Abstract
Purpose
A woman’s risk for sexual disruption after breast cancer recurrence has received little clinical or research attention.
Patients and Methods
Breast cancer patients recently diagnosed with recurrence (n = 60) were initially assessed at baseline and completed follow-ups at 4, 8, and 12 months. They were compared by age, stage, and duration and frequency of follow-up with matched patients who remained disease free (n = 120). Using linear mixed modeling, the groups were compared in their trajectories of change on measures of sexuality, relationship satisfaction, cancer-specific stress, and physical functioning. Recurrence subgroups, those with locoregional versus distant disease and those younger versus older than 52 years, were also compared.
Results
At baseline, the recurrence group had significantly lower intercourse frequency and physical functioning compared with the disease-free group and these differences were maintained. There were no significant differences in the frequencies of kissing or sexual and relationship satisfactions. For the recurrence group patients, the heightened stress of the diagnostic/early recurrence treatment period declined to the lower disease-free levels by 12 months. This effect was largely due to improvement of the patients with distant disease. Finally, sexual changes were most notable for younger patients.
Conclusion
To our knowledge, this is the first longitudinal, controlled study of sexuality—sexuality in the context of other quality of life domains—for women coping with recurrence. Despite disruption, patients maintained their sexual lives. Younger and distant recurrence patients, however, may have greatest risk of sexual disruption. The factors contributing to sexual disruption remain unknown, and studies investigating strategies to help patients maintain this aspect of quality of life are needed.
INTRODUCTION
All cancer diagnoses, including recurrence,1 are associated with significant psychological stress.2 Up to 35% of patients may have mood, anxiety, or adjustment disorders.3,4 Moreover, significant deficits in health-related quality of life—cancer-specific domains in particular—are evident in patients with breast cancer recurrence.5,6 There is extensive literature on sexual morbidities associated with breast cancer; however, most studies are cross-sectional with newly diagnosed patients and focus on the impact of mastectomy7 or adjuvant treatments.8,9 In contrast, there have only been two studies of women with breast cancer recurrence. Silberfarb and colleagues10 found that patients with recurrent disease were 50% more likely to experience significant disruptions in desire and intercourse frequency than their newly diagnosed counterparts. Hanson Frost et al11 contrasted newly diagnosed women, undergoing chemotherapy, post-treatment, or with recurrent disease, and reported no group differences in sexuality or marital outcomes.
In sum, there are minimal data, and this may be related, in part, to the assumption that sexuality might be the least of a patient’s concerns. Alternatively, after recurrence, the need for intimacy and support may be considerable as the stress is great. We conducted a controlled, longitudinal study of patients with newly diagnosed breast cancer recurrence. We monitored behavioral and subjective dimensions of sexuality, including sexual behavior (frequency of intercourse and kissing) and global sexual and relationship satisfaction for coincident change. Sexuality is examined in context, as measures of stress, functional status, and symptoms/signs were included. There are significant decrements in sexuality after an initial diagnosis of breast cancer.5,12–16 As we were interested in determining any added burden of recurrence, a similar cohort of patients remaining disease free was selected as the referent group. The study aims were: to describe the trajectories of behavioral and subjective sexuality outcomes for those diagnosed with recurrence; to describe their stress and physical functioning trajectories for contextual reference; to compare these trajectories with those of patients remaining disease free and observed similarly; and to test for individual differences within the recurrence group, specifically patients differing in the extent of disease and age at recurrence diagnosis.
PATIENTS AND METHODS
An ongoing, randomized, clinical trial testing the efficacy of a psychological intervention for newly diagnosed breast cancer patients (N = 227) provided the context for this study. Details of accrual, procedures, and outcomes have been published.2,17 Briefly, consecutive patients from a university-affiliated comprehensive cancer center were accrued. Study arms were assessment only or psychological intervention and assessment. Intervention sessions were conducted during the first 12 months after accrual and patients were monitored with assessments every 4 months. When a patient experienced a recurrence, she was approached and consented to the recurrence study, which had required a separate review and approval from the local human investigations committee in accordance with an assurance filed with and approved by the Department of Health and Human Services.
Patient Groups
Recurrence group
Eleven years after the start of the randomized trial, data analyses for this recurrence substudy began. By then, 55 of 227 patients (24%) had experienced a recurrence. Women without a significant other (ie, romantic partner), diagnosed with a second primary tumor, or who experienced a recurrence fewer than 12 months after the initial diagnosis were not eligible. The latter criterion effectively excludes women with rapid disease progression. Of the 55 patients, 20 (36%) were not eligible. Of the remaining 35, seven (20%) had withdrawn from the randomized trial before their recurrence and three (9%) declined participation. Thus, 25 of 35 (71%) partnered, recurrent cancer patients were accrued. Of these patients, approximately one half (52%) were randomly assigned to the intervention arm.
During this same period, 59 other consecutive patients referred to the same medical oncology clinic for recurrent breast cancer were also accrued. Inclusion criteria were identical with one exception—eligibility in the randomized trial was restricted to women initially diagnosed with stage II or III disease, whereas women originally diagnosed with stage I-III were accrued from the clinic. Of the 59 accrued, 48 (81%) were partnered. In combination, 73 partnered patients with recurrence were enrolled.
After informed consent, patients completed face-to-face interviews and questionnaires. The baseline assessment was performed a median 10 weeks after diagnosis and then repeated 4, 8, and 12 months later. By the 12-month follow-up, six patients (8%) discontinued participation and seven (10%) had a second recurrence or died. Thus, data from 60 of 73 patients (82%) were analyzed.
Disease-free group
Patients from the randomized trial that had a partner, no evidence of disease, and no diagnosis of a second primary cancer were matched to the recurrence group patients. To increase the reliability of the estimates for the mixed-effects models and statistical power, each recurrence patient was matched to two disease-free patients. Patients were matched on age, follow-up duration, and, to the extent possible, initial stage. Of the patients identified as matches (n = 120), approximately one half (55%) were randomly assigned to the intervention arm.
Measures
Sexual behavior
Patients reported the frequency of sexual intercourse and kissing during the past 2 months, using an 8-point scale ranging from 0 (did not occur at all) to 7 (once/d for intercourse; 10-point scale ranging from 0 to 9 > 4 times/d for kissing).18 Four-month test-retest reliability was 0.74 for intercourse and 0.85 for kissing.
Sexual satisfaction
Participants were asked to rate their current sexual life using a 9-point scale ranging from 0 (could not be worse) to 8 (could not be better).19
Relationship satisfaction
Using the satisfaction item from the dyadic adjustment scale,20 participants rated their current overall satisfaction on a 7-point scale, ranging from 0 (extremely unhappy) to 3 (happy representing the degree of happiness in most relationships) to 6 (perfect). Correlations among the sexuality and relationship measures are provided in Table 1.
Table 1.
Correlations Among Sexuality and Relationship Variables at Initial Assessment for the Recurrence and Disease-Free Groups
| Group | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Recurrence | ||||
| Intercourse frequency | — | |||
| Kissing frequency | 0.19 | — | ||
| Sexual satisfaction | 0.58* | 0.31† | — | |
| Relationship satisfaction | 0.35† | 0.71* | 0.33† | — |
| Disease free | ||||
| Intercourse frequency | — | |||
| Kissing frequency | 0.44* | — | ||
| Sexual satisfaction | 0.65* | 0.36* | — | |
| Relationship satisfaction | 0.42* | 0.48* | 0.52* | — |
Correlation is significant at the .01 level (two tailed).
Correlation is significant at the .05 level (two tailed).
Cancer-Specific Stress
The impact of events scale (IES)21 examines anxious cognitions and avoidant thoughts and behaviors related to traumatic stress. Items were slightly reworded for patients to focus on cancer-related stresses. Total scores can range from 0 to 75. Distinctions among scores have been made (<10 = low; 10 to 19 = moderate; and >19 = high stress levels) in studies contrasting nonclinic and clinic patients seeking treatment for stress disorders.22 Internal consistency was 0.90.
Physical Functioning
Measures were completed by a nurse after a patient interview, with chart review and physician consultation as needed.
Functional status
The Karnofsky performance status (KPS)23 11-point scale ranges from 100 (normal) to 0 (dead). Inter-rater reliability ranges from 0.70 to 0.97.24,25
Symptoms, signs, illnesses, and toxicities
The type and severity of treatment toxicities and common symptoms/signs and illnesses (eg, fever, numbness) were rated.26 Items are grouped in 19 body categories (eg, cardiovascular), rated on a 0 to 4 scale, and then averaged. Internal consistency ranges from 0.67 to 0.83.1,17
Analytic Strategy
Descriptive statistics are provided and one-way analysis of variance or χ2 tests compare the groups on sociodemographic, disease, and treatment variables when initially diagnosed. Linear mixed-effects models27 tested for group differences and changes over time in trajectories. Within this framework, the fixed effects (group-average effects) were estimated to test for group differences at baseline (recurrence v disease free), changes over time within the recurrence group, and group by time interaction effects. Specifically, four coefficients were estimated for each variable: an intercept and a linear slope for the reference group and differences in intercept and slope in the comparison group (disease free) when contrasted with the recurrence group. The repeated assessments were coded as 0, 4, 8, and 12 so that the time elapsed in months between assessments is reflected in the estimations.
As there are individual differences in patients’ risk for sexual disruption,28,29 we used the same procedures in follow-up analyses with the recurrence group, contrasting the trajectories of patients with local (n = 21) versus distant (n = 39) metastatic disease and patients younger (n = 30; mean, 43 years; range, 29 to 51) versus older (n = 30; mean, 60 years; range, 52 to 81) when diagnosed with recurrence. We note that the age data were distributed normally with an equal mean and median of 51.5 and the median (52) was used to create the two groups. Thus, we contrasted women generally younger and older when in midlife (ie, 43 v 60 years). The following variables were controlled: age, education, original surgery (lumpectomy v mastectomy), location of recurrent disease (local v distant), and any therapies at recurrence (surgery, chemotherapy, and radiation therapy).
RESULTS
Preliminary Analyses and Description of the Groups
Table 2 presents sociodemographic, disease, and treatment characteristics. The groups did not differ on any sociodemographic variable (P > .07). The average disease-free interval for the recurrence group was 52 months (median, 33) and the average time since initial diagnosis for the disease-free group was 45 months (median, 37). As described, imbalance between groups in disease stage at initial diagnosis was anticipated (χ22 = 38.6; P <.001). Approximately 30% of the recurrence group had been initially diagnosed with stage I disease. Consistent with this, fewer of the recurrence group patients had been originally treated with chemotherapy (χ21, 7.2; P = .007) and/or hormone therapies (χ21, 4.4; P = .04).
Table 2.
Sociodemographic, Prognostic, and Treatment Variables for the Recurrence and Disease-Free Groups
| Recurrence (n = 60)
|
Disease Free (n = 120)
|
|||
|---|---|---|---|---|
| Variable | Mean (%) | SD | Mean (%) | SD |
| Sociodemographic | ||||
| Age, years | 51.7 | 10.0 | 51.4 | 9.2 |
| Race, white | 92 | 93 | ||
| Education, years | 14.8 | 3.0 | 15.4 | 2.9 |
| Marital status, married | 91 | 81 | ||
| Relationship duration, years | 26.8 | 12.4 | 25.4 | 12.2 |
| Family income, thousand $/year | 75.3 | 67.5 | 82.8 | 87.1 |
| Initial diagnosis | ||||
| Stage, % | ||||
| I | 28 | 0 | ||
| II | 60 | 90 | ||
| III | 12 | 10 | ||
| No. of positive nodes | 2.9 | 5.1 | 2.9 | 4.6 |
| ER/PR, % positive | 70 | 73 | ||
| Initial treatment | ||||
| Surgery, % modified radical mastectomy | 53 | 49 | ||
| Radiation therapy, % yes | 54 | 60 | ||
| Chemotherapy, % yes | 77 | 92 | ||
| Hormone therapy, % yes | 61 | 77 | ||
| Recurrence diagnosis | ||||
| Disease-free interval, months | 51.6 | 49.4 | ||
| Location of recurrent disease | ||||
| Local | 25 | |||
| Regional | 10 | |||
| Distant | 65 | |||
| Bone | 47 | |||
| Viscera | 27 | |||
| Treatments for recurrence* | ||||
| Surgery, % yes | 33 | |||
| Radiation therapy, % yes | 33 | |||
| Chemotherapy, % yes | 75 | |||
| Hormone therapy, % yes | 62 | |||
| Bone marrow transplantation, % yes | 7 | |||
Abbreviations: SD, standard deviation; ER, estrogen receptor status; PR, progesterone receptor status.
Refers to treatments received during the first 12 months after recurrence diagnosis.
Comparison of Trajectories: Recurrence Versus Disease-Free Groups
Table 3 provides the observed means and standard deviations for all measures at baseline. Table 4 summarizes the results from the fixed-effects models comparing the mean trajectories of the groups. For each outcome, the values in the first row are the estimates of intercept and linear slope for the reference group (recurrence), and those in the second row are the estimates of differences in intercept and slope of the comparison group (disease free) from the reference group.
Table 3.
Observed Means and Standard Deviations for Sexuality, Stress, and Physical Functioning at Recurrence Diagnosis for Disease-Free and Recurrence Subgroups
| Outcome
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Free (n =120)
|
Recurrence (n = 60
|
Local Recurrence (n =21)
|
Distant Recurrence (n =39)
|
Younger Recurrence (n =30)
|
Older Recurrence (n =30)
|
|||||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Sexuality | ||||||||||||
| Intercourse frequency | 2.03 | 1.52 | 1.55 | 1.52 | 1.67 | 1.45 | 1.48 | 1.57 | 2.05 | 1.61 | 1.13 | 1.33 |
| Kissing frequency | 6.14 | 2.52 | 6.61 | 2.58 | 6.27 | 2.25 | 6.79 | 2.76 | 7.20 | 2.07 | 6.13 | 2.89 |
| Sexual satisfaction | 3.44 | 2.07 | 3.34 | 2.07 | 3.53 | 1.60 | 3.24 | 2.29 | 4.00 | 2.10 | 2.79 | 1.91 |
| Relationship satisfaction | 3.80 | 1.19 | 4.07 | 1.23 | 3.90 | 1.17 | 4.17 | 1.28 | 4.41 | 0.98 | 3.70 | 1.38 |
| Cancer-specific stress | ||||||||||||
| IES | 13.30 | 13.04 | 20.60 | 14.86 | 20.60 | 15.17 | 20.61 | 14.89 | 22.61 | 13.93 | 18.73 | 15.67 |
| Physical functioning | ||||||||||||
| SymS/Tox | 0.22 | 0.11 | 0.26 | 0.11 | 0.25 | 0.11 | 0.26 | 0.12 | 0.24 | 0.12 | 0.27 | 0.11 |
| KPS | 90.88 | 8.47 | 78.98 | 10.45 | 80.00 | 8.37 | 78.42 | 11.51 | 77.59 | 12.44 | 80.33 | 8.09 |
Abbreviations: SD, standard deviation; IES, impact of events scale; SymS/Tox, symptoms, signs, illnesses, and toxicities; KPS, Karnofsky performance status.
Table 4.
Significance Tests for Recurrence Versus Disease-Free Groups on Intercept and Slope Estimates and Their Differences
| Intercept
|
Slope
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Estimate | SE | t | P | Estimate | SE | t | P |
| Sexuality | ||||||||
| Intercourse frequency | ||||||||
| Recurrence | 1.59 | 0.19 | 8.22 | .00 | −0.02 | 0.01 | −1.21 | .23 |
| Disease free | 0.50 | 0.24 | 2.13 | .03* | 0.00 | 0.01 | 0.27 | .79 |
| Kissing frequency | ||||||||
| Recurrence | 6.80 | 0.34 | 19.92 | .00 | −0.04 | 0.03 | −1.57 | .12 |
| Disease free | −0.81 | 0.42 | −1.95 | .053 | 0.06 | 0.03 | 1.83 | .07 |
| Sexual satisfaction | ||||||||
| Recurrence | 3.27 | 0.25 | 12.84 | .00 | −0.01 | 0.02 | −0.36 | .72 |
| Disease free | 0.26 | 0.31 | 0.86 | .39 | 0.02 | 0.02 | 0.69 | .49 |
| Relationship satisfaction | ||||||||
| Recurrence | 3.94 | 0.14 | 27.41 | .00 | 0.00 | 0.01 | 0.31 | .76 |
| Disease free | −0.24 | 0.18 | −1.33 | .19 | −0.02 | 0.01 | −1.36 | .18 |
| Cancer-specific stress | ||||||||
| IES | ||||||||
| Recurrence | 19.23 | 1.70 | 11.29 | .00 | −0.36 | 0.14 | −2.68 | .01† |
| Disease free | −7.06 | 2.09 | −3.38 | .00* | 0.18 | 0.17 | 1.09 | .28 |
| Physical functioning | ||||||||
| SymS/Tox | ||||||||
| Recurrence | 0.26 | 0.01 | 18.51 | .00 | 0.00 | 0.00 | −1.85 | .07 |
| Disease free | −0.04 | 0.02 | −2.45 | .02* | 0.00 | 0.00 | 0.84 | .40 |
| KPS | ||||||||
| Recurrence | 80.29 | 1.01 | 79.78 | .00 | 0.11 | 0.13 | 0.88 | .38 |
| Disease free | 10.50 | 1.23 | 8.56 | .00* | −0.18 | 0.15 | −1.18 | .24 |
Abbreviations: IES, impact of events scale; SymS/Tox, symptoms, signs, illnesses, and toxicities; KPS, Karnofsky performance status.
Significant group difference at baseline.
Significant change over time within recurrence group.
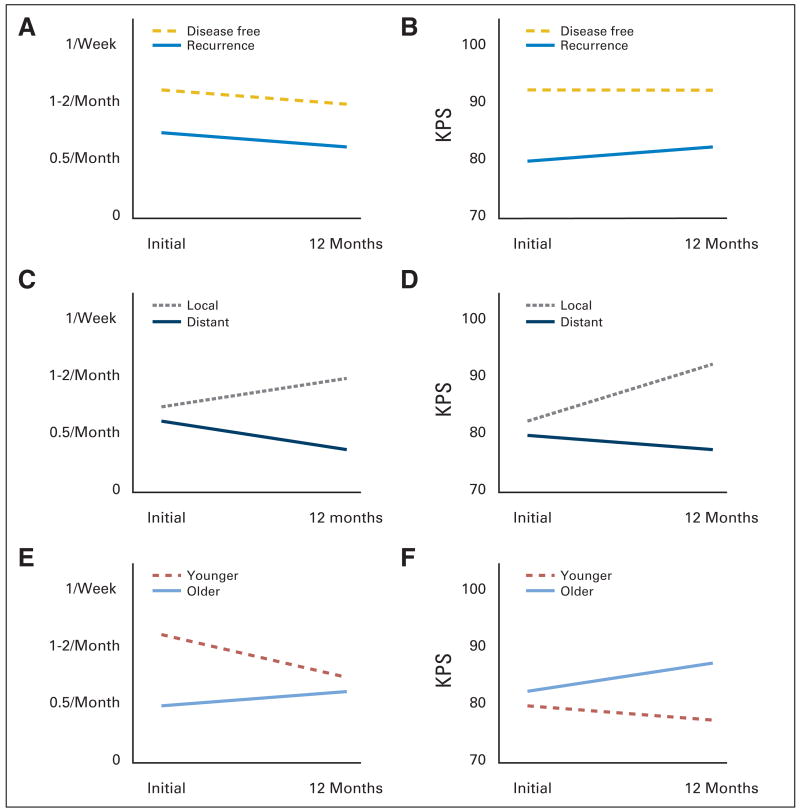
For the sexual outcomes, the recurrence group had a significantly lower intercourse frequency at baseline than the disease-free group (P = .03; corresponding to intercourse once per month v once to twice per month, respectively; Fig 1A). This difference between groups remained, as there was no significant change over time for the recurrence group and the rate of change for the disease-free group was not significantly different from that of the recurrence group. In contrast, the groups were equivalent in their reports of partner kissing (once per day) and their global evaluations of their current sexual life (somewhat inadequate) and relationship (very happy).
Fig 1.
Comparison of group trajectories from initial to 12-month follow-up for (A, C, E) frequency of intercourse and (B, D, E) functional status (Karnofsky performance status [KPS]) outcomes.
Regarding cancer-specific stress, the recurrence group had significantly higher stress at baseline than the disease-free group (P = .001). According to normative IES estimates,22 values were high for the recurrence group and moderate for the disease-free group. This heightened stress among recurrence group patients significantly declined (P = .008) to the moderate range (estimated mean, 14.87) by 12 months. The interaction was not significant. For physical functioning, the recurrence group had significantly more symptoms/signs (symptoms, signs, illnesses, and toxicities) and lower functional status (KPS) at baseline than the disease-free group (P < .02; Fig 1B), and these differences remained stable. For KPS, the mean value at baseline for the recurrence group corresponded to normal activity with effort (some signs/symptoms of disease), whereas the disease-free group corresponded to be able to carry on normal activity (minor signs/symptoms).
Comparison of Trajectories: Local Versus Distant Recurrence Groups
With regards to intercourse frequency, there were no group differences at baseline, with a frequency equivalent to intercourse once per month (Table 3); however, there were both significant time (P = .04) and interaction effects (P = .02; Fig 1C). While frequency of intercourse increased among the local recurrence group to once to twice per month, it declined further for the distant recurrence group to 0.5/month. Also, while the groups did not differ in the frequency of kissing at baseline (once per day), there was a significant decline for the distant group (P = .03; 4 to 6/week). Despite these behavioral differences, the groups did not differ in their global evaluations of sexual life or relationship satisfaction.
An interesting pattern was observed for the stress measure: there was a significant time effect for the distant group (P = .048). Their cancer-specific stress (IES) declined from a high (mean, 19.2) to a moderate level (mean, 14.1).22 There were no significant effects with regard to symptoms/signs (symptoms, signs, illnesses, and toxicities); however, there was a significant interaction effect for functional status (P = .01; Fig 1D). KPS of the local group improved (from 80 at initial to 88 at 12 months), whereas for the distant group it remained at a lower, stable level (from 80 at initial to 79 at 12 months).
Comparison of Trajectories: Younger Versus Older Patients at Recurrence
Table 3 presents the baseline data. The younger recurrence group had significantly higher intercourse frequency at baseline (P = .01; 1 to 2/month) versus the older recurrence group (0.5/month). However, this higher frequency of intercourse declined significantly across time (P = .01) and did so differentially (P = .01). By 12 months, intercourse frequency for the younger group declined to the stable level of the older group (0.5/month; Fig 1E). Also, the frequency of kissing significantly declined among the younger group from once per day to four to six times per week by 12 months (P = .03); the four to six times per week level of the older group remained stable. There were no significant effects for sexual and relationship satisfaction.
Regarding cancer-specific stress, the younger group had significantly higher cancer-specific stress at baseline than the older group (P = .03) and this difference remained stable. For physical functioning, there was a marginally significant interaction effect for both symptoms/signs (P = .06) and functional status (P = .09; Fig 1F). For the younger group, there were no changes, but symptoms/signs declined and functional status improved for the older group.
DISCUSSION
Currently, metastatic breast cancer is considered incurable, and the goals of treatment are generally palliative.30 Nevertheless, with the development of newer, more effective agents, such as trastuzumab31 and bevacizumab in combination with chemotherapy, improvements in time to progression are realized.32 While long-term survival rates remain modest, many patients are living longer with minimal disease-related symptoms. Thus, quality of life issues are salient.
To our knowledge, this is the first longitudinal, controlled study of sexuality, and sexuality in the context of physical health and other quality of life domains, for women coping with their recent recurrence diagnosis. Overall, both disease-free and recurrence group patients rated their sexual lives as somewhat inadequate. How these patients viewed their sexual lives before the diagnosis of breast cancer is unknown; however, data does show that sexuality declines after a cancer diagnosis and does not improve over time.14 The only significant difference between the groups was on the frequency of intercourse, suggesting that is the aspect of sexuality most vulnerable to disruption after recurrence diagnosis. An important correlate of sexual well-being is the characteristics of one’s relationship, and the patients in both groups reported, on average, that they were very happy with their partner. To what extent a women’s positive view of her relationship is a buffer to further deteriorations in her sexual life or that of the couple is unknown, but raises an interesting hypothesis that could be tested in future studies.
The results of this study demonstrate the resilience of patients as they cope with recurrence. The recurrence diagnosis produces substantial stress and quality of life disruption.1,5,6 However, during the 12 months after diagnosis stress declined to eventually match that of the disease-free survivors—an effect occurring in large measure because of the emotional improvement for those with distant metastases. Unfortunately, there was no salutary effect on sexuality; both the frequency of intercourse and kissing declined significantly. While negative psychological effects of all kinds—depression, anxiety, stress—disrupt sexual desire, reduce the frequency of activities, and produce sexual problems and dysfunctions,33 these data suggest that psychological improvement does not necessarily prevent sexual behaviors from declining. This occurs even for the frequency of kissing, which is strongly correlated (r = 0.71) with patients’ reports of relationship satisfaction. Because of the limited focus of these data, we do not know if these declines mirror those of embracing, hand holding, and related affectionate behaviors. Finally, comparison of recurrence patients differing in age at diagnosis—patients in their 40s versus their 60s—suggests sexual activity changes most notably for younger women. The change is equivalent to having intercourse once or twice per month to once every 2 months. This change is also unwelcome, as trends (P = .10) in the observed data show their view of their sexual life declines from average to somewhat inadequate.
A strength of the study is the addition of time-equivalent assessments from a matched, disease-free sample, providing comparison analyses of patients’ trajectories across sexuality, stress, and health domains. Both behavioral and subjective aspects of sexuality were included. The primary measures were brief, but they are commonly used, robust indicators.18,34 The pattern of correlations among variables provided evidence for their separate consideration, with correlations in the moderate range with kissing most strongly related to relationship satisfaction and intercourse most strongly related to sexual satisfaction. With this foundation, future research might consider measures sampling a range of behavioral sexual expression that contribute to global satisfaction, such as sexual responsiveness, partner sexual functioning, and quality of communication, among others. Also, participation of sexual partners would provide additional insights as to how couples cope and attempt to maintain sexual activity when their partners have symptoms, are recovering from treatment, and/or have stress regarding body changes.35
The limitations of study were the restricted ethnic and educational sampling, and we are uncertain of the applicability of the findings to lesbian couples. Regarding generalizability, these findings may represent the best case scenario, as the patients’ relationships were predominantly long-term (20+ years) ones in which women reported being happy. Future studies should also include unpartnered women. Having limited these data to only partnered women may implicitly suggest to the reader that we view a woman’s sexual life as equivalent to that which she shares with a partner. We do not; indeed, women view themselves as the same sexual person whether or not they have a current partner,29 and future research needs to address the sexual needs of all patients, whether they happen to have a partner or not.
This study provides an important, although preliminary, view of the impact of breast cancer recurrence on women’s sexual activity and satisfaction. The presence of symptoms from the illness or treatment may play an influential role in the extent to which women can maintain (or recover) their sexual life during the first year after recurrence diagnosis. Younger patients may be most vulnerable to sexual disruption. Women coping with recurrence are attempting to maintain their quality of life—including their sexual life—as they undergo cancer treatment and re-establish emotional equilibrium.
Acknowledgments
Supported in part by the American Cancer Society Grants No. PBR-89 and RSGPB-03-248-01-PBP; Longaberger Company-American Cancer Society Grant No. PBR-89A for Breast Cancer Research; the Walther Cancer Institute; the U.S. Army Medical Research Acquisition Activity Grants No. DAMD17-94-J-4165, DAMD17-96-1-6294, and DAMD17-97-1-7062; National Institutes of Mental Health Grant No. 1 RO1 MH51487; the National Cancer Institute Grants No. KO5 CA098133 and RO1 CA92704; the General Clinical Research Center Grant No. MO1-RR0034; and the Ohio State University Comprehensive Cancer Center Grant No. P30 CA16058.
Footnotes
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Barbara L. Andersen, Kristen M. Carpenter, Hae-Chung Yang, Charles L. Shapiro
Financial support: Barbara L. Andersen
Administrative support: Barbara L. Andersen
Provision of study materials or patients: Barbara L. Andersen, Charles L. Shapiro
Collection and assembly of data: Barbara L. Andersen, Kristen M. Carpenter, Hae-Chung Yang
Data analysis and interpretation: Barbara L. Andersen, Kristen M. Carpenter, Hae-Chung Yang
Manuscript writing: Barbara L. Andersen, Kristen M. Carpenter, Hae-Chung Yang, Charles L. Shapiro
Final approval of manuscript: Barbara L. Andersen, Kristen M. Carpenter, Hae-Chung Yang, Charles L. Shapiro
References
- 1.Andersen BL, Shapiro CL, Farrar WB, et al. A controlled prospective study of psychological responses to cancer recurrence. Cancer. 2005;104:1540–1547. doi: 10.1002/cncr.21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenkins PL, May VE, Hughes LE. Psychological morbidity associated with local recurrence of breast cancer. Int J Psychiatry Med. 1991;21:149–155. doi: 10.2190/UU5G-MDJ3-U55G-33GQ. [DOI] [PubMed] [Google Scholar]
- 4.Okamura H, Watanabe T, Narabayashi M, et al. Psychological distress following first recurrence of disease in patients with breast cancer: Prevalence and risk factors. Breast Cancer Res Treat. 2000;61:131–137. doi: 10.1023/a:1006491417791. [DOI] [PubMed] [Google Scholar]
- 5.Oh S, Heflin L, Meyerowitz BE. Quality of life of breast cancer survivors after a recurrence: A follow-up study. Breast Cancer Res Treat. 2004;87:45–57. doi: 10.1023/B:BREA.0000041580.55817.5a. [DOI] [PubMed] [Google Scholar]
- 6.Thornton AA, Madlensky L, Flatt SW, et al. The impact of a second breast cancer diagnosis on health related quality of life. Breast Cancer Res Treat. 2005;92:25–33. doi: 10.1007/s10549-005-1411-7. [DOI] [PubMed] [Google Scholar]
- 7.Henson HK. Breast cancer and sexuality. Sex Disabil. 2002;20:261–275. [Google Scholar]
- 8.Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: Understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 9.Ganz PA, Desmond KA, Belin TR, et al. Predictors of sexual health in women after a breast cancer diagnosis. J Clin Oncol. 1999;17:2371–2380. doi: 10.1200/JCO.1999.17.8.2371. [DOI] [PubMed] [Google Scholar]
- 10.Silberfarb PM, Maurer LH, Crouthamel CS. Psychosocial aspects of neoplastic disease: I. Functional status of breast cancer patients during different treatment regimens. Am J Psychiatry. 1980;137:450–455. doi: 10.1176/ajp.137.4.450. [DOI] [PubMed] [Google Scholar]
- 11.Hanson Frost M, Suman VJ, Rummans TA, et al. Physical, psychological and social well-being of women with breast cancer: The influence of disease phase. Psychooncology. 2000;9:221–231. doi: 10.1002/1099-1611(200005/06)9:3<221::aid-pon456>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Wolberg WH, Romsaas EP, Tanner MA, et al. Psychosexual adaptation to breast cancer surgery. Cancer. 1989;63:1645–1655. doi: 10.1002/1097-0142(19890415)63:8<1645::aid-cncr2820630835>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Bull AA, Meyerowitz BE, Hart S, et al. Quality of life in women with recurrent breast cancer. Breast Cancer Res Treat. 1999;54:47–57. doi: 10.1023/a:1006172024218. [DOI] [PubMed] [Google Scholar]
- 14.Yurek D, Farrar W, Andersen BL. Breast cancer surgery: Comparing surgical groups and determining individual differences in postoperative sexuality and body change stress. J Consult Clin Psychol. 2000;68:697–709. doi: 10.1037//0022-006X.68.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen BL. Sexual functioning morbidity among cancer survivors. Current status and future research directions. Cancer. 1985;55:1835–1842. doi: 10.1002/1097-0142(19850415)55:8<1835::aid-cncr2820550832>3.0.co;2-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen BL, Jochimsen PR. Sexual functioning among breast cancer, gynecologic cancer, and healthy women. J Consult Clin Psychol. 1985;53:25–32. doi: 10.1037//0022-006x.53.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen BL, Broffitt B. Is there a reliable and valid self-report measure of sexual behavior? Arch Sex Behav. 1988;17:509–525. doi: 10.1007/BF01542339. [DOI] [PubMed] [Google Scholar]
- 19.Derogatis LR, Melisaratos N. The DSFI: A multidimensional measure of sexual functioning. J Sex Marital Ther. 1979;5:244–281. doi: 10.1080/00926237908403732. [DOI] [PubMed] [Google Scholar]
- 20.Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976;38:15–28. [Google Scholar]
- 21.Horowitz M, Wilner N, Alvarez W. Impact of event scale: A measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz MJ. Stress response syndromes and their treatment. In: Goldberger L, Breznitz S, editors. Handbook of Stress. New York, NY: The Free Press; 1982. pp. 711–732. [Google Scholar]
- 23.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod C, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- 24.Mor V, Laliberte L, Morris JN, et al. The Karnofsky performance status scale: An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 25.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45:2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Moinpour CM, Feigl P, Metch B, et al. Quality of life end points in cancer clinical trials: Review and recommendations. J Natl Cancer Inst. 1989;81:485–495. doi: 10.1093/jnci/81.7.485. [DOI] [PubMed] [Google Scholar]
- 27.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 28.Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol. 1997;65:221–229. doi: 10.1037//0022-006x.65.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen BL, Cyranowski JM. Women’s sexual self-schema. J Pers Soc Psychol. 1994;67:1079–1100. doi: 10.1037//0022-3514.76.4.645. [DOI] [PubMed] [Google Scholar]
- 30.Chung CT, Carlson RW. Goals and objectives in the management of metastatic breast cancer. Oncologist. 2003;8:514–520. doi: 10.1634/theoncologist.8-6-514. [DOI] [PubMed] [Google Scholar]
- 31.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 32.Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer. 2004;100:44–52. doi: 10.1002/cncr.11859. [DOI] [PubMed] [Google Scholar]
- 33.Dunn KM, Croft PR, Hackett GI. Association of sexual problems with social, psychological, and physical problems in men and women: A cross sectional population survey. J Epidemiol Community Health. 1999;53:144–148. doi: 10.1136/jech.53.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laumann EO, Gagnon J, Michael RT, et al. The Social Organization of Sexuality: Sexual Practices in the United States. Chicago, IL: University of Chicago Press; 1994. [Google Scholar]
- 35.Andersen BL, Anderson B, deProsse C. Controlled prospective longitudinal study of women with cancer: I. Sexual functioning outcomes. J Consult Clin Psychol. 1989;57:683–691. doi: 10.1037//0022-006x.57.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]