Abstract
The effect of resuscitation with varying levels of O2 on pulmonary hemodynamics at birth is not well known. We hypothesized that the decrease in pulmonary vascular resistance (PVR) and subsequent response to pulmonary vasoconstrictors and vasodilators will differ following resuscitation with 21%, 50%, or 100%O2 for 30 min at birth in normal term lambs. Lambs at 141 d gestation were delivered by cesarean section and ventilated with 21% (21% Res; n = 6), 50% (50% Res; n = 6), or 100% O2 (100% Res; n = 7) for 30 min followed by ventilation with 21% O2 in all three groups. A greater decrease in PVR was seen with 50% and 100% O2 ventilation than with 21% O2 (0.21 ± 0.02, 0.21 ± 0.02, and 0.34 ± 0.05 mm Hg/mL/min/kg, respectively). Subsequent pulmonary vasoconstriction to hypoxia (10% O2) and the thromboxane analog U46619 (0.5 and 1 μg/kg/min) was similar in all three groups. After inducing a stable elevation in PVR with U46619, impaired pulmonary vasodilation to inhaled NO (59 ± 4, 65 ± 4, and 74 ± 5% of baseline PVR with 21, 50, and 100%Res, respectively) and acetylcholine infusion (67 ± 8, 75 ± 6, and 87 ± 4% of baseline PVR with 21, 50, and 100%Res, respectively) and rebound pulmonary hypertension following their withdrawal were observed in the 100%Res group. We conclude that, while ventilation with 100% O2 at birth results in a greater initial decrease in PVR, subsequent pulmonary vasodilation to NO/acetylcholine is impaired.
Fetal pulmonary vascular resistance is high and O2 plays a crucial role in mediating the pulmonary vascular transition at birth (1). However, the optimal level of O2 supplementation during resuscitation of a newborn infant remains controversial (2) despite publication of new guidelines (3). During resuscitation of a depressed newborn infant with potentially high PVR, there is concern that room air resuscitation may result in inadequate pulmonary vasodilation. It has been suggested that breathing 100% O2 dilates constricted pulmonary arteries more efficiently than room air.
Previous studies have demonstrated conflicting results regarding the effect of ventilation with different gas mixtures on PVR. Some studies indicate that ventilation with nitrogen, air, and O2 are similarly effective in reducing fetal PVR in lambs (4,5). Other studies indicate that PVR drops more effectively with 100% O2 (6) or air (7,8) than with a nitrogen-rich gas. It may be important to find an intermediate level of O2 that has the advantages of short-term reduction in PVR without the toxicity of 100% O2 (9,10). Recent studies examined pulmonary hemodynamic responses to 21% or 100% O2 ventilation, but were conducted on hypoxemic lambs only after 12–72 h of age, after PVR had dropped from high fetal levels immediately after birth (11–13). Understanding the precise pulmonary hemodynamic response to ventilation with room air, 100% O2, and an intermediate level of O2 exposure (such as 50% O2) in animals at birth is vital for determining the safest protocol for delivery room resuscitation of newborns. We chose 50% O2 as the intermediate level for this study because preliminary data from our laboratory indicate that 50% O2 is as effective as 100% O2 in improving oxygenation in lambs with persistent pulmonary hypertension of the newborn (PPHN) induced by antenatal ductal ligation.
We performed the current study to determine whether ventilation with 21%, 50%, or 100% O2 for the first 30 min (referred to as “resuscitation” in this article) following birth alters the rate and extent of decrease in PVR in normal term lambs. We recently reported that even a brief exposure to 100% O2 for 30 min after birth alters the reactivity of isolated pulmonary arteries 24 h later (14). Thus, we also wanted to determine whether initial ventilation with 21%, 50%, or 100% O2 influenced subsequent in vivo pulmonary vasoconstriction to hypoxia or a thromboxane analog and pulmonary vasodilation to either inhaled nitric oxide (NO) or infusion of acetylcholine. We hypothesized that newborn lambs initially resuscitated with 100% O2 would have more pulmonary vasoconstriction in response to known vasoconstrictors such as hypoxia and thromboxane analog and less vasodilation to known vasodilators such as inhaled NO and acetylcholine infusion compared with lambs exposed to 21% O2.
METHODS
This study was approved by the Institutional Animal Care Committee at the State University of New York at Buffalo. Pregnant ewes approaching term (141 d gestation; term being approximately 145 d) were obtained from the Swartz family farm, Attica, NY. After 12 h of fasting, ewes were anesthetized with Pentothal and halothane. Newborn lambs were exteriorized by cesarean section. A small incision was made in the neck and systemic arterial and venous access was established through the carotid artery and jugular vein, respectively. Newborn lambs were intubated with a cuffed endotracheal tube. A left thoracotomy was performed and polyvinyl catheters were placed in the main pulmonary artery and left atrium. An ultrasonic flow transducer (Transonics Systems Inc., Ithaca, NY) was placed around the main pulmonary artery. The ductus arteriosus was ligated just before delivery. Mean pulmonary arterial pressure, left atrial pressure (in mm Hg), and pulmonary blood flow (in mL/min) were continuously measured and recorded. PVR was calculated as [(pulmonary arterial pressure – left atrial pressure)/pulmonary blood flow] and corrected for body weight. Arterial blood gases were analyzed from the carotid arterial catheter before delivery. The cord was clamped and tied; the lamb was delivered, weighed and transferred to a preheated servo-controlled radiant warmer. Lambs were sedated with fentanyl 5 μg/kg/ dose every 2 h PRN and received an initial dose of pancuronium bromide 0.1 mg/kg/dose at birth, which was repeated only if necessary for spontaneous movement despite adequate sedation. Rectal temperature was maintained between 37.9 and 39°C. Intravenous fluids (dextrose 10% solution with 25 mEq of sodium chloride, 20 mEq of potassium chloride, and 10 mEq of sodium bicarbonate per liter) were initially administered at 120 mL/kg/d. Fluid composition and rate were adjusted based on serum electrolyte values. The lambs were ventilated with Servo 300 ventilators (Siemens, Mississauga, ON, Canada) with the following initial settings: positive end-expiratory pressure (PEEP) 4, rate 40/min, peak inspiratory pressure (PIP) approximately 25 cm of water (adjusted to deliver 10 mL/kg tidal volume using a BiCore CP-100 Monitor (BiCore Monitoring systems, Irvine, CA). Arterial blood gases were monitored frequently (every 5–10 min) during initial stabilization. Ventilator settings (PIP and rate) were adjusted to maintain arterial PCO2 between 35 and 50 mm Hg.
The lambs were allocated randomly to the three resuscitation protocols before delivery. The lambs were initially ventilated with 21% (21% Res, n = 6), 50% (50% Res, n = 6), or 100% O2 (100% Res, n = 7) for 30 min. Subsequently, all lambs were ventilated with 21% O2 for 60 min. Hypoxic pulmonary vasoconstriction was then induced by ventilation with 10% O2 for 10 min. The lambs were allowed to recover in 21% O2 for approximately 20 min until the PVR returned to prehypoxic baseline.
To evaluate whether pulmonary vasoconstriction to exogenous agents is altered by prior ventilation with 21%, 50%, or 100% O2, an intravenous infusion of a thromboxane analog, U46619 (Sigma Chemical Co. Aldrich, St. Louis MO) was administered, initially at 0.5 μg/kg/min for 20 min followed by an increase to 1 μ g/kg/min. All lambs were ventilated with 21% O2 for the duration of the protocol. When the increase in PVR was stable, inhaled NO at 20 ppm was administered through the INOvent for 10 min. After a 20-min recovery period, acetylcholine was infused at 1 μg/kg/min over 10 min. The sequence of inhaled NO and acetylcholine infusion was not randomized. NO has a very short half-life and was infused before acetylcholine, a longer-acting stimulant of endothelial NO synthase. At the completion of the study, lambs were killed by an i.v. injection of 1 mL of Fatal-Plus (390 mg/mL pentobarbital, Vortech Pharmaceuticals, Dearborn, MI).
Statistical analysis
Differences among groups at a given time point were compared with ANOVA with Fisher’s protected least significant difference (PLSD) post hoc test when appropriate. Sequential changes in PVR over time were compared with ANOVA repeated measures. Changes in PVR with interventions within a group were analyzed using paired t test. A p value of < 0.05 was considered significant. We used Statview 4.5 software (Abacus Concepts, Berkeley, CA) for the analysis.
RESULTS
Changes in PVR with ventilation at birth
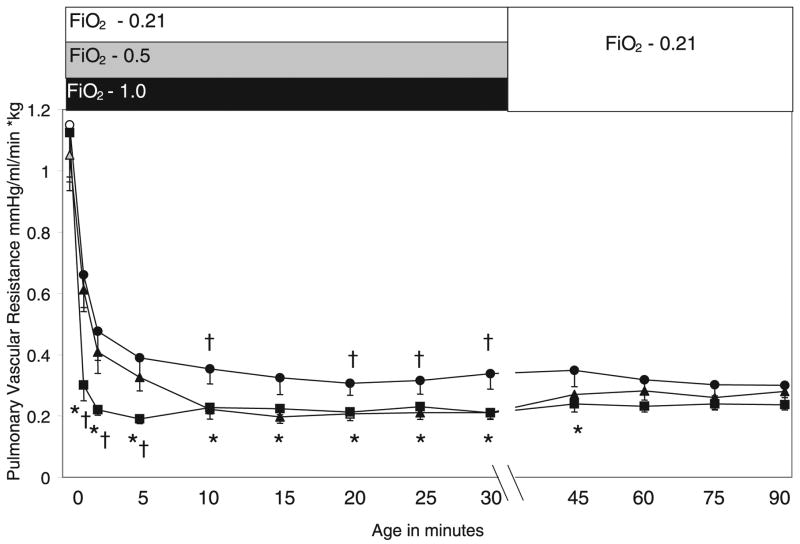
There were no significant differences in the birth weights or fetal blood gases among the three groups of lambs (Table 1). Following the initial 30 min of ventilation with different concentrations of O2 there was a significant difference in the arterial O2 (PaO2) levels that paralleled inspired O2 concentration (Table 2). At cord clamping, PVR was similar in the three groups of lambs before the onset of ventilation (0-min point on Fig. 1). The decrease in PVR following ventilation was more marked in the 100% Res group than in the 21% group throughout the first 30 min of life. The PVR in the 50% Res lambs was similar to the 21% Res lambs for the first 5 min of life (and significantly different from 100% Res lambs), but subsequently was similar to 100% Res lambs at 30 min. After 30 min, all lambs were ventilated with 21% O2 for 60 min (see Fig. 1). At 90 min of life, PVR was not significantly different between the three groups of lambs.
Table 1.
Birth weight and fetal arterial blood gases among the three groups (mean ± SEM)
| Groups | Birth weight (kg) | pH | Pco2 (mm Hg) | Po2 (mm Hg) | Base excess (mEq/L) |
|---|---|---|---|---|---|
| 21% Res | 3.403 ± 0.396 | 7.30 ± 0.02 | 59 ± 5 | 20 ± 1 | 1 ± 0.8 |
| 50% Res | 3.351 ± 0.158 | 7.29 ± 0.05 | 55 ± 5 | 16 ± 3 | −2 ± 2 |
| 100% Res | 3.768 ± 0.328 | 7.26 ± 0.05 | 59 ± 3 | 18 ± 2 | −2 ± 1.6 |
Table 2.
Arterial blood gases at the end of the initial 30 minutes of resuscitation among the three groups (mean ± SEM)
| Groups | pH | Pco2 (mm Hg) | Po2 (mm Hg) | Base excess (mEq/L) |
|---|---|---|---|---|
| 21% Res | 7.41 ± 0.04 | 38 ± 4 | 56 ± 7 | −3 ± 0.7 |
| 50% Res | 7.39 ± 0.02 | 38 ± 2 | 129 ± 13* | −2 ± 1 |
| 100% Res | 7.34 ± 0.04 | 38 ± 5 | 408 ± 24*† | −5 ± 1 |
p < 0.01 compared with 21% Res.
p < 0.01 compared with 50% Res.
Figure 1.
Changes in PVR during the first 90 min of life. Lambs were ventilated/resuscitated with 21% (●, n = 6), 50% (▲, n = 6), and 100% (■, n = 7) O2 for the first 30 min of life. Subsequently, lambs were ventilated with 21% O2 for 60 min. The bar at the top of the figure indicates the fraction of inspired oxygen. *p < 0.05 compared with 21% Res lambs, †p < 0.05 compared with 50% Res lambs. Please note a change in the x axis scale after 30 min.
Hypoxic pulmonary vasoconstriction
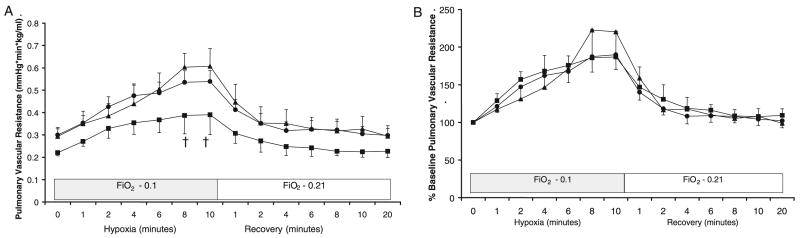
At 90 min of life, all groups of lambs were ventilated with 10% O2 for 10 min to induce hypoxic pulmonary vasoconstriction. Arterial blood gases and maximal PVR values were recorded during the final 2 min of hypoxic ventilation. The arterial oxygen tensions were similar in the three groups of lambs during this period of hypoxia (Table 3). Hypoxia induced a significant increase in PVR and PAP in all three groups (Table 3). There were no significant differences in the increase in PVR among the three groups when expressed as a percentage of baseline PVR (Fig. 2B). However, PVR values were significantly higher in 50% Res lambs compared with 100% Res lambs during the last 2 min of hypoxia (Fig. 2A). There was no significant change in systemic blood pressure during hypoxic ventilation (data not shown). During the recovery period (20 min of ventilation in 21% O2), PVR returned to baseline in all three groups of lambs (Fig. 2).
Table 3.
Arterial blood gases during the last 2 min of hypoxia (10% O2 ventilation for 10 min) and changes in PVR and PAP among the three groups of lambs (mean ± SEM)
| Protocol | pH | Pco2 (mm Hg) | Po2 (mm Hg) | Base excess (mEq/L) | Increase in PAP (mm Hg) |
|---|---|---|---|---|---|
| 21% Res | 7.39 ± 0.03 | 39 ± 2 | 16 ± 2 | −2 ± 1 | 20 ± 4* |
| 50% Res | 7.44 ± 0.02 | 34 ± 2 | 18 ± 1 | −2 ± 1 | 22 ± 2* |
| 100% Res | 7.39 ± 0.02† | 38 ± 2 | 16 ± 1.5 | −3 ± 1 | 21 ± 2* |
This increase in PAP reflects p < 0.05 compared with baseline.
p < 0.05 compared with 50% Res.
Figure 2.
Changes in PVR during 10 min of hypoxic (10% O2) ventilation and 20 min of recovery expressed as units (A) or percentage of baseline PVR before initiation of hypoxia (B). 21% Res (●, n = 6), 50% Res (▲, n = 6), and 100% Res (■, n = 7) O2; †p < 0.05 compared with 50% Res lambs.
Effect of U46619 infusion
Systemic infusion of U46619 at both 0.5 μg/kg/min and 1 μg/kg/min increased PVR and mean PAP significantly in the three groups of lambs (Table 4). The mean systemic blood pressure tended to increase during U46619 infusion (9 ± 3 mm Hg increase at 1 μg/kg/min in 21% Res, 7 ± 2 mm Hg in 50% Res, and 12 ± 3 mm Hg in 100% Res groups, statistically insignificant). The final PVR on U46619 infusion was not significantly different between the three groups.
Table 4.
Arterial blood gases during U 46619 infusion (1 μg/kg/min) and changes in PVR and PAP among the three groups of lambs (mean ± SEM)
| Protocol | pH | Pco2 (mm Hg) | Po2 (mm Hg) | Base excess (mEq/L) |
|---|---|---|---|---|
| 21% Res | 7.34 ± 0.03 | 42 ± 4 | 36 ± 5 | −4 ± 1 |
| 50% Res | 7.31 ± 0.02 | 45 ± 3 | 46 ± 7 | −4 ± 1 |
| 100% Res | 7.37 ± 0.02† | 40 ± 2 | 42 ± 5 | −3 ± 1 |
|
| ||||
| Protocol | Baseline PVR immediately prior to infusion of U46619 | PVR during U46619 0.5μg/kg/min | % Change in PVR from baseline (0.5 μg/kg/min) | |
|
| ||||
| 21% Res | 0.31 ± 0.03 | 0.72 ± 0.2‡ | 258 ± 70 | |
| 50% Res | 0.28 ± 0.05 | 0.6 ± 0.2‡ | 275 ± 54 | |
| 100% Res | 0.22 ± 0.02* | 0.75 ± 0.2‡ | 321 ± 81 | |
|
| ||||
| Protocol | PVR during U46619 1 μg/kg/min | % Change in PVR from baseline (1 μg/kg/min) | Increase in PAP with U46619 at 1 μg/kg/min (mm Hg) | |
|
| ||||
| 21% Res | 1.07 ± 0.25‡ | 343 ± 77 | 35 ± 5 | |
| 50% Res | 1.03 ± 0.4‡ | 401 ± 121 | 36 ± 3 | |
| 100% Res | 1.12 ± 0.3‡ | 496 ± 112 | 36 ± 3 | |
p < 0.05 compared with 21% Res.
p < 0.05 compared with 50% Res.
p < 0.01 compared with baseline PVR in the same group.
Inhaled NO
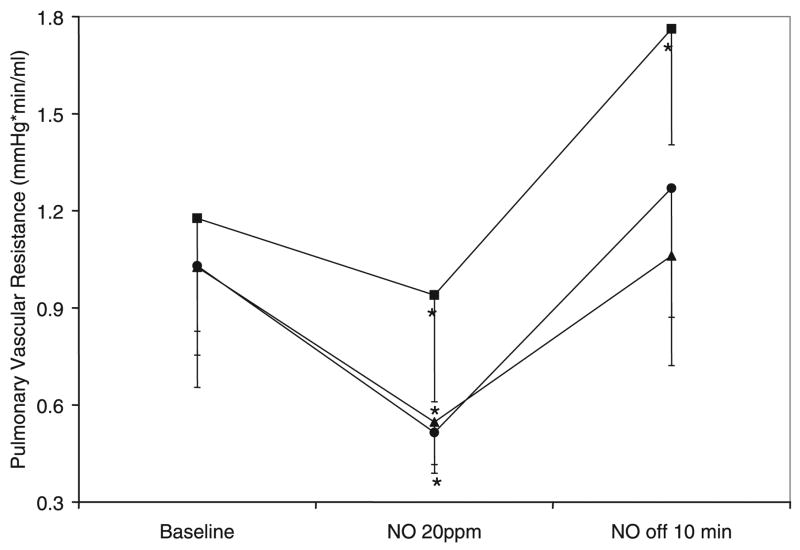
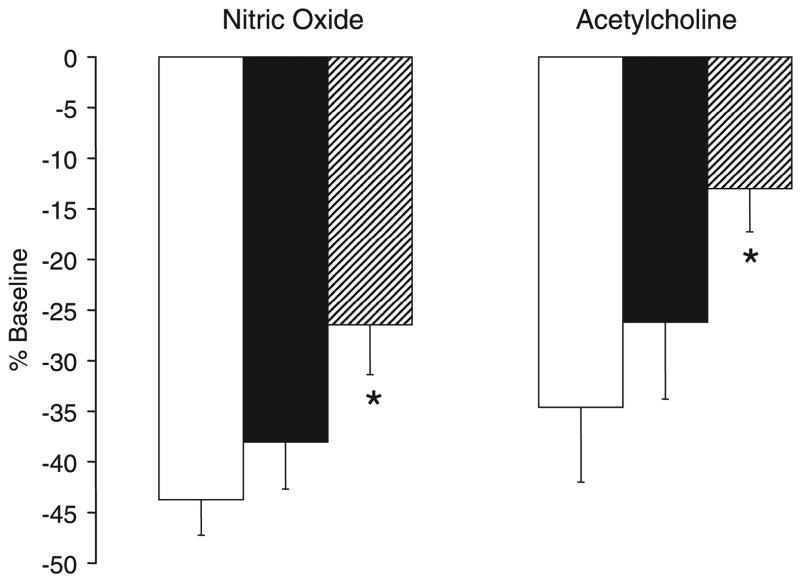
Inhaled NO at 20 ppm administered with 21% O2 ventilation and continuous U46619 infusion at 1 μg/kg/min significantly decreased PVR in all three groups of lambs (Fig. 3). When expressed as a percentage of baseline PVR, the decrease induced by inhaled NO was significantly impaired in lambs that were exposed to 100% O2 at birth (100% Res) compared with 21% Res group (Fig. 4). Moreover, 100% Res lambs had significant rebound pulmonary hypertension (defined for this study as increase in PVR compared with baseline before NO) following withdrawal of inhaled NO, whereas the other groups did not (Fig. 2). Response to inhaled NO in 50% Res lambs was similar to 21% Res lambs.
Figure 3.
Decrease in PVR with inhaled NO at 20 ppm. Decrease in PVR during 10 min of NO inhalation with 21% O2 ventilation and infusion of U46619 at 1 μg/kg/min. Rebound pulmonary hypertension was recorded 10 min after cessation of NO inhalation. 21% Res (●, n = 6), 50% Res (▲, n = 6), and 100% Res (■, n = 7) O2, *p < 0.05 compared with corresponding baseline PVR.
Figure 4.
Decrease in PVR following inhaled NO (20 ppm) and infusion of acetylcholine at 1 μg/kg/min expressed as a percentage of baseline PVR. 21% Res (open bars), 50% Res (solid bars), and 100% Res (hatched bars) O2. *p < 0.05 compared with 21% Res group.
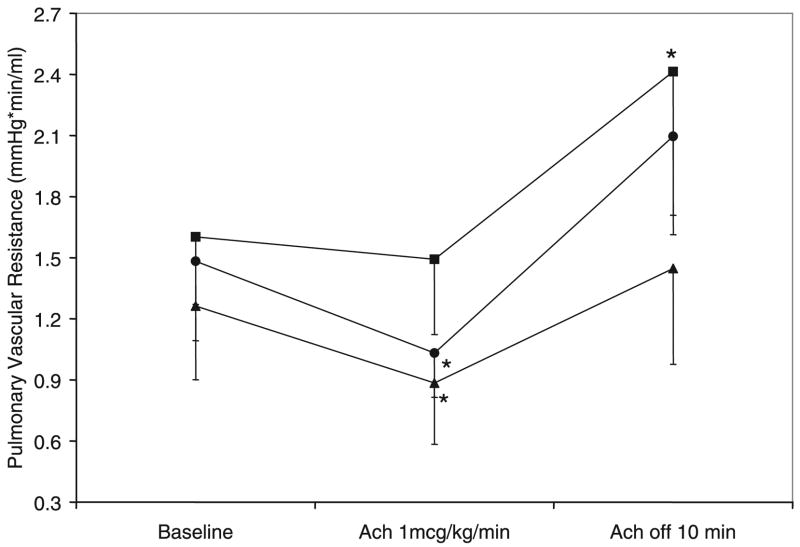
Acetylcholine infusion
PVR returned to a stable elevated level 10 –15 min following the end of NO inhalation while they continued to be ventilated with 21% O2 and receive a systemic venous infusion of U46619 at 1 μg/kg/min. Then, all groups of lambs received an intrapulmonary arterial infusion of acetylcholine at 1 μg/kg/min. Acetylcholine infusion resulted in a significant decrease in PVR in 50% Res and 21% Res groups but not in 100% Res lambs (Fig. 5). The decrease in PVR as expressed as a percentage of baseline was significantly less in the 100% Res group compared with the 21% Res group (Fig. 4). Following cessation of acetylcholine infusion, PVR increased significantly above the baseline before acetylcholine in the 100% Res group but not in the other groups (Fig. 5).
Figure 5.
Changes in PVR during infusion of acetylcholine at 1 μg/kg/min. Rebound pulmonary hypertension was recorded 10 min after cessation of acetylcholine infusion. 21% Res (●, n = 6), 50% Res (▲, n = 6), and 100% Res (■, n = 7) O2. *p < 0.05 compared with corresponding baseline PVR.
DISCUSSION
While the new guidelines for neonatal resuscitation do not mandate the use of 100% O2, it is still the recommended strategy for term neonates (3,15) despite considerable amount of human and animal data demonstrating toxicity of exposure to 100% O2 (2). Data suggesting that ventilation with O2 reduces PVR better than a nitrogen-rich gas at birth in lambs (6) have been used as an argument to promote 100% O2 use in the resuscitation of depressed newborn infants. In the current study, we have demonstrated that ventilation of newborn lambs with 100% O2 for 30 min does decrease PVR significantly more than ventilation with 21% O2. However, it is not clear this difference is beneficial. For instance, the rapid fall in PVR following 100% O2 ventilation may lead to pulmonary overcirculation, particularly in the presence of an open ductus arteriosus. The slower decrease in PVR observed with 21% O2 may be more physiologic. Moreover, there is impairment of pulmonary vasodilation to inhaled NO and acetylcholine more than 2 h after a 30-min exposure to 100% O2. We have also shown for the first time that ventilation with an intermediate level of O2 (50%), results in a slower decrease in PVR, that is similar in magnitude to 100% O2 at birth, and is not associated with impairment of pulmonary vasodilation to NO and acetylcholine.
The enhanced decrease we observed in PVR with 100% O2 ventilation at birth is contrary to data from Dawes et al. (4). In this widely quoted study, Dawes and co-workers ventilated 12 fetal lambs for 10 min (1.1–5.7 kg, 111–141 d gestation) and reported similar decreases in PVR with nitrogen, air, and 100% O2. However, the numbers in each group were small and the lambs were selected from a wide range of gestational age and birth weights that would have included lambs with extremely immature lungs and surfactant deficiency. Cassin et al. (7,8) ventilated ovine fetuses with nitrogen/7% carbon dioxide (a mixture referred to as “fetal gas”), pure nitrogen, or air and studied the effect on pulmonary blood flow. Ventilation with air (mechanical ventilation with decrease in PaCO2 and increase in PaO2) resulted in a significant increase in pulmonary blood flow. Ventilation with the fetal gas (mechanical ventilation without any change in PaCO2 or PaO2) resulted in one-third of the drop in PVR as air ventilation. Ventilation with nitrogen (mechanical ventilation without any change in PaO2 but decrease in PaCO2) resulted in two-thirds of the decrease in PVR as air ventilation. Unlike the Dawes study, these results clearly indicate that air ventilation is more effective than nitrogen ventilation and are similar to those reported by Teitel et al. (6). However, the effect of an intermediate level of O2 was not tested in any of these studies. Our study demonstrates that ventilation with 50% or 100% O2 results in rapid decline in PVR. However, by 90 min of age there is no significant difference in PVR regardless of the initial O2 concentration used during resuscitation (Fig. 1).
Contrary to our hypothesis, lambs previously exposed to 100% O2 did not have enhanced pulmonary vasoconstriction to hypoxic ventilation. This hypothesis was based on studies suggesting a role of reactive oxygen species (ROS) in mediating hypoxic pulmonary vasoconstriction (16). However, hypoxic pulmonary vasoconstriction is a complex phenomenon mediated by multiple factors such as endothelin, potassium channels, and the rhokinase pathway (17–22), which may not be altered by brief exposure to high O2 concentrations.
Intravenous infusion of a thromboxane analog (U46619) significantly increased PVR in all lambs (Table 2). The increase tended to be greater in 100% Res lambs, but this trend did not reach statistical significance. We have previously shown that a 30-min exposure to 100% O2 significantly increases fifth-generation pulmonary arterial contractility in vitro to norepinephrine and potassium chloride compared with 21% O2 (14). We speculate that we could not replicate this finding in vivo because of a) use of a different vasoconstrictor agent (thromboxane analog, U46619 instead of norepinephrine and potassium chloride); b) a higher degree of biologic variability in the intact lamb model, including the complex contribution of pulmonary arteries, arterioles and veins to PVR in the intact lung and lamb (23); and c) measurement at different points in time (24 h in our previous study versus 2–3 h in the current study). For instance, it is possible that a change in gene expression may require a longer interval to manifest as increased contractility. In the previous manuscript, by study design, lambs in the 100% Res group were exposed to 100% O2 for 30 min and then gradually weaned to 21% over the next 6 h. This resulted in exposure to higher concentrations of O2 for several hours compared with the current study. In the current study, O2 concentration was abruptly turned down from 100% to 21% at the end of 30 min.
Pulmonary vasodilation induced by inhaled NO (an endothelium-independent pulmonary vasodilator) and acetylcholine (an endothelium-dependent pulmonary vasodilator that stimulates endothelial NO synthase) was significantly reduced in lambs resuscitated with 100% O2 (Figs. 3–5). Rebound pulmonary hypertension after discontinuation of these agents was also significantly higher in 100% Res group, but not in the other groups (Figs. 3 and 4). These differences were observed more than 2 h after initial ventilation with 100% O2. We speculate that ROS formation associated with 100% O2 resuscitation results in impaired vasodilation to NO and acetylcholine. An alternate possibility is that a ROS-induced increase in phosphodiesterase 5 (PDE5) enzyme activity following 100% O2 ventilation (Farrow KN et al., American Heart Association Scientific Sessions 2006, Nov 11–15, Chicago IL) results in lower concentrations of cGMP in pulmonary arteries in response to NO inhalation or acetylcholine infusion. One hundred percent oxygen ventilation is injurious to the developing lung (24) by the formation of ROS such as superoxide anions. ROS can react with arachidonic acid, leading to the formation of isoprostanes, which are potent constrictors in pulmonary arteries (25,26). Superoxide anions react avidly with NO to produce peroxynitrite, a potent oxidant with the potential to cause vasoconstriction, cytotoxicity, and damage to surfactant proteins and lipids (27,28). Newborn rat pulmonary arteries exposed to peroxynitrite increase isoprostane production by 10-fold, implicating these products as possible mediators of peroxynitrite-induced vascular constriction (29). These free radicals may also contribute to rebound pulmonary hypertension following withdrawal of inhaled NO and acetylcholine infusion as seen in our 100% O2 resuscitation lambs. It has been recently reported that ROS play an important role in mediating rebound pulmonary hypertension following acute withdrawal of NO by inactivating NO synthase (30). In our study, ventilation with 50% O2 was not associated with impaired vasodilator response to NO or rebound pulmonary hypertension.
There are several limitations to this study. The period of hypoxic ventilation may itself contribute to formation of ROS in the lung (31) and could have influenced the responses to NO and acetylcholine. However, we subjected all lambs to the same sequence of events, including hypoxic ventilation. We also did not perform any ROS measurements in the pulmonary vasculature. Hence, this study is descriptive in nature and we can only speculate about the possible role of ROS in these responses. The current observations in term ovine lambs may not be applicable to humans because of species differences. Similarly, these findings may be limited to term neonates with healthy lungs and not apply to neonates with diseased lungs or premature newborn infants with surfactant deficiency. Lastly, we ligated the ductus arteriosus before delivery, obviously an unusual event during normal transition. However, an open ductus arteriosus invalidates the measurement of pulmonary blood flow by a flow transducer on the main pulmonary artery. In addition, varied levels of O2 may influence the rapidity of ductal closure, thus changing pulmonary blood flow and PVR calculation independent of pulmonary vascular tone. Hence, we decided to ligate the ductus just before clamping the cord.
We conclude that ventilation at birth with 21% O2 results in rapid reduction in PVR and does not interfere with subsequent vasodilation to NO and acetylcholine, whereas resuscitation with 100% O2 impairs subsequent vasodilation. We speculate that when supplemental O2 is required, an intermediate level of O2 such as 50% may be preferable to 100% O2. Ventilation with 50% O2 reduces PVR to levels seen with 100% O2 by 5 min of age and does not significantly impair vasodilation to NO and acetylcholine. Future studies assessing the role of hyperoxic ventilation in an ovine model of PPHN are planned.
Acknowledgments
The authors thank INO Therapeutics for providing inhaled NO and INOvents for this study.
Supported by American Academy of Pediatrics/Neonatal Resuscitation Program 2006 grant (SL) and Grant #HL-54705 (RHS). AQ: Please write out/provide full names of funding institutions.
Abbreviations
- PAP
pulmonary arterial pressure
- PVR
pulmonary vascular resistance
References
- 1.Lakshminrusimha S, Steinhorn RH. Pulmonary vascular biology during neonatal transition. Clin Perinatol. 1999;26:601–619. [PubMed] [Google Scholar]
- 2.Saugstad OD, Ramji S, Vento M. Oxygen for newborn resuscitation: how much is enough? Pediatrics. 2006;118:789–792. doi: 10.1542/peds.2006-0832. [DOI] [PubMed] [Google Scholar]
- 3.International Liaison Committee on Resuscitation. The International Liaison Committee on Resuscitation (ILCOR) consensus on science with treatment recommendations for pediatric and neonatal patients: neonatal resuscitation. Pediatrics. 2006;117:e978–e988. doi: 10.1542/peds.2006-0350. [DOI] [PubMed] [Google Scholar]
- 4.Dawes GS, Mott JC, Widdicombe JG, Wyatt DG. The effect of ventilation on pulmonary blood flow in the newborn lamb. J Physiol. 1952;118:45P–46P. [PubMed] [Google Scholar]
- 5.Dawes GS, Mott JC, Widdicombe JG, Wyatt DG. Changes in the lungs of the new-born lamb. J Physiol. 1953;121:141–162. doi: 10.1113/jphysiol.1953.sp004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teitel DF, Iwamoto HS, Rudolph AM. Changes in the pulmonary circulation during birth-related events. Pediatr Res. 1990;27:372–378. doi: 10.1203/00006450-199004000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Cassin S, Dawes GS, Mott JC, Ross BB, Strang LB. The vascular resistance of the foetal and newly ventilated lung of the lamb. J Physiol. 1964;171:61–79. doi: 10.1113/jphysiol.1964.sp007361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassin S, Dawes GS, Ross BB. Pulmonary blood flow and vascular resistance in immature foetal lambs. J Physiol. 1964;171:80–89. doi: 10.1113/jphysiol.1964.sp007362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vento M, Asensi M, Sastre J, Lloret A, Garcia-Sala F, Vina J. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J Pediatr. 2003;142:240–246. doi: 10.1067/mpd.2003.91. [DOI] [PubMed] [Google Scholar]
- 10.Vento M, Sastre J, Asensi MA, Vina J. Room-air resuscitation causes less damage to heart and kidney than 100% oxygen. Am J Respir Crit Care Med. 2005;172:1393–1398. doi: 10.1164/rccm.200412-1740OC. [DOI] [PubMed] [Google Scholar]
- 11.Fugelseth D, Borke WB, Lenes K, Matthews I, Saugstad OD, Thaulow E. Restoration of cardiopulmonary function with 21% versus 100% oxygen after hypoxaemia in newborn pigs. Arch Dis Child Fetal Neonatal Ed. 2005;90:F229–F234. doi: 10.1136/adc.2004.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medbo S, Yu XQ, Asberg A, Saugstad OD. Pulmonary hemodynamics and plasma endothelin-1 during hypoxemia and reoxygenation with room air or 100% oxygen in a piglet model. Pediatr Res. 1998;44:843–849. doi: 10.1203/00006450-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Tollofsrud PA, Solas AB, Saugstad OD. Newborn piglets with meconium aspiration resuscitated with room air or 100% oxygen. Pediatr Res. 2001;50:423–429. doi: 10.1203/00006450-200109000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, 3rd, Swartz DD, Kumar VH. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2006;59:137–141. doi: 10.1203/01.pdr.0000191136.69142.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Heart Association; American Academy of Pediatrics. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: neonatal resuscitation guidelines. Pediatrics. 2006;117:e1029–e1038. doi: 10.1542/peds.2006-0349. [DOI] [PubMed] [Google Scholar]
- 16.Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2003;285:L322–L333. doi: 10.1152/ajplung.00337.2002. [DOI] [PubMed] [Google Scholar]
- 17.Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothe-lin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res. 2005;57:631–636. doi: 10.1203/01.PDR.0000159512.55862.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCulloch KM, Osipenko ON, Gurney AM. Oxygen-sensing potassium currents in pulmonary artery. Gen Pharmacol. 1999;32:403–411. doi: 10.1016/s0306-3623(98)00219-5. [DOI] [PubMed] [Google Scholar]
- 19.Medbo S, Froen JF, Saugstad OD. Effects of selective inhibition of the endothelin A and B receptors on hypoxic pulmonary vasoconstriction in newborn piglets. J Perinat Med. 2001;29:344–350. doi: 10.1515/JPM.2001.049. [DOI] [PubMed] [Google Scholar]
- 20.Osipenko ON, Tate RJ, Gurney AM. Potential role for kv3.1b channels as oxygen sensors. Circ Res. 2000;86:534–540. doi: 10.1161/01.res.86.5.534. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Morio Y, Morris KG, Rodman DM, McMurtry IF. Mechanism of hypoxic pulmonary vasoconstriction involves ET(A) receptor-mediated inhibition of K(ATP) channel. Am J Physiol Lung Cell Mol Physiol. 2000;278:L434–L442. doi: 10.1152/ajplung.2000.278.3.L434. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Lanner MC, Jin N, Swartz D, Li L, Rhoades RA. Hypoxia inhibits myosin phosphatase in pulmonary arterial smooth muscle cells: role of Rhokinase. Am J Respir Cell Mol Biol. 2003;29:465–471. doi: 10.1165/rcmb.2002-0157OC. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Raj JU. Role of veins in regulation of pulmonary circulation. Am J Physiol Lung Cell Mol Physiol. 2005;288:L213–L226. doi: 10.1152/ajplung.00103.2004. [DOI] [PubMed] [Google Scholar]
- 24.Bush A. Update in pediatrics 2005. Am J Respir Crit Care Med. 2006;173:585–592. doi: 10.1164/rccm.2601002. [DOI] [PubMed] [Google Scholar]
- 25.Janssen LJ, Catalli A, Helli P. The pulmonary biology of isoprostanes. Antioxid Redox Signal. 2005;7:244–255. doi: 10.1089/ars.2005.7.244. [DOI] [PubMed] [Google Scholar]
- 26.Janssen LJ, Premji M, Netherton S, Coruzzi J, Lu-Chao H, Cox PG. Vasoconstrictor actions of isoprostanes via tyrosine kinase and Rho kinase in human and canine pulmonary vascular smooth muscles. Br J Pharmacol. 2001;132:127–134. doi: 10.1038/sj.bjp.0703784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 28.Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S. Mechanisms of peroxynitrite-induced injury to pulmonary surfactants. Am J Physiol. 1993;265:L555–L564. doi: 10.1152/ajplung.1993.265.6.L555. [DOI] [PubMed] [Google Scholar]
- 29.Belik J, Jankov RP, Pan J, Tanswell AK. Peroxynitrite inhibits relaxation and induces pulmonary artery muscle contraction in the newborn rat. Free Radic Biol Med. 2004;37:1384–1392. doi: 10.1016/j.freeradbiomed.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Oishi P, Grobe A, Benavidez E, Ovadia B, Harmon C, Ross GA, Hendricks-Munoz K, Xu J, Black SM, Fineman JR. Inhaled nitric oxide induced NOS inhibition and rebound pulmonary hypertension: a role for superoxide and peroxynitrite in the intact lamb. Am J Physiol Lung Cell Mol Physiol. 2006;290:L359–L366. doi: 10.1152/ajplung.00019.2005. [DOI] [PubMed] [Google Scholar]
- 31.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]