Abstract
A retrospective medical record review was established to test the hypothesis that in children with sickle cell anemia (SCA), a daytime oxygen saturation (SpO2) ≤94% is associated with nocturnal desaturation with or without obstructive sleep apnea (OSA). Twenty children had a resting SpO2 ≤94% and an abnormal polysomnogram (PSG). Seven patients had OSA and thirteen patients had nocturnal desaturation. The average daytime SpO2 correlated with the average nighttime SpO2 (Spearman correlation coefficient = 0.453; P = 0.045). Our results indicate that in children with SCA, a daytime SpO2 ≤94% is a reasonable threshold to recommend a pulmonary evaluation, including a PSG. Pediatr Blood Cancer
Keywords: nocturnal desaturation, obstructive sleep apnea, polysomnogram, sickle cell anemia
INTRODUCTION
Increased attention has recently been focused upon the prevalence of sleep-disordered breathing, specifically nocturnal desaturation and obstructive sleep apnea (OSA) in children with sickle cell anemia (SCA) [1-5]. Recent studies have demonstrated that a decreased mean nocturnal oxygen saturation (SpO2) is associated with an increased rate of both painful episodes and cerebrovascular accidents in children with sickle cell disease [6,7]. In healthy children, a normal physiological decrease in nighttime versus daytime SpO2 is expected [8-10]. Daytime SpO2 assessments are becoming a routine practice in many sickle cell disease clinics, yet few studies have explored if daytime SpO2 correlates to nighttime SpO2 in SCA or can help identify children at risk for sleep-disordered breathing. Moreover, no physiological parameters have been established to identify children with SCA at risk for sleep-disordered breathing. This study was designed to test the hypothesis that children with a low daytime SpO2 ≤94% is associated with sleep-disordered breathing.
METHODS
Study Design
A 5-year retrospective medical record review was conducted on 39 patients with sickle cell disease at Washington University School of Medicine/St. Louis Children’s Hospital (WUSM/SLCH) from August 2000 to August 2005. Demographic data were collected. Inclusion criteria for our current cohort included (1) individuals less than 22 years of age, (2) HbSS or HbSB0-thalassemia on hemoglobin analyses, (3) no chronic blood transfusion therapy or on hydroxyurea during study period, (4) a one time SpO2 ≤94% in the pulmonary/sickle cell disease clinic at clinical baseline, and (5) full pulmonary evaluation which included a detailed history and examination, allergy testing, and spirometry if >5 years old (Fig. 1). Patients with comorbid pulmonary disease such as asthma or allergies were asymptomatic at the time of sleep referral.
Fig. 1.
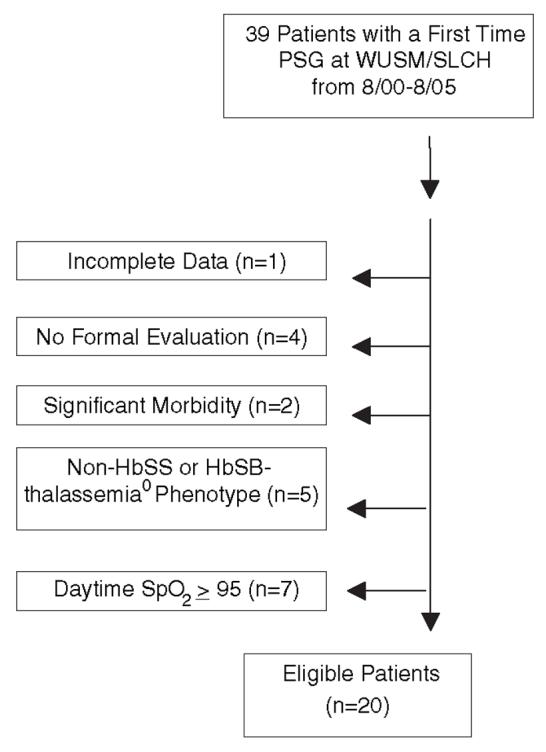
Patient selection for subjects based upon study inclusion or exclusion criteria.
Study Procedure and Standard Care
Daytime SpO2 was obtained in clinic and measured by peripheral pulse oximetry with a Welch Allyn® (Beaverton, OR) vital signs monitor 300 Series Model 53N00 with a Nellcor® (Puritan Bennett; Pleasanton, CA) probe. After a stable waveform was obtained for 1 min duration, the highest value was recorded. Low daytime SpO2 was defined as ≤94% while breathing room air (FIO2 = 0.21) when well. Daytime oximetry readings were collected on the children within a 12-month sampling frame from the overnight polysomnogram (PSG). Average daytime SpO2 was calculated from all available recordings within the sampling frame when the patient was free of illness and at clinical baseline. All children underwent an all-night PSG at the SLCH sleep laboratory. PSG data were obtained using the Alice III® (Respironics; Murrysville, PA) from 8/00 to 4/05 or the SomnoStar Pro® (Viasys/Sensor Medics; Yorba Linda, CA) from 4/05 to 8/05 sleep systems and analyzed following the guidelines and parameters of the general consensus statement and standards set by the American Thoracic Society [11]. Natural sleep was observed overnight and each patient monitored continuously by infrared video camera. Sleep stage, heart rate, respiratory events including apnea and hypopnea, adequacy of gas exchange, and oxygen saturations were recorded and scored by each 30 sec epoch.
Definitions
Sleep-disordered breathing is a broad clinical term defined as any ventilatory abnormality occurring during sleep, including but not limited to OSA [9,11-13]. OSA was defined as a disorder of breathing during sleep characterized by prolonged upper airway obstruction (either partial or complete) that disrupts normal ventilation and normal sleep patterns during sleep [9,11-13]. Oxygen desaturation (daytime or nighttime) was defined as the mean value of SpO2 <94% while breathing room air (FIO2 = 0.21) analyzed by peripheral pulse oximetry. Respiratory disturbance index (RDI) was defined as the number of apneas, hypopneas, and arousals per hour of total sleep time. Asthma was defined as a previous doctor diagnosis of asthma and current use of asthma medication (use of bronchodilators and/or inhaled steroids) to control clinical symptoms.
Data Analysis
All data were entered into a database. Data were analyzed using SPSS 11.0 (Statistical Package for the Social Sciences, Chicago, IL). Spearman two-tailed correlation was used to determine if average daytime oximetry was associated with average nighttime oximetry.
RESULTS
From August 2000 to August 2005, 39 patients underwent a first time PSG at WUSM/SLCH. Nineteen patients did not meet inclusion criteria and were excluded from the analysis. Twenty patients met our inclusion criteria. Eleven patients were male. The ages ranged between 1 and 19 years of age with a mean of 9.6 years and median of 9.5 years. Body mass index (BMI) ranged from 14 to 21 kg/m2 (age-gender adjusted percentiles ranged from 3 to 70%). No patient was on supplemental oxygen at the time of PSG. Five patients were asthmatic. One patient had adenotonsillectomy prior to the overnight PSG (Table 1).
TABLE I.
Demographic Data on Patients with Sickle Cell Anemia Referred For a One-Time Low Oxygen Saturation ≤94% at Baseline
| Patient | Age (years) | Gender | Asthma diagnosis | Average daytime SpO2 | Median daytime SpO2 | Average nighttime SpO2 | OSA |
|---|---|---|---|---|---|---|---|
| 1 | 7 | F | No | 94 | 94 | 93 | Yes |
| 2 | 6 | M | No | 93 | 93 | 87 | Yes |
| 3 | 3 | M | Yes | 97.2 | 97 | 88 | Yes |
| 4 | 9 | F | No | 90 | 90 | 83 | Yes |
| 5 | 4 | M | No | 95.5 | 95.5 | 92 | Yes |
| 6 | 7 | M | Yes | 95.25 | 95 | 94 | Yes |
| 7 | 9 | F | No | 94 | 94 | 88 | Yes |
| 8 | 17 | M | No | 91.25 | 92 | 87 | No |
| 9 | 18 | M | No | 91 | 91 | 84 | No |
| 10 | 2 | M | No | 92 | 92 | 91 | No |
| 11 | 19 | M | No | 93 | 93 | 90 | No |
| 12 | 11 | F | No | 94.7 | 97 | 90 | No |
| 13 | 13 | F | No | 90 | 90 | 89 | No |
| 14 | 10 | M | Yes | 93 | 93 | 86 | No |
| 15 | 8 | F | No | 92 | 92 | 87 | No |
| 16 | 11 | F | Yes | 95 | 95 | 91 | No |
| 17 | 14 | F | Yes | 91.5 | 91.5 | 90 | No |
| 18 | 12 | M | No | 91 | 91 | 89 | No |
| 19 | 12 | M | No | 89 | 89 | 91 | No |
| 20 | 1 | F | No | 94 | 94 | 91 | No |
OSA, obstructive sleep apnea.
All 20 patients referred for PSG for a one time daytime SpO2 ≤94% in clinic had an abnormal all-night PSG defined as either nocturnal desaturation with OSA or nocturnal desaturation without OSA. Seven of twenty patients (35%) had confirmed OSA with an apnea index (AI) ranging from 1 to 8 respiratory events/hr (mean = 3.4, median = 3.0)of the total sleep time and a RDI ranging from 6.0 to 41.4 respiratory events/hr (mean = 14.3, median = 10)of the total sleep time. The average of nighttime SpO2 was 89.3% (range 83-94%) in the seven patients with OSA. Hypercarbia was not observed in our cohort. Seven patients with OSA had an average end-tidal CO2 recording of 43.1 mmHg (range of 36-47 mmHg).
Nocturnal desaturation was a common finding based upon all-night PSG in children with SCA. Average nighttime SpO2 was 89.1% for the entire cohort with a range of 83-94%. In the 13 patients with nocturnal desaturation and without OSA, nighttime SpO2 ranged from 84 to 91% (mean 88.9% median 89.5%).
Average daytime SpO2 correlated with the average nighttime SpO2 (Spearman correlation coefficient = 0.453; P = 0.045). The mean daytime and nighttime SpO2 was 92.8% (89-97.2%) and 88.8% (83-94%), respectively. Individuals with the lowest daytime SpO2 also had the lowest mean nighttime SpO2. Two patients had a mean nighttime SpO2 between 80 and 85%, 11 patients between 85 and 90%, and 7 patients between 91 and 94%.
DISCUSSION
The results of our study suggest a resting daytime SpO2 ≤94% is a reasonable threshold to screen for sleep-disordered breathing in children with SCA. All 12 patients had an abnormal sleep study with 35% having OSA. Sleep-disordered breathing is a common clinical finding encountered in children with SCA. Although the exact prevalence, etiology and natural history of sleep-disordered breathing in children with SCA remains poorly defined, our study is similar to Samuels et al. [3] which showed a high proportion of patients with OSA. The expected rate of OSA in the general pediatric population is between 2 and 3% [9,12,13] suggesting that children with SCA may have a higher risk of sleep-disordered breathing when compared to the general pediatric population. Based on increasing evidence that untreated OSA is associated with significant morbidities, including cognitive and behavioral abnormalities, growth delay, and cardiovascular complications, early evaluation and treatment of OSA notably in children with sickle cell disease may be clinically relevant [9,12,13].
Screening patients with daytime oximetry may also help predict who is at risk for a low mean oxygen desaturation that is associated with an increase in sickle cell disease related morbidity. Hargrave et al. demonstrated an association between five variables and high rate of painful episodes in children with sickle cell disease. After adjusting for clinically significant variables, mean nighttime SpO2 remained significantly associated with an increased pain rate (P < 0.005) [6]. In the same cohort, Kirkham et al. [7] reported that a previous transient ischemic attack, high velocity on Transcranial Doppler, and a low mean nighttime SpO2 were all significantly associated with a greater risk of a central nervous system event.
As with any retrospective study, there were several inherent study design limitations. Patients were highly selected, as only patients who were referred to a pediatric pulmonologist and had a PSG, were identified and studied. Perhaps families of children that were more symptomatic were the only ones that followed through with the PSG. All patients met our definition of sleep-disordered breathing and, even if highly selected, showed an association between daytime and nighttime oxygen saturation. A second potential limitation is only first night PSG were performed with the bias of poor sleep or altered sleep physiology due to firstnight effects. Each PSG reported was deemed an adequate study with sleep efficiency scores ranging from 61 to 97%. In addition, all children were evaluated in the same manner and at the same hospital sleep laboratory (WUSM/SLCH). A perceived significant limitation is that pulse oximeter in sickle cell disease is inaccurate when compared to the gold standard, co-oximetry, as a result of hemoglobin and oxygen binding properties, the oxygen-hemoglobin dissociation curve, and the high levels of carboxyhemoglobin, methemoglobin, and other factors unique in the sickle cell patient [14-18]. Arterial blood gas sampling was infrequently obtained to document hypoxemia in patients with a low SpO2. However, regardless of the perceived limitations of SpO2 in SCA, the mean nocturnal SpO2 has been associated with sickle cell disease related morbidity and not arterial oxygen saturation measured by continuous co-oximetery [6,7].
Our preliminary results indicate that in children with SCA, a baseline daytime SpO2 ≤94% is a reasonable screening threshold to recommend a full pulmonary evaluation that includes an all-night PSG. Further studies are underway to better evaluate the clinical implications of OSA and nocturnal desaturation within this vulnerable patient population.
Footnotes
Grant sponsor: Training grant; Grant numbers: T32 HL07873, R01 HL079937-01.
REFERENCES
- 1.Sidman JD, Fry TL. Exacerbation of sickle cell disease by obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1988;114:916–917. doi: 10.1001/archotol.1988.01860200100028. [DOI] [PubMed] [Google Scholar]
- 2.Maddern BR, Ohene-Frempong K, Reed HT, et al. Obstructive sleep apnea syndrome in sickle cell disease. Ann Otol Rhinol Laryngol. 1989;98:174–178. doi: 10.1177/000348948909800302. [DOI] [PubMed] [Google Scholar]
- 3.Samuels MP, Stebbens VA, Davies SC, et al. Sleep related upper airway obstruction and hypoxaemia in sickle cell disease. Arch Dis Child. 1992;67:925–929. doi: 10.1136/adc.67.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks LJ, Koziol SM, Chiarucci KM, et al. Does sleep-disordered breathing contribute to the clinical severity of sickle cell anemia. J Pediatr Hematol Oncol. 1996;18:135–139. doi: 10.1097/00043426-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Needleman JP, Franco ME, Varlotta L, et al. Mechanisms of nocturnal oxyhemoglobin desaturation in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28:418–422. doi: 10.1002/(sici)1099-0496(199912)28:6<418::aid-ppul6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Hargrave DR, Wade A, Evans JPM, et al. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101:846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 7.Kirkham FJ, Hewes DKM, Prengler M, et al. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357:1656–1659. doi: 10.1016/s0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- 8.Uliel S, Tauman R, Greenfeld M, et al. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–878. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 9.Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med. 2001;164:16–30. doi: 10.1164/ajrccm.164.1.2008171. [DOI] [PubMed] [Google Scholar]
- 10.Gries RE, Brooks LJ. Normal oxyhemoglobin saturation during sleep. How low does it go. Chest. 1996;110:1489–1492. doi: 10.1378/chest.110.6.1489. [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–878. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics Technical report: Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–712. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 13.Guilleminault C, Bio ID, Lee JH, et al. Pediatric obstructive sleep apnea syndrome. Arch Pediatr Adolesc Med. 2005;159:775–785. doi: 10.1001/archpedi.159.8.775. [DOI] [PubMed] [Google Scholar]
- 14.Craft JA, Alessandrini E, Kenney LB, et al. Comparisons of oxygenation measurements in pediatric patients during sickle cell crises. J Pediatr. 1994;124:93–95. doi: 10.1016/s0022-3476(94)70260-8. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz FO, Aldrich TK, Nagel RL, et al. Accuracy of pulse oximetry in sickle cell disease. Am J Respir Crit Care Med. 1999;159:447–451. doi: 10.1164/ajrccm.159.2.9806108. [DOI] [PubMed] [Google Scholar]
- 16.Blaisdell CJ, Goodman S, Clark K, et al. Pulse oximetry is a poor predictor of hypoxemia in stable children with sickle cell disease. Arch Pediatr Adolesc Med. 2000;154:900–903. doi: 10.1001/archpedi.154.9.900. [DOI] [PubMed] [Google Scholar]
- 17.Comber JT, Lopez BL. Evaluation of pulse oximetry in sickle cell anemia patients presenting to the emergency department in acute vasoocclusive crisis. Am J Emerg Med. 1996;14:16–18. doi: 10.1016/S0735-6757(96)90005-4. [DOI] [PubMed] [Google Scholar]
- 18.Rackoff WR, Kunkel N, Silber JH, et al. Pulse oximetry and factors associated with hemoglobin oxygen desaturation in children with sickle cell disease. 1993;81:3422–3427. [PubMed] [Google Scholar]


