation by the targeted expression of cell cycle control genes. Oncogene. 2001;20:1825–1831. doi: 10.1038/sj.onc.1204248. [DOI] [PubMed] [Google Scholar]
- Nguyen H., Gitig D.M., Koff A. Cell-free degradation of p27(kip1), a G1 cyclin-dependent kinase inhibitor, is dependent on CDK2 activity and the proteasome. Mol. Cell. Biol. 1999;19:1190–1201. doi: 10.1128/mcb.19.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hagan R., Ohh M., David G., Moreno de Alboran I., Alt F., Kaelin W., Jr., DePinho R. Myc-enhanced expression of Cul1 promotes ubiquitin-dependent proteolysis and cell cycle progression. Genes Dev. 2000;14:2185–2191. doi: 10.1101/gad.827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Michel J., Schottelius A., Xiong Y. ROC1, a homolog of APC11, represents a family of cullin partners with an associated ubiquitin ligase activity. Mol. Cell. 1999;3:535–541. doi: 10.1016/s1097-2765(00)80482-7. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Theodoras A., Schumacher J., Roberts J.M., Pagano M. Human cyclin E, a nuclear protein essential for the G1 to S phase transition. Mol. Cell. Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orend G., Hunter T., Ruoslahti E. Cytoplasmic displacement of cyclin E-cdk2 inhibitors p21Cip1 and p27Kip1 in anchorage-independent cells. Oncogene. 1998;16:2575–2583. doi: 10.1038/sj.onc.1201791. [DOI] [PubMed] [Google Scholar]
- Pagano M., Draetta G., Jansen-Dürr P. Association of cdk2 kinase with the transcription factor E2F during S phase. Science. 1992;255:1144–1147. doi: 10.1126/science.1312258. [DOI] [PubMed] [Google Scholar]
- Pagano M., Tam S., Theodoras A., Beer P., Delsal S., Chau I., Yew R., Draetta G., Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Piva R., Cancelli I., Cavalla P., Bortolotto S., Dominguez J., Draetta G.F., Schiffer D. Proteasome-dependent degradation of p27/kip1 in gliomas. J. Neuropathol. Exp. Neurol. 1999;58:691–696. doi: 10.1097/00005072-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Resnitzky D. Ectopic expression of cyclin D1 but not cyclin E induces anchorage-independent cell cycle progression. Mol. Cell. Biol. 1997;17:5640–5647. doi: 10.1128/mcb.17.9.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G.M., Yandell M.D., Wortman J.R., Gabor Miklos G.L., Nelson C.R., Hariharan I.K., Fortini M.E., Li P.W., Apweiler R., Fleischmann W. Comparative genomics of the eukaryotes. Science. 2000;287:2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman B., Carrano A.C., Kinnucan E., Jeffrey P., Bowen Z., Elledge S., Harper W., Pagano M., Pavletich N. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature. 2000;408:381–386. doi: 10.1038/35042620. [DOI] [PubMed] [Google Scholar]
- Schulze A., Zerfass-Thome K., Berges J., Middendorp S., Jansen-Durr P., Henglein B. Anchorage-dependent transcription of the cyclin A gene. Mol. Cell. Biol. 1996;16:4632–4638. doi: 10.1128/mcb.16.9.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaff R., Groudine M., Gordon M., Roberts J., Clurman B. Cyclin E-Cdk2 is a regulator of p27Kip1 . Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- Sherr C.J., Roberts J.M. CDK inhibitorspositive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Spruck C., Strohmaier H., Watson M., Smith A., Ryan A., Krek W., Reed S. A CDK-independent function of mammalian Cks1targeting of SCFSkp2 to the CDK inhibitor p27. Mol. Cell. 2001;7:639–650. doi: 10.1016/s1097-2765(01)00210-6. [DOI] [PubMed] [Google Scholar]
- Steeg P., Abrams J. Cancer prognosticpast, present and p27. Nat. Med. 1997;3:152–154. doi: 10.1038/nm0297-152. [DOI] [PubMed] [Google Scholar]
- Sutterluty H., Chatelain E., Marti A., Wirbelauer C., Senften M., Muller U., Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- Tam S.W., Theodoras A.M., Shay J.W., Draetta G.F., Pagano M. Differential expression and regulation of cyclin D1 protein in normal and tumor human cellsassociation with Cdk4 is required for cyclin D1 function in G1 progression. Oncogene. 1994;9:2663–2674. [PubMed] [Google Scholar]
- Thomas G.V., Szigeti K., Murphy M., Draetta G., Pagano M., Loda M. Down-regulation of p27 is associated with development of colorectal adenocarcinoma metastases. Am. J. Pathol. 1998;153:681–687. doi: 10.1016/S0002-9440(10)65610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda K., Kubota Y., Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27 is instigated by Jab1. Nature. 1999;398:160–164. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- Tsvetkov L.M., Yeh K.H., Lee S., Sun H., Zhang H. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr. Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- Vlach J., Hennecke S., Amati B. Phosphorylation-dependent of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J.T., Koepp D.M., Zhu C., Elledge S.J., Harper J.W. A family of mammalian F-box proteins. Curr. Biol. 1999;9:1180–1182. doi: 10.1016/S0960-9822(00)80021-4. [DOI] [PubMed] [Google Scholar]
- Wirbelauer C., Sutterluty H., Blondel M., Gstaiger M., Peter M., Reymond F., Krek W. The F-box protein Skp2 is a ubiquitylation target of a Cul1-based core ubiquitin ligase complexevidence for a role of Cul1 in the suppression of Skp2 expression in quiescent fibroblasts. EMBO J. 2000;19:5362–5375. doi: 10.1093/emboj/19.20.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae W., Nasmyth K. Whose end is destructioncell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- Zhang H., Kobayashi R., Galaktionov K., Beach D. p19Skp-1 and p45Skp-2 are essential elements of the cyclin A-Cdk2 S phase kinase. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- Zhu X., Ohtsubo M., Bohmer R., Roberts J., Assoian R. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Roovers K., Davey G., Assoian R. Methods for Analysis of Adhesion-dependent Cell Cycle Progression 1999. CRC Press; Boca Raton, FL: pp. 129–140 [Google Scholar]