Abstract
In nonneuronal cells, the cell surface protein dystroglycan links the intracellular cytoskeleton (via dystrophin or utrophin) to the extracellular matrix (via laminin, agrin, or perlecan). Impairment of this linkage is instrumental in the pathogenesis of muscular dystrophies. In brain, dystroglycan and dystrophin are expressed on neurons and astrocytes, and some muscular dystrophies cause cognitive dysfunction; however, no extracellular binding partner for neuronal dystroglycan is known. Regular components of the extracellular matrix, such as laminin, agrin, and perlecan, are not abundant in brain except in the perivascular space that is contacted by astrocytes but not by neurons, suggesting that other ligands for neuronal dystroglycan must exist. We have now identified α- and β-neurexins, polymorphic neuron-specific cell surface proteins, as neuronal dystroglycan receptors. The extracellular sequences of α- and β-neurexins are largely composed of laminin-neurexin–sex hormone–binding globulin (LNS)/laminin G domains, which are also found in laminin, agrin, and perlecan, that are dystroglycan ligands. Dystroglycan binds specifically to a subset of the LNS domains of neurexins in a tight interaction that requires glycosylation of dystroglycan and is regulated by alternative splicing of neurexins. Neurexins are receptors for the excitatory neurotoxin α-latrotoxin; this toxin competes with dystroglycan for binding, suggesting overlapping binding sites on neurexins for dystroglycan and α-latrotoxin. Our data indicate that dystroglycan is a physiological ligand for neurexins and that neurexins' tightly regulated interaction could mediate cell adhesion between brain cells.
Keywords: neurexin; dystroglycan; synapse; LNS domain; laminin
Introduction
Neurexins are neuron-specific cell surface proteins (for review see Missler and Südhof, 1998a). Neurexins are expressed in hundreds of alternatively spliced isoforms that are developmentally and spatially regulated, suggesting a role in regulated cell–cell interactions (Ushkaryov et al., 1992, 1994; Ushkaryov and Südhof, 1993; Ullrich et al., 1995). All three vertebrate genes for neurexins contain two independent promoters that direct synthesis of the longer α-neurexins and the shorter β-neurexins. α-Neurexins are composed of a large extracellular sequence that includes six laminin-neurexin–sex hormone–binding globulin (LNS)*/laminin G (LG) domains with three interspersed EGF-like regions; in contrast, α-neurexins contain only a single LNS domain and no EGF-like sequences (see Fig. 1) . LNS domains are widely distributed globular domains that are named for the proteins in which they were originally defined and are also referred to as LG domains (for review see Missler and Südhof, 1998a; Timpl et al., 2000). Following the LNS domains, both α- and β-neurexins contain a glycosylation sequence, a single transmembrane region, and a short intracellular tail (see Fig. 1).
Figure 1.
Domain structures of a- and b-neurexins (top) and locations of Ig and GST fusion proteins used for binding studies (bottom). Principal features of neurexins are indicated on top: SP, signal peptide; LNS, LNS domains, named after laminin G-domain repeats, neurexins, and sex hormone–binding globulin, and also referred to as LG domains; EGF, EGF-like sequences; CHO, carbohydrate attachment site; T, transmembrane region; numbered arrows, positions of canonical alternative splice sites (Ullrich et al., 1995). The regions of neurexins included in the various Ig and GST fusion proteins are displayed below the neurexin diagram; these proteins are named after the neurexin from which they were derived, followed by a unique identifying number. The splice site inserts contained in the fusion proteins are indicated above the lines. A single variant for splice site no. 1 (which is not in an LNS domain) was analysed (+, B-variant; Ullrich et al., 1995) and three variants for splice site no. 2 in which “−” corresponds to the no-insert variant, “+” to the small insert (sequence in neurexin 1α: HSGIgHAM), and “++” to the large insert (sequence in neurexin 1α: HSGIgHAMVNKLHCS). Two variants were studied for splice site nos. 3 and 4 (“+,” 3 residue insert for splice site no 3 and 30 residues for splice site no. 4; “−,” no insert).
The receptor-like structures of neurexins, their highly polymorphic nature, and the spatial segregation of their splice forms led to the suggestion that neurexins function as cell adhesion and cell recognition molecules whose extracellular interactions are regulated by alternative splicing. In support of this hypothesis, β-neurexins bind to neuroligins, neuronal cell surface proteins that are concentrated in the postsynaptic density of excitatory synapses (Ichtchenko et al., 1995, 1996; Song et al., 1999). Binding of β-neurexins to neuroligins is dependent on alternative splicing and results in the formation of a cell adhesion junction (Irie et al., 1997; Nguyen and Südhof, 1997; Butz et al., 1998; Scheiffele et al., 2000). In contrast to β-neurexins, α-neurexins have no currently known extracellular cell adhesion partner, although they are more polymorphic than β-neurexins and are at least as abundant. The only characterized physiological ligands for α-neurexins are neurexophilins, small secreted glycoproteins that bind to the second LNS domain of α-neurexins in a splice site–independent manner (Petrenko et al., 1996; Missler and Südhof, 1998b; Missler et al., 1998). In addition, both α- and β-neurexins are high affinity receptors for α-latrotoxin, an excitatory neurotoxin from black widow spider venom that triggers synaptic vesicle exocytosis (Davletov et al., 1995; Sugita et al., 1999). Based on their structure and distribution, it appears likely that α-neurexins can also function as cell adhesion molecules, but no binding partner for such a role is known.
Like neurexins, dystroglycan is also a cell surface protein. However, unlike neurexins dystroglycan is expressed ubiquitously and is composed of two invariable subunits, α- and β-dystroglycan, that are derived by proteolytic cleavage from a single dystroglycan precursor and remain bound to each other on the cell surface (Ibraghimov-Beskrovnaya et al., 1992, 1993). The α-dystroglycan subunit contains an autonomously folded NH2-terminal domain and a highly glycosylated sequence (Brancaccio et al., 1997) and accounts for almost the entire extracellular part of dystroglycan. In contrast, the β-dystroglycan subunit is composed of a single transmembrane region and a cytoplasmic tail with little extracellular sequence. The function of dystroglycan has been well defined in embryonic development and in mature muscle cells (for reviews see Hemler, 1999; Henry and Campbell, 1999). Extracellularly, dystroglycan binds to the LNS domains of several extracellular matrix proteins, namely laminin, agrin, and perlecan (Ervasti and Campbell, 1993; Bowe et al., 1994; Campanelli et al., 1994; Gee et al., 1994; Peng et al., 1998; Henry et al., 2001). Intracellularly, it binds to dystrophin and utrophin, which in turn are connected to the actin cytoskeleton (Ibraghimov-Beskrovnaya et al., 1992, 1993; Ahn and Kunkel, 1993). By virtue of these interactions, dystroglycan links the intracellular actin cytoskeleton to the extracellular matrix, especially the basal lamina, and thereby connects cells mechanically in the context of an overall tissue. Dystroglycan function is essential, since its deletion leads to early embryonic lethality (Henry and Campbell, 1998). In addition, muscle dystroglycan is required for normal neuromuscular junction formation (Peng et al., 1999; Grady et al., 2000).
Dystrophin and dystroglycan are expressed abundantly in neurons where their function is unknown (Lidov et al., 1990; Kim et al., 1992; Ibraghimov-Beskrovnaya et al., 1993; Tian et al., 1996; Blake and Kröger, 2000). The extracellular space between neurons in adult brain lacks a basal lamina and does not contain the classical extracellular matrix proteins laminin, perlecan, collagen, or agrin (Jucker et al., 1996; Ruoslahti, 1996). Basal lamina in brain delineate blood vessels, which are contacted by astrocytic “feet” extensions but have no direct connections with neurons. Thus, dystroglycan in neurons has no known extracellular-binding partner, and its role is obscure. However, the function of neuronal dystroglycan may potentially be important not only for insight into neuronal cell biology but also for understanding muscular dystrophies, since these are often associated with cognitive impairment (Bresolin et al., 1994; Davies, 1997). In the present study, in a search for endogenous ligands of α-neurexins we identified dystroglycan as the major interacting partner. Extensive studies showed that dystroglycan binds tightly to single LNS domains in both α- and β-neurexins in a manner regulated by alternative splicing. Our studies suggest a novel role for dystroglycan in neurons as intercellular cell adhesion molecules by binding to neurexins.
Results
We chose a biochemical approach to search for endogenous ligands of α-neurexins. Using transfected COS cells, we produced a series of Ig fusion proteins in which sequences from α- and β-neurexins were linked to the human Ig Fc fragment (Fig. 1). The largest neurexin Ig fusion protein (Ig–N1α-1) contained almost the complete extracellular sequence of neurexin 1α, whereas the control Ig fusion protein (Ig–control) included only the signal peptide and 18 NH2-terminal amino acids of neurexin 1α (Fig. 1). To identify α-neurexin ligands, we immobilized purified Ig–N1α-1 and Ig–control protein on protein A–Sepharose and employed them in affinity chromatography experiments. Proteins in total rat brain homogenates were applied to the Ig fusion protein–Sepharose matrix in the presence of Ca2+, the Sepharose was washed extensively, and bound proteins were eluted in high salt buffer with and without Ca2+. The eluents were analyzed on silver-stained SDS-gels, revealing two major proteins in the eluents from the Ig neurexin–Sepharose that were absent from the control eluents (molecular weights ∼43 and ∼120 kD; Fig. 2) . These proteins were identified as α- and β-dystroglycan by amino acid sequencing and immunoblotting (unpublished data). In addition, both the neurexin and control eluents contained IgG heavy (IgG–HC) and light chains (IgG–LC) that were presumably bound to the protein A–Sepharose from the rat brain homogenate and minor bands of unknown provenance.
Figure 2.
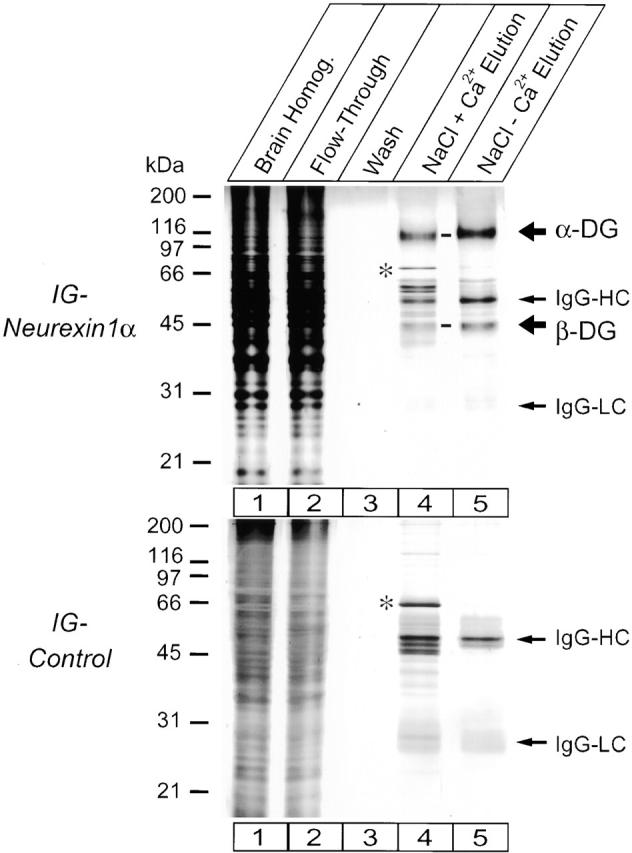
Affinity purification of dystroglycan on Ig–neurexin analyzed by SDS-PAGE and silver staining. Solubilized proteins from total rat brain homogenate (lane 1) were applied in 0.1 M NaCl and 2.0 mM Ca2+ buffer to a column containing immobilized Ig–N1α-1 or Ig–control proteins, and the flow-through was collected (lane 2). The column was washed extensively with loading buffer (lane 3) and 0.2 M NaCl, 2.0 mM Ca2+ buffer (not shown), and eluted with 1.0 M NaCl, 2.0 mM Ca2+ buffer (lane 4) followed by 1.0 M NaCl, 5 mM EGTA buffer (lane 5). Fractions were analyzed by SDS-PAGE and silver staining. The 120- and 43-kD proteins only present in the Ig–N1α-1 eluent correspond to α- and β-dystroglycan (α-DG and β-DG). IgG-HC and IgG-LC, respectively, and minor contaminants (for example, the 75-kD band labeled with an asterisk) were equally retained on the neurexin and the control column, presumably by binding to protein A–Sepharose.
The affinity chromatography experiments suggested that α- and β-dystroglycan are the only abundant rat brain proteins which interact strongly with the neurexin 1α Ig fusion protein (Fig. 2). To explore the specificity and tightness of dystroglycan binding to neurexin 1α, we probed the flow-through, washes, and high salt eluents of similar affinity chromatography experiments by immunoblotting. These results confirmed that the neurexin 1α column but not the control column strongly bound α- and β-dystroglycan (Fig. 3) . Strikingly, α- and β-dystroglycan were quantitatively removed from the flow-through of the neurexin 1α column, demonstrating that dystroglycan binding to neurexin 1α is of high affinity (Fig. 3, lane 2). No binding of neuroligin 1 to neurexin 1α was observed as expected from previous results showing that only β-neurexins bind to neuroligins (Ichtchenko et al., 1995, 1996).
Figure 3.
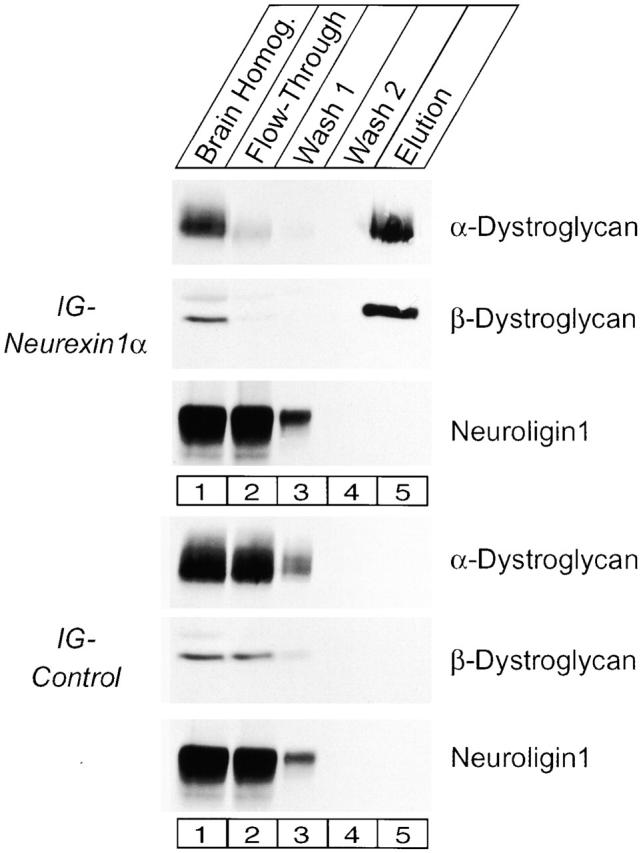
Quantitative binding of rat brain dystroglycan to immobilized neurexin 1α analyzed by immunoblotting. Rat brain proteins were bound to columns containing immobilized Ig–N1α-1 or Ig–control proteins, washed twice in binding buffer (Wash 1 and 2), and eluted with 1.0 M NaCl, 5 mM EGTA (Elution). Identical amounts of the various fractions were analyzed by immunoblotting for α- and β-dystroglycan and neuroligin 1. Note that dystroglycan is quantitatively depleted from the homogenates in the flow-through of the Ig–neurexin column, whereas neuroligin does not bind to neurexin 1α as shown previously (Ichtchenko et al., 1995, 1996). Numbers at the left in both panels indicate positions of molecular weight markers.
We next asked if native neurexin and dystroglycan form a physiological complex in brain. For this purpose, we immunoprecipitated α- and β-neurexins from brain homogenates with antibodies to the intracellular COOH terminus that is identical for both classes of neurexins (Fig. 1), using preimmune serum as a negative control (Hata et al., 1993). Proteins coimmunoprecipitated with α- and β-neurexins were eluted with Ca2+-free high salt buffer. Immunoblotting of the eluted proteins revealed that α-dystroglycan and neuroligin 1 were highly enriched in the immunoprecipitates with the neurexin antibody but were absent from the immunoprecipitates with preimmune serum (Fig. 4) . Synaptophysin and syntaxin, abundant synaptic proteins that are relatively “sticky,” were not brought down, confirming the specificity of the immunoprecipitation.
Figure 4.

Coimmunoprecipitation of neurexins with dystroglycan and neuroligin 1 from brain. Neurexin antibodies or preimmune sera were immobilized on protein A–Sepharose and incubated with rat brain proteins solubilized from synaptosomes (lane 1). After extensive washing (lanes 2–4), the protein A–Sepharose column was eluted with buffer containing 1.0 M NaCl, 5 mM EDTA (lane 5). The final wash and the EDTA eluent were concentrated with TCA, and equivalent aliquots were analyzed by immunoblotting with antibodies to the indicated proteins.
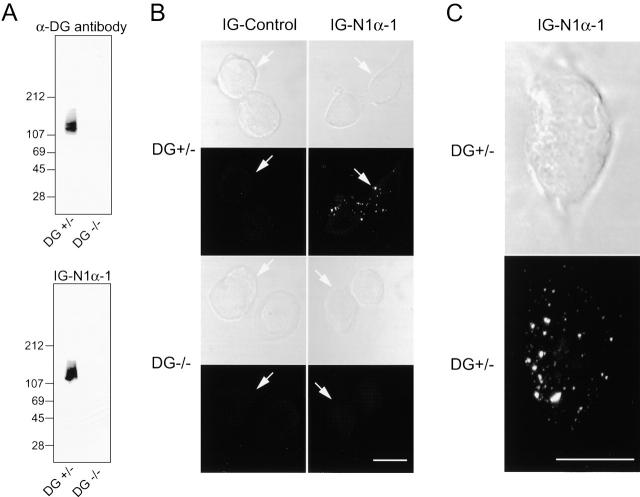
The immunoprecipitation and affinity chromatography experiments suggest that neurexins and dystroglycan form a stoichiometric complex in brain and that dystroglycan could constitute a cell surface receptor for α-neurexins. To test this directly, we made use of embryonic stem (ES) cells that are heterozygous (DG+/−) or homozygous (DG−/−) for a null mutation in the dystroglycan gene and either express or lack dystroglycan on the surface (Henry and Campbell, 1998). We partially purified dystroglycan from the ES cells by WGA affinity chromatography, separated the ES cell proteins on an SDS-gel, and blotted them onto PVDF membranes. The membranes were then probed with purified Ig–N1α-1 in an overlay assay and with antibodies to α-dystroglycan as a control (Fig. 5 A). We detected a single 130-kD protein that was bound by neurexin 1α Ig fusion protein and was absent from the dystroglycan-deficient cells (Fig. 5 A). The same 130-kD protein reacted with α-dystroglycan antibodies, suggesting that neurexin 1α directly binds to α-dystroglycan. We next examined if the neurexin Ig fusion protein binds specifically to the surface of cultured ES cells, using Ig–control protein as a negative control. Indeed, neurexin 1α Ig fusion protein only bound to ES cells containing dystroglycan but not to ES cells lacking dystroglycan; no binding of the Ig–control protein was observed (Fig. 5 B). Binding occurred in patches on the cell surface, suggesting that the neurexin 1α Ig fusion protein may cluster dystroglycan (Fig. 5 C). Together, these data demonstrate that endogenous dystroglycan on ES cells is a functional cell surface receptor for exogenous neurexin 1α.
Figure 5.
Binding to neurexin 1α to ES cells that express or lack dystroglycan. (A) Neurexin overlay analysis. Proteins were isolated by WGA affinity chromatography from ES cells that are heterozygous (DG+/−) or homozygous mutant (DG−/−) for the dystroglycan gene and analyzed by immunoblotting with an antibody to α-dystroglycan (top) or by the neurexin overlay assay using Ig–N1α-1 (bottom). Note that the neurexin Ig fusion protein only reacts with α-dystroglycan in the heterozygous mutant but not the homozygous mutant ES cells. (B and C) Cell surface binding. Cultured heterozygous and homozygous mutant ES cells containing or lacking dystroglycan (DG+/− or DG−/−, respectively) were reacted with Ig–control or Ig–N1α-1. Phase–contrast images are shown above the corresponding immunofluorescence images for each condition; cell boundaries are identified by arrows. Note that both dystroglycan and neurexin 1α are required to obtain cell surface reactivity. C depicts a high magnification view of a single dystroglycan-positive ES cell reacted with Ig–N1α-1 to highlight clustered neurexin/dystroglycan complexes on the cell surface. Bars, 10 μm.
Dystroglycan is a glycoprotein in which the α-subunit carries an uncommon O-mannosyl modification that is required for laminin binding and exhibits tissue-specific differences (Ibraghimov-Beskrovnaya et al., 1992, 1993; Ervasti and Campbell, 1993; Chiba et al., 1997). To test if dystroglycan glycosylation is involved in neurexin binding, we partially purified dystroglycan from mouse brain, skeletal and cardiac muscle, and lung tissue by WGA affinity chromatography. The partially purified proteins were then analysed with the neurexin overlay assay, using immunoblotting for α- and β-dystroglycan as a control. In the various tissues examined, α-dystroglycan exhibited distinct electrophoretic mobility patterns consistent with differential glycosylation, whereas β-dystroglycan was constant as expected (Fig. 6 A). With the neurexin Ig fusion protein overlay assay, we observed specific binding of neurexin 1α to α-dystroglycan in brain and muscle but not in lung. In brain and muscle, α-dystroglycan was the only protein in the WGA protein fraction that bound neurexin 1α (Fig. 6 A). Although fully reactive with antibodies, lung α-dystroglycan did not bind to neurexin 1α. To test the effect of dystroglycan glycosylation on neurexin 1α binding, we first deglycosylated the WGA-purified proteins from brain and lung with a mixture of glycolytic enzymes that cleave N- and O-linked sugars (endoglycosidase F, sialidase, and O-glycanase) (Fig. 6 B). Enzymatic deglycosylation led to a small shift in the apparent size of α-dystroglycan, presumably because only part of the complex carbohydrates are removed (Ervasti and Campbell, 1993). Enzymatic deglycosylation had little effect on neurexin 1α binding to brain α-dystroglycan but surprisingly activated neurexin 1α binding to lung α-dystroglycan, suggesting that lung α-dystroglycan was inactive because of a particular form of glycosylation (Fig. 5 B). We also performed complete deglycosylations of α-dystroglycan using trifluoromethanesulfonic acid (TFMS). Chemical deglycosylation caused a major decrease in the electrophoretic mobility of α-dystroglycan as described (Ervasti and Campbell, 1993) and completely abolished neurexin 1α binding as measured by the overlay assay in both brain and lung (Fig. 6 B). These data indicate that neurexin 1α interacts with α-dystroglycan only when α-dystroglycan is properly and specifically glycosylated.
Figure 6.

Tissue-specific glycosylation controls binding of neurexin 1α to α-dystroglycan. (A) Tissue distribution. Equivalent amounts of proteins isolated by WGA affinity chromatography from mouse brain, skeletal muscle, cardiac muscle, and lung were analyzed by immunoblotting with antibodies to β- and α-dystroglycan (β-DG and α-DG) and by the neurexin overlay assay with Ig–N1α-1. (B) Effect of deglycosylation. Mouse brain and lung proteins were enzymatically deglycosylated by treatment with N-glycosidase F, sialidase and O-glycosidase (left) or chemically deglycosylated by treatment with trifluoromethanesulfonic acid (right). Proteins were separated by SDS-PAGE, and α-dystroglycan was detected by immunoblotting (α-DG antibody) or by neurexin 1α overlay assay with Ig–N1α-1. Note that enzymatic deglycosylation (which only removes part of the complex sugars) activates neurexin 1α binding to lung dystroglycan, whereas complete chemical deglycosylation inhibits neurexin 1α binding without abolishing immunoreactivity. For all panels, numbers on the left indicate positions of the molecular weight markers.
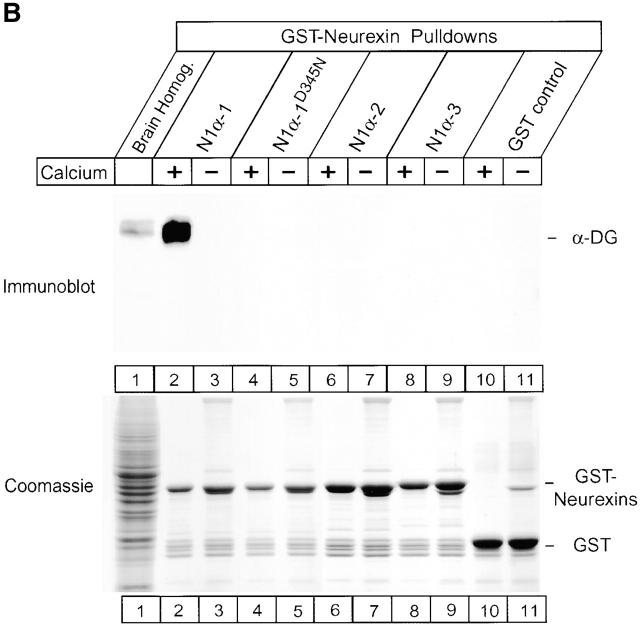
Which domain of neurexin 1α binds to dystroglycan, do other neurexins also bind to dystroglycan, and are the interactions of dystroglycan with neurexins regulated by alternative splicing of neurexins? To address these questions, we employed a series of shorter Ig fusion proteins of neurexins that incorporate a subset of the neurexin domains with selected splice variants (schematically described in Fig. 1). We tested which of these proteins bind to dystroglycan by immobilizing equivalent amounts of Ig fusion proteins on protein A–Sepharose for affinity chromatography experiments. Rat brain proteins were then applied to the columns in the presence and absence of Ca2+, and bound proteins were analysed by immunoblotting. A highly selective pattern of dystroglycan-binding emerged: only α-neurexins containing the second or the sixth LNS domain captured dystroglycan (Fig. 7 A). A single isolated LNS domain without flanking sequences fully bound to dystroglycan in a Ca2+-dependent reaction. The second LNS domain of all three α-neurexins (neurexin 1α, 2α, and 3α) formed tight Ca2+-dependent complexes with dystroglycan, suggesting that all α-neurexins bind to dystroglycan (Fig. 7 A). Furthermore, neurexin 1β (which contains only a single LNS domain that corresponds to the sixth LNS domain of neurexin 1α) also captured dystroglycan. These results suggest that both α- and β-neurexins bind to dystroglycan and confirm that a single LNS domain contains all information required for dystroglycan binding. Nevertheless, not all LNS domains interact with dystroglycan, since the first, third, fourth, and fifth LNS domains of neurexin 1α were inactive.
Figure 7.
Determinants of α-dystroglycan binding in the neurexin LNS domains. (A) Effect of alternative splicing. Equivalent amounts of the immobilized Ig–neurexin proteins as indicated (see Fig. 1 for a precise location of these proteins) were incubated with total rat brain homogenate in the presence and absence of Ca2+. Bound proteins were analysed by SDS-PAGE and immunoblotting for α-dystroglycan (α-DG; top) or Coomassie blue staining (bottom; arrows indicate the positions of respective Ig–neurexins). The highly glycosylated α-dystroglycan is poorly visible on the Coomassie-stained gel because small amounts of brain extracts were used, whereas all columns bind relatively large amounts of immunoglobulins (IgG-HC) via protein A–Sepharose. α-Dystroglycan binding is specific for constructs containing either the second or sixth LNS domain of α-neurexins or the single LNS domain of β-neurexins, but binding only occurs when these LNS domains include no alternatively spliced insert. (B) A Ca2+-binding site mutant abolishes binding. GST fusion proteins containing the second LNS domain of neurexin 1α without an insert with the wild-type sequence (N1α-1) or with a point mutation in a putative Ca2+-binding site (N1α-1D345N) and of the second LNS domain with the short (N1α-2) or long insert (N1α-3) and GST alone were used in pulldown experiments with brain homogenates with and without Ca2+. Bound proteins were analysed by immunoblotting with antibodies to α-dystroglycan (top) or Coomassie blue staining (bottom).
The neurexin Ig fusion proteins employed for the experiments in Fig. 7 A not only incorporate different LNS domains but also different splice variants of these domains. The second and sixth LNS domain of α-neurexins (the latter being identical with the single LNS domain of β-neurexins) are both alternatively spliced (Ullrich et al., 1995). Interestingly, for both LNS domains alternative splicing tightly regulated dystroglycan binding. Only the splice variants of these LNS domains that lack an insert interacted with dystroglycan (Fig. 7, A and B). Although the sites of alternative splicing are at different positions in the sequences of the second and sixth LNS domains, the crystal structure of the sixth LNS domain of neurexin 1α (Rudenko et al., 1999) showed that both splice sites are located in close proximity on the surface of the folded domain, suggesting that this location represents the binding site for dystroglycan. In the second LNS domain, the smallest splice insert contains only eight amino acids, indicating that a small change in the binding surface abolishes binding. These findings indicate that the regulated alternative splicing of neurexins can control their interactions with dystroglycan (Fig. 7).
The binding of neurexins to dystroglycan resembles binding of laminin, which is also Ca2+-dependent and mediated by LNS domains (for review see Timpl et al., 2000) but differs from laminin binding in that the alternative splicing of the neurexin LNS domains controls dystroglycan binding (Fig. 7 A). In the LNS domains from laminin, a Ca2+-binding site was defined structurally, and the residues involved in Ca2+-binding are conserved in the neurexin LNS domains (Hohenester et al., 1999; Rudenko et al., 1999; Tisi et al., 2000). To examine if the second LNS domain of neurexin 1α contains an analogous Ca2+-binding site, we mutated one of the aspartate residues of the putative Ca2+-binding site to asparagine. We then used glutathione S-transferase (GST) fusion proteins of wild-type and mutant LNS domains to test α-dystroglycan binding (Fig. 7 B). As with the Ig fusion proteins, the GST fusion protein bound dystroglycan in a Ca2+-dependent manner; binding was completely abolished by the Ca2+-binding site mutant. Furthermore, GST fusion proteins of the splice variants of the second LNS domain that were inactive in the Ig fusion proteins were also inactive in the GST fusion proteins (Fig. 7 B). Among others, these experiments suggest that neurexin LNS domains have Ca2+-binding sites similar to those of laminin but do not have to be glycosylated in order to bind to dystroglycan, since the bacterial GST fusion proteins are unlikely to be glycosylated.
Our data indicate the possibility that dystroglycan, currently known as an anchor protein for the extracellular matrix in nonneuronal cells but not as a cell adhesion molecule, may function in brain by interacting with neuron-specific neurexins. This hypothesis implies that brain dystroglycan should be expressed in a developmental profile similar to that of neurexins if they functionally cooperate. Indeed, immunoblot analysis of rat brain proteins at different stages of postnatal development revealed parallel expression of dystroglycan and neurexins (Fig. 8) . α-Dystroglycan and α- and β-neurexins were of low abundance prenatally, increased dramatically after birth, and experienced peak expression during the second postnatal week, a period of intense synaptogenesis. Thereafter, the levels of dystroglycan and neurexins declined together to a steady-state level that was approximately half of peak abundance at the end of the second postnatal week (Fig. 8, P15). This expression profile was different from that of laminin, a ligand for dystroglycan in basement membranes, which was expressed at a relatively constant low level similar to the ubiquitous control protein VCP. By contrast, synaptotagmin exhibited a cumulative increase in expression during development, in parallel with the continuous accumulation of synaptic vesicles in presynaptic nerve terminals as synapses mature.
Figure 8.

Developmental expression of α-dystroglycan. Dynamics of developmental expression of α-dystroglycan and neurexins. Brain homogenates from rats of the indicated ages were analyzed by SDS-PAGE (50 μg protein/lane) and immunoblotting with antibodies to the proteins shown. E17, embryonic day 17; P1–36, postnatal days 1–36. Note the multiple bands observed for α-neurexins, including very large proteins (>200 kD), which are due to the extensive alternative splicing of the α-neurexins. Asterisk identifies prominent proteolytic breakdown product of synaptotagmin I.
Together, these results suggest that α- and β-neurexins represent ligands for dystroglycan via interactions of their LNS domains, analogous to interaction of the LNS-domain in laminin, agrin, and perlecan with dystroglycan. The tight regulation of the neurexin–dystroglycan interaction by alternative splicing of neurexins is similar to the binding of α-latrotoxin to neurexins, which also requires variants lacking an insert in the site of alternative splicing in the sixth LNS domain (Sugita et al., 1999). We therefore tested if α-latrotoxin competes with dystroglycan for neurexin binding. Dystroglycan from rat brain homogenates was bound to immobilized neurexin 1α, and the ability of α-latrotoxin to displace dystroglycan in a concentration-dependent manner was tested (Fig. 9) . In the absence of α-latrotoxin, dystroglycan was stably bound to the Ig neurexin fusion protein. However, addition of increasing concentrations of α-latrotoxin released dystroglycan into the supernatant, suggesting that α-latrotoxin and dystroglycan bind to the same site on neurexins. α-Latrotoxin binds to two separate high affinity receptors in brain, neurexins, and CLs (CIRL/latrophilins) (Ushkaryov et al., 1992; Krasnoperov et al., 1997; Lelianova et al., 1997; Sugita et al., 1998, 1999). To investigate if dystroglycan may be a general endogenous ligand for high affinity α-latrotoxin–binding sites, we tested binding of dystroglycan to an Ig fusion protein of the extracellular sequences of CL1 but detected no binding (unpublished data). This is consistent with the fact that CL1 contains no LNS domains and binds to α-latrotoxin via a distinct mechanism.
Figure 9.
Dystroglycan and α-latrotoxin compete for the same binding site in neurexins. Ig–N1α-1 was loaded with α-dystroglycan by incubation with an excess of total rat brain homogenate (lane 1) and extensive washing to remove nonbound proteins. Beads containing Ig–N1α-1 with bound dystroglycan were incubated with buffer containing the indicated amounts of α-latrotoxin and then centrifuged to separate unbound proteins in the supernatant (S) from bound proteins in the pellet (P). Fractions were analysed by immunoblotting for α-dystroglycan (top) and α-latrotoxin (bottom). Note that α-latrotoxin quantitatively displaces bound α-dystroglycan. The slight difference in migration of α-dystroglycan on the SDS-gels between supernatant (S) and pellet (P) fractions may be due to differences in the buffer composition of the samples loaded or to preferential binding of a subset of glycosylated forms of dystroglycan by neurexin 1α.
Discussion
Our data demonstrate that dystroglycan and neurexins form a stoichiometric Ca2+-dependent complex in brain. This conclusion is supported by the following evidence. (a) Affinity chromatography on immobilized neurexin 1α purified α- and β-dystroglycan as major binding proteins (Fig. 2). (b) Immobilized neurexin 1α quantitatively removed α-dystroglycan from brain homogenates, indicating complete binding (Fig. 3). (c) Neurexins are coimmunoprecipitated with α- and β-dystroglycans from brain homogenates (Fig. 4). (d) The neurexin 1α overlay assay revealed specific binding of neurexin 1α to α-dystroglycan in ES cells (Fig. 5 A) and in mouse tissues (Fig. 6 A). (e) A soluble recombinant neurexin 1α Ig fusion protein specifically bound to the surface of cells expressing dystroglycan but not to cells lacking dystroglycan (Fig. 5 B). (f) Finally, the excitatory neurotoxin α-latrotoxin, which is a natural ligand for neurexins (Ushkaryov et al., 1992; Sugita et al., 1999), competes with dystroglycan for binding to neurexins (Fig. 9).
The neurexin–dystroglycan complex has several potentially interesting characteristics. The second and sixth LNS domains of α-neurexins and the single LNS domain of β-neurexins specifically bind to α-dystroglycan in a manner that is regulated by alternative splicing in an all-or-none fashion (Fig. 7, A and B). Thus, a single α-neurexin molecule can potentially bind multiple dystroglycans, which may explain the patchy appearance of bound neurexin on the surface of dystroglycan-expressing cells (Fig. 5 C). The fact that only an alternatively spliced subset of neurexins is capable of complexing with dystroglycan on the cell surface indicates that use of splice variants regulates neuronal interactions with dystroglycan. Glycosylation of dystroglycan is required for neurexin to bind. Dystroglycan exhibits tissue-specific differences in glycosylation (Ibraghimov-Beskrovnaya et al., 1992, 1993) that may be functionally relevant, since native lung dystroglycan was unable to bind to neurexin 1α but was activated by partial deglycosylation (Fig. 6 B). However, neurexin glycosylation is not required for dystroglycan binding, since bacterially expressed LNS domain from neurexin 1α was fully active (Fig. 7 B).
Neurexins constitute the first neuronal dystroglycan ligands that are also cell surface proteins. Conversely, dystroglycan is the first transmembrane ligand for α-neurexins that could involve α-neurexins in cell–cell interactions similar to the binding of neuroligins to β-neurexins (Ichtchenko et al., 1995, 1996; Irie et al., 1997; Nguyen and Südhof, 1997). The binding of neuroligins to β-neurexins has been implicated in synaptogenesis because neuroligins are highly enriched in postsynaptic densities (Ichtchenko et al., 1995; Song et al., 1999), the interaction of neuroligins with β-neurexins results in the formation of an intercellular junction that is coated on both sides by PDZ domain proteins (Irie et al., 1997; Butz et al., 1998), and expression of neuroligin in fibroblasts induces presynaptic specializations in nerve terminals that contact these fibroblasts (Scheiffele et al., 2000). It is possible that the interaction of dystroglycan with neurexins has a similar role in synaptogenesis or that this interaction serves in other contacts of neurons with each other or with astrocytes. Ultrastructural localization of neurexins and dystroglycan would be very helpful here but has proved exceedingly difficult especially for neurexins because we and others have been unable until now to raise antibodies with high enough affinity for EM.
In nonneuronal cells, the primary function of dystroglycan is to provide a transmembrane link between the extracellular matrix, especially basal lamina, and the intracellular actin cytoskeleton (for reviews see Hemler, 1999; Henry and Campbell, 1999). However, in fully developed brain neurons are not in direct contact with basement membranes or other types of extracellular matrix–containing laminin, agrin, or perlecan, the currently known ligands for dystroglycan (Ervasti and Campbell, 1993; Gee et al., 1993; Campanelli et al., 1996; Gesemann et al., 1996; Jucker et al., 1996; Ruoslahti, 1996; Tian et al., 1996; Peng et al., 1998; Serpinskaya et al., 1999; Talts et al., 1999). Thus, the primary function of dystroglycan in nonneuronal cells, to attach cells to the extracellular matrix, does not apply to neurons. Our results suggest that one of the ligands with which dystroglycan interacts in brain may be neurexins, thereby providing an explanation for the abundant expression of dystroglycan in neurons where its biological role is unknown (Lidov et al., 1990; Ibraghimov-Beskrovnaya et al., 1992, 1993; Tian et al., 1996; Blake and Kröger, 2000).
Although functionally different, the binding of dystroglycan to the LNS domains of neurexins follows the same paradigm as the binding of dystroglycan to the LNS domains in laminin and agrin, all of which bind to dystroglycan in a Ca2+-dependent reaction that requires glycosylation of dystroglycan and that is mediated by single LNS domains. The three-dimensional structures of LNS domains uncovered a similarity with lectins (Hohenester et al., 1999; Rudenko et al., 1999; Grishkovskaya et al., 2000), consistent with the notion that the carbohydrates on dystroglycan are bound directly by the LNS domain. Furthermore, a Ca2+-binding site was observed in the laminin LNS domain (Hohenester et al., 1999), and our mutational analysis indicates that neurexin LNS domains have a similar Ca2+-binding site (Fig. 7 B). Together, these data suggest a unitary structural mechanism by which LNS domains bind to dystroglycan; possibly other LNS domain proteins, such as NG2, CASPR, or slit (Rothberg et al., 1990; Nishiyama et al., 1991; Peles et al., 1997), may also bind. However, our results also reveal an unexpected versatility of LNS domains. The second LNS domain of α-neurexins interacts with two proteins, dystroglycan and neurexophilin, via distinct mechanisms. Neurexophilin binds to the second LNS domain independent of Ca2+ and irrespective of alternative splicing (Missler and Südhof, 1998b; Missler et al., 1998), whereas dystroglycan binding requires Ca2+ and is regulated by alternative splicing. This suggests that neurexophilin and dystroglycan contact distinct sites in the second LNS domain. The sixth LNS domain of α-neurexins, simultaneously the only LNS domain of β-neurexins, also participates in multiple interactions, namely with dystroglycan, neuroligins, and α-latrotoxin (Ichtchenko et al., 1995, 1996; Sugita et al., 1999). These interactions share the same Ca2+ requirement and regulation by alternative splicing, indicating that all three ligands may contact the same site on the surface of this LNS domain. Dystroglycan and α-latrotoxin bind to either α- and β-neurexins without regard to flanking sequences, in agreement with the finding that a single LNS domain is sufficient for dystroglycan binding (Fig. 7). However, neuroligins only bind to the β-neurexin LNS domain when it is preceded by the short sequence unique to β-neurexins, which thus confers specificity (Ichtchenko et al., 1995, 1996).
What is the function of the dystroglycan–neurexin complex? The stoichiometric nature and abundance of the complex suggest that it is physiologically important, but its localization is unclear. Indirect evidence links neurexins and dystroglycan to the synapse (Lidov et al., 1990; Ushkaryov et al., 1992; Hsueh et al., 1998; Song et al., 1999; Sugita et al., 1999; Blake and Kröger, 2000), an interesting possibility in view of the cognitive disorders associated with some cases of muscular dystrophy in which the dystroglycan-binding protein dystrophin is mutated (Bresolin et al., 1994). Both dystroglycan and α-neurexins are essential genes required for viability (Henry and Campbell, 1998). Although in mice dystrophin is not necessary for normal neuronal function, possibly because of redundancy, dystrophin-deficient mice exhibit a mislocalization of synaptic γ-aminobutyric acid receptors (Knuesel et al., 1999, 2001). This indicates that the dystrophin-related complex may be required for the proper localization of proteins to postsynaptic density in central synapses, similar to the role of postsynaptic dystroglycan in neuromuscular junctions (Grady et al., 2000). A synaptic cell adhesion complex composed of dystroglycan and neurexins would be ideally suited for synaptic interactions because similar to synapses this complex is asymmetric. Furthermore, the regulation of this complex by alternative splicing of neurexins could provide a mechanism of control whereby the type of alternative splicing would dictate the intercellular interactions of a neuron, and the polymorphism of neurexins would translate into a recognition code. Finally, our results suggest a mechanism by which some forms of muscular dystrophy could give rise to cognitive dysfunction, namely via a defective linkage of the dystroglycan–neurexin complex to dystrophin. However, these are speculative hypotheses at present that have to be explored in future studies.
Materials and methods
Construction of expression vectors and production of recombinant proteins
The following expression vectors for neurexin–Ig proteins constructed in pCMV-Ig (Davletov et al., 1995; Ichtchenko et al., 1995, 1996; Missler et al., 1998) were used (see Fig. 1 for the various splice variants present in these constructs): pCMVIgL-C = Ig–control (residues 1–48 of rat neurexin 1α; includes signal peptide and NH2-terminal 18 amino acids); pCMVIgbN1α-1 = Ig–N1α-1 (residues 1–1361 of bovine neurexin 1α); pCMVIgbN1α-14 and -15 = Ig–N1α-14 and -15 (residues 1–611 of bovine neurexin 1α); pCMVIgbN1α-18 = Ig–N1α-18 (residues 1–207 of bovine neurexin 1α); pCMVIgbN1α-21, -24, and -25 = Ig–N1α-21, -24, and -25 (residues 298–506 of bovine neurexin 1α fused to residues 1–48 of rat neurexin 1α as a signal peptide); pCMVbN1α-31 and -32 = Ig–N1α-31 and -32 (residues 1–42 [as signal peptide] fused to residues 920–1,361 of bovine neurexin 1α); pCMVIgbN1α-37 and -38-2 = Ig–N1α-37 and -38 (residues 1–42 fused to residues 738–931 of bovine neurexin 1α); pCMVIgbN1α-39 and -40-2 = Ig–N1α-39 and -40 (residues 1–42 fused to residues 486–931 of bovine neurexin 1α); pCMVIgN1β-1 and -3 = Ig–N1β-1 and -3 (residues 1–299 of rat neurexin 1β); pCMVIgN2α-5 = Ig–N2α-5 (residues 288–493 of neurexin 2α); pCMVIgN3α-6 = Ig–N3α-6 (residues 265–455 of neurexin 3α). Ig fusion proteins were produced by transfection of the pCMVIg constructs into COS cells using DEAE-dextran with chloroquine and a 2-min glycerol shock (Ichtchenko et al., 1995). The supernatant of transfected COS cells was harvested 3–4 d after transfection, adjusted to 10 mM Hepes-NaOH, pH 7.4, and 0.1 g/liter PMSF, cleared by centrifugation (2,500 g), incubated overnight with protein A–Sepharose (Amersham Pharmacia Biotech), and washed three times with PBS before use in affinity chromatography experiments. The GST protein vectors pGexbN1α(2LNSA), pGexbN1α(2LNSB), pGexbN1α(2LNSC), and pGexbN1α(2LNSAD345N) were constructed in pGEX-KG and encode GST-N1α-1, GST-N1α-2, GST-N1α-3, and GST-N1α-1D345N that contain the second LNS domain of bovine neurexin 1α (residues 293–491) with the indicate splice variants and point mutation (Fig. 1).
Affinity chromatography experiments
For Ig fusion protein affinity chromatography, two frozen rat brains were homogenized in 11 ml of 20 mM Hepes-NaOH, pH 7.4, 1 mM EGTA, and 0.1 g/liter PMSF, and an equal volume of the same buffer containing 0.2 M NaCl, 2% Triton X-100 was added. The homogenate was extracted for 1 h at 4°C, insoluble material was removed by centrifugation (30 min at 100,000 g), and 2.5 mM MgCl2 and 3.0 mM CaCl2 were added. Protein A–Sepharose containing Ig–N1α-1 or Ig–control (∼80 μg protein in 0.2 ml) were preequilibrated with buffer A (20 mM Hepes-NaOH, pH 7.4, 0.1 M NaCl, 1% Triton X-100, 2.5 mM MgCl2, 2 mM CaCl2), incubated overnight at 4°C with 10 ml of the brain extract, centrifuged (800 g for 2 min), and washed with 15 ml buffer A. Washed Sepharose was packed into polypropylene columns with paper discs (Quick-Sep; Isolab), washed again with buffer A (5 ml), and sequentially eluted with 4 ml of buffer A containing 0.2 M NaCl, 1.0 M NaCl, and 1.0 M NaCl with 5 mM EGTA instead of CaCl2. Eluted proteins were TCA precipitated, resuspended in 200 μl sample buffer, and 20 μl were analyzed by SDS-PAGE and silver staining. The 120-kD protein that was affinity purified on the Ig–N1α-1 was cut out of the gel, digested with trypsin, and tryptic fragments were purified by high performance liquid chromatography and analyzed by Edman degradation as described (Hata et al., 1993), identifying α-dystroglycan. The identity of both dystroglycan subunits was then confirmed by immunoblotting with specific antibodies. For analysis of how much of the dystroglycan in brain extracts bound to Ig–neurexins, 1 ml each of the brain extract was incubated with 20 μg of Ig–N1α-1 or Ig–control protein immobilized on protein A–Sepharose, affinity chromatography was performed essentially as above, and samples were analysed by SDS-PAGE and immunoblotting with antibodies to α- and β-dystroglycan (VIA4-1, Upstate Biotechnologies; and 43DAG/8D5, Novocastra) and to neuroligin (4C12; Song et al., 1999), with equal amounts of the total samples to allow precise comparisons. For domain analyses, 1 ml of brain extract containing 1 mM EGTA or 2 mM Ca2+ was incubated overnight with 30 μl protein A–Sepharose containing ∼8 μg of the various Ig neurexin fusion proteins (Fig. 1). The Sepharose beads were washed five times with 1 ml of the incubation buffers, resuspended in 250 μl of SDS-sample buffer, and 40 μl were analyzed by SDS-PAGE and Coomassie blue staining or immunoblotting. GST affinity chromatography experiments were carried out with GST fusion proteins immobilized on glutathione agarose (Sigma Aldrich) essentially as described above for the Ig affinity chromatography procedures. To test if α-latrotoxin can displace dystroglycan bound to neurexin, 10 ml brain extract were incubated for 5 h at 4°C with 200 μl of protein A–Sepharose containing 5 μg of Ig–N1α-1 to saturate binding of dystroglycan to neurexin 1α. After washing, the Sepharose beads were divided into nine tubes containing 0.1 ml buffer A with 0.5 μg of BSA and 0.01–40 nM α-latrotoxin. After overnight incubation, the supernatant was recovered by centrifugation (800 g), the Sepharose was washed three times with 0.5 ml buffer A, resuspendend in 80 μl sample buffer, and 10 μl of the supernatant and the bound material were analyzed by SDS-PAGE and immunoblotting.
Immunoprecipitations
Eight frozen rat brains were homogenized in 80 ml of 10 mM Hepes-NaOH, pH 7.2, 0.32 M sucrose, and 1 mM CaCl2, centrifuged 10 min at 800 g to remove debris, and recentrifuged for 20 min at 14,000 g. The resulting crude synaptosomes were resuspended for 30 min at 4°C in 40 ml buffer B (20 mM Hepes-NaOH, pH 7.4, 0.1 M NaCl, 1% Triton X-100, 1 mM CaCl2, and 2.5 mM MgCl2) with protease inhibitors (0.1 g/liter PMSF, 10 mg/liter leupeptin, 10 mg/liter aprotinin, and 1 mg/liter pepstatin A). Insoluble material was removed by centrifugation (30 min at 100,000 g), and 20 ml of the synaptosome extract were incubated overnight with 150 μl of a mixture of antibodies directed against the COOH terminus of all neurexins (A473, B186, G393, G394) (Ushkaryov et al., 1992) or of the preimmune serum for B186, followed by 2 h incubation with 200 μl of protein A–Sepharose preequilibrated with buffer B. Afterwards, samples were centrifuged (800 g for 2 min), the Sepharose beads were washed with 15 ml of buffer B, and packed into a polypropylene column. The column was washed with 5 and 4 ml buffer B, and eluted with 4 ml buffer B containing 1.0 M NaCl and 5 mM EDTA. The final wash and eluted materials were TCA precipitated, resuspended in 160 μl sample buffer, and 40 μl were analyzed by SDS-PAGE and immunoblotting using ECL detection.
Partial purification of dystroglycan
Brain, skeletal muscle, heart, and lung tissues from SVB/NJFVB mice were disrupted with a polytron followed by Dounce homogenization in 50 mM Tris-HCl, pH 7.4, 500 mM NaCl, 1% Triton X-100, 0.6 μg/ml pepstatin A, 0.5 μg/ml leupeptin, 0.5 μg/ml aprotinin, 0.75 mM benzamidine, 0.1 mM PMSF, 0.4 μg/ml calpain inhibitor 1, and 0.4 μg/ml calpeptin (buffer C). The homogenate was incubated for 2 h at 4°C, centrifuged at 140,000 g for 30 min at 4°C, and the supernatant was incubated with WGA agarose (Vector Laboratories) overnight at 4°C. The WGA agarose was washed with buffer C containing 0.1% Triton X-100 and eluted with 50 mM Tris-HCl, pH 7.4, 0.1% Triton X-100, and 0.3 M N-acetyl glucosamine. The eluate was concentrated with Centricon-30 (Amicon). Extraction of dystroglycan from ES cells was performed accordingly except that 50 mM Hepes, pH 7.4, 0.2 M NaCl, 1% NP-40, and protease inhibitor mixture was used instead of buffer C.
Deglycosylation
For enzymatic deglycosylation, partially purified dystroglycan (30 μl) was boiled for 5 min in the presence of 0.7% SDS. 1% Triton X-100 was added, and the pH was adjusted to 5.5 with 50 mM sodium-acetate. The preparation was incubated with 100 mU of sialidase and 2 mU of O-glycosidase (Roche) at 37°C for 16 h, the pH was neutralized with 0.1 M sodium-phosphate buffer, and the mixture was incubated with 10 mU of N-glycosidase F (Glyko) at 37°C for 16 h and boiled for 3 min. Control samples were treated identically without enzymes. For chemical deglycosylation (Ervasti and Campbell, 1993), dystroglycan (30 μl) was lyophilized, resuspended under nitrogen in 0.9 ml TFMS (Sigma-Aldrich) and 0.6 ml anisole (Sigma-Aldrich), and incubated 5 h on ice. The reaction was quenched with 1.6 ml ice-cold pyridin/H2O (3:5), dialyzed against cold distilled water, and lyophilized. Control samples were treated identically without TFMS. Samples were analyzed with the neurexin overlay assay or dystroglycan immunoblotting using mouse monoclonal antibody IIH6 and affinity purified rabbit and goat polyclonal antibodies to β- and α-dystroglycan, respectively (Ibraghimov-Beskrovnaya et al., 1992; Ervasti and Campbell, 1993).
Neurexin overlay assays
Proteins were separated by SDS-PAGE on 3–12% gradient gels and transferred to PVDF membranes. Membranes were blocked for 1 h in 5% dry milk buffer D (10 mM triethanolamine, pH 7.6, 140 mM NaCl, 1 mM CaCl2, 0.1% Tween 20), incubated overnight at room temperature in buffer D containing 0.9 mg/liter Ig–N1α-1 in 5% BSA, and washed three times with buffer D. Membranes were then incubated with HRP-conjugated protein A (Roche) for 1 h at room temperature and washed again three times with buffer D.
Cell culture and immunofluorescence analysis
Dystroglycan heterozygous (DG+/−) and homozygous mutant (DG−/−) R1 ES cells were cultured (Henry and Campbell, 1998) on coverslips coated with 5 μg/cm2 human fibronectin (Collaborative Biomedical Products). After 24 h, the culture medium was replaced with medium containing 10 mM CaCl2 and 75 nM Ig–N1α-1 or Ig–control, incubated overnight, washed with PBS, and fixed in 4% paraformaldehyde. Bound Ig fusion proteins were detected by Texas red–conjugated anti–human (Fc domain) antibody (Jackson ImmunoResearch Laboratories), and viewed by confocal microscopy (Henry and Campbell, 1998; Henry et al., 2001).
Acknowledgments
We are grateful to Drs. M. Missler, M.S. Brown, and J.L. Goldstein for advice, Drs. M. Missler and Y. Hata for the pCMVIg vectors, and Dr. M. Khvotchev for providing purified recombinant α-latrotoxin.
This study was supported by grants from the National Institutes of Health (RO1-MH52804 to T.C. Sudhof), by investigatorships from the Howard Hughes Medical Institute to K. Cambell and T.C. Sudhof, and by a postdoctoral fellowship from the Muscular Dystrophy Association to S. Sugita.
Footnotes
Abbreviations used in this paper: CL, CIRL/latrophilin; ES, embryonic stem; GST, glutathione S-transferase; HC, heavy chain; LC, light chain; LG, laminin G; LNS, laminin-neurexin–sex hormone–binding globulin; TFMS, trifluoromethanesulfonic acid.
References
- Ahn, A.H., and L.M. Kunkel. 1993. The structural and functional diversity of dystrophin. Nat. Genet. 3:283–291. [DOI] [PubMed] [Google Scholar]
- Blake, D.J., and S. Kröger. 2000. The neurobiology of Duchenne muscular dystrophy: learning lessons from muscles? Trends Neurosci. 23:92–99. [DOI] [PubMed] [Google Scholar]
- Bowe, M.A., K.A. Deyst, J.D. Leszyk, and J.R. Fallon. 1994. Identification and purification of an agrin receptor from torpedo postsynaptic membranes: a heteromeric complex related to the dystroglycans. Neuron. 12:1173–1180. [DOI] [PubMed] [Google Scholar]
- Brancaccio, A., T. Schulthess, M. Gesemann, and J. Engel. 1997. The N-terminal region of α-dystroglycan is an autonomous globular domain. Eur. J. Biochem. 246:166–172. [DOI] [PubMed] [Google Scholar]
- Bresolin, N., E. Castelli, G.P. Comi, G. Felisari, A. Bardoni, D. Perani, F. Grassi, A. Turconi, F. Mazzucchelli, and D. Gallotti. 1994. Cognitive impairment in Duchenne muscular dystrophy. Neuromuscul. Disord. 4:359–369. [DOI] [PubMed] [Google Scholar]
- Butz, S., M. Okamoto, and T.C. Südhof. 1998. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 94:773–782. [DOI] [PubMed] [Google Scholar]
- Campanelli, J.T., S.L. Roberds, K.P. Campbell, and R.H. Scheller. 1994. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AchR clustering. Cell. 77:663–674. [DOI] [PubMed] [Google Scholar]
- Campanelli, J.T., G.G. Gayer, and R.H. Scheller. 1996. Alternative RNA splicing that determines agrin activity regulates binding to heparin and α-dystroglycan. Development. 122:1663–1672. [DOI] [PubMed] [Google Scholar]
- Chiba, A., K. Matsumura, H. Yamada, T. Inazu, T. Shimizu, S. Kusunoki, I. Kanazawa, A. Kobata, and T. Endo. 1997. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve α-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of α-dystroglycan with laminin. J. Biol. Chem. 272:2156–2162. [DOI] [PubMed] [Google Scholar]
- Davies, K.E. 1997. Challenges in Duchenne muscular dystrophy. Neuromuscul. Disord. 7:482–486. [DOI] [PubMed] [Google Scholar]
- Davletov, B.A., V. Krasnoperov, Y. Hata, A.G. Petrenko, and T.C. Südhof. 1995. High affinity binding of α-latrotoxin to recombinant neurexin Iα. J. Biol. Chem. 270:23903–23905. [DOI] [PubMed] [Google Scholar]
- Ervasti, J.M., and K.P. Campbell. 1993. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 122:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, S.H., R.W. Blacher, P.J. Douville, P.R. Provost, P.D. Yurchenco, and S. Carbonetto. 1993. Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J. Biol. Chem. 268:14972–14980. [PubMed] [Google Scholar]
- Gee, S.H., F. Montanaro, M.H. Lindenbaum, and S. Carbonetto. 1994. Dystroglycan-α, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 77:675–686. [DOI] [PubMed] [Google Scholar]
- Gesemann, M., V. Cavalli, A. Denzer, A. Brancaccio, B. Schumacher, and M.A. Ruegg. 1996. Alternative splicing of agrin alters its binding to heparin, dystroglycan, and the putative agrin receptor. Neuron. 16:755–767. [DOI] [PubMed] [Google Scholar]
- Grady, R.M., H. Zhou, J.M. Cunningham, M.D. Henry, K.P. Campbell, and J.R. Sanes. 2000. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin-glycoprotein complex. Neuron. 25:279–293. [DOI] [PubMed] [Google Scholar]
- Grishkovskaya, I., G.V. Avvakumov, G. Sklena, D. Dales, G.L. Hammond, and Y.A. Muller. 2000. Crystal structure of human sex hormone-binding globulin: steroid transport by a laminin G-like domain. EMBO J. 19:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata, Y., C.A. Slaughter, and T.C. Südhof. 1993. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 366:347–351. [DOI] [PubMed] [Google Scholar]
- Hemler, M.E. 1999. Dystroglycan versatility. Cell. 97:543–546. [DOI] [PubMed] [Google Scholar]
- Henry, M.D., and K.P. Campbell. 1998. A role for dystroglycan in basement membrane assembly. Cell. 95:859–870. [DOI] [PubMed] [Google Scholar]
- Henry, M.D., and K.P. Campbell. 1999. Dystroglycan inside and out. Curr. Opin. Cell Biol. 11:602–607. [DOI] [PubMed] [Google Scholar]
- Henry, M.D., J.S. Satz, C. Brakebusch, M. Costell, E. Gustafsson, R. Fässler, and K.P. Campbell. 2001. Distinct roles for dystroglycan, α1 integrin and perlecan in cell surface laminin organization. J. Cell Sci. 114:1137–1144. [DOI] [PubMed] [Google Scholar]
- Hohenester, E., D. Tisi, J.F. Talts, and R. Timpl. 1999. The crystal structure of a laminin G-like module reveals the molecular basis of α-dystroglycan binding to laminins, perlecan, and agrin. Mol. Cell. 4:783–792. [DOI] [PubMed] [Google Scholar]
- Hsueh, Y.P., F.C. Yang, V. Kharazia, S. Naisbitt, A.R. Cohen, R.J. Weinberg, and M. Sheng 1998. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J. Cell Biol. 142:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya, O., J.M. Ervasti, C.J. Leveille, C.A. Slaughter, S.W. Sernett, and K.P. Campbell. 1992. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 355:696–702. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya, O., A. Milatovich, T. Ozcelik, B. Yang, K. Koepnick, U. Francke, and K.P. Campbell. 1993. Human dystroglycan: skeletal muscle cDNA, genomic structure, origin of tissue specific isoforms and chromosomal localization. Hum. Mol. Genet. 2:1651–1657. [DOI] [PubMed] [Google Scholar]
- Ichtchenko, K., Y. Hata, T. Nguyen, B. Ullrich, M. Missler, C. Moomaw, and T.C. Südhof. 1995. Neuroligin 1: A splice-site specific ligand for α-neurexins. Cell. 81435–443. [DOI] [PubMed]
- Ichtchenko, K., T. Nguyen, and T.C. Südhof. 1996. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J. Biol. Chem. 271:2676–2682. [DOI] [PubMed] [Google Scholar]
- Irie, M., Y. Hata, M. Takeuchi, K. Ichtchenko, A. Toyoda, K. Hirao, Y. Takai, T.W. Rosahl, and T.C. Südhof. 1997. Binding of neuroligins to PSD-95. Science. 277:1511–1515. [DOI] [PubMed] [Google Scholar]
- Jucker, M., M. Tian, and D.K. Ingram. 1996. Laminins in the adult and aged brain. Mol. Chem. Neuropathol. 28:209–218. [DOI] [PubMed] [Google Scholar]
- Kim, T.W., K. Wu, J.L. Xu, and I.B. Black. 1992. Detection of dystrophin in the postsynaptic density of rat brain and deficienty in a mouse model of Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA. 89:11642–11644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel, I., M. Mastrocola, R.A. Zuellig, B. Bornhauser, M.C. Schaub, and J.M. Fritschy. 1999. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice). Eur. J. Neurosci. 11:4457–4462. [DOI] [PubMed] [Google Scholar]
- Knuesel, I., R.A. Zuellig, M.C. Schaub, and J.M. Fritschy. 2001. Alterations in dystrophin and utrophin expression parallel the reorganization of GABAergic synapses in a mouse model of temporal lobe epilepsy. Eur. J. Neurosci. 13:1113–1124. [DOI] [PubMed] [Google Scholar]
- Krasnoperov, V.G., M.A. Bittner, R. Beavis, Y. Kuang, K.V. Salnikow, O.G. Chepurny, A.R. Little, A.N. Plotnikov, D. Wu, R.W. Holz, et al. 1997. α-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron. 18:925–937 [DOI] [PubMed] [Google Scholar]
- Lelianova, V.G., B.A. Davletov, A. Sterling, M.A. Rahman, E.V. Grishin, N.F. Totty, and Y.A. Ushkaryov. 1997. α-Latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J. Biol. Chem. 272:21504–21508. [DOI] [PubMed] [Google Scholar]
- Lidov, H.G., T.J. Byers, S.C. Watkins, and L.M. Kunkel. 1990. Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature. 348:725–728. [DOI] [PubMed] [Google Scholar]
- Missler, M., and T.C. Südhof. 1998. a. Neurexins: three genes and 1001 products. Trends Genet. 14:20–26. [DOI] [PubMed] [Google Scholar]
- Missler, M., and T.C. Südhof. 1998. b. Neurexophilins form a conserved family of neuropeptide-like glycoproteins. J. Neurosci. 18:3630–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler, M., R.E. Hammer, and T.C. Südhof. 1998. Neurexophilin binding to α-neurexins: a single LNS-domain functions as independently folding ligand-binding unit. J. Biol. Chem. 273:34716–34723. [DOI] [PubMed] [Google Scholar]
- Nguyen, T., and T.C. Südhof. 1997. Binding properties of neuroligin 1 and neurexin 1α reveal function as heterophilic cell adhesion molecules. J. Biol. Chem. 272:26032–26039. [DOI] [PubMed] [Google Scholar]
- Nishiyama, A., K.J. Dahlin, J.T. Prince, S.R. Johnstone, and W.B. Stallcup. 1991. The primary structure of NG2, a novel membrane-spanning proteoglycan. J. Cell Biol. 114:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles, E., M. Nativ, M. Lustig, M. Grumet, J. Schilling, R. Martinez, G.D. Plowman, and J. Schlessinger. 1997. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 16:978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, H.B., A.A. Ali, D.F. Daggett, H. Rauvala, J.R. Hassell, and N.R. Smalheiser. 1998. The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes. Comm. 5:475–489. [DOI] [PubMed] [Google Scholar]
- Peng, H.B., H. Xie, S.G. Rossi, and R.L. Rotundo. 1999. Acetylcholinesterase clustering at the neuromuscular junction involves perlecan and dystroglycan. J. Cell Biol. 145:911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko, A.G., B. Ullrich, M. Missler, V. Krasnoperov, T.W. Rosahl, and T.C. Südhof. 1996. Structure and evolution of neurexophilin. J. Neurosci. 16:4360–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg, J.M., J.R. Jacobs, C.S. Goodman, and S. Artavanis-Tsakonas. 1990. Slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 4:2169–2187. [DOI] [PubMed] [Google Scholar]
- Rudenko, G., T. Nguyen, Y. Chelliah, T.C. Südhof, and J. Deisenhofer. 1999. The structure of the ligand-binding domain of neurexin α: regulation of LNS domain function by alternative splicing. Cell. 99:93–101. [DOI] [PubMed] [Google Scholar]
- Ruoslahti, E. 1996. Brain extracellular matrix. Glcyobiology. 6:489–492. [DOI] [PubMed] [Google Scholar]
- Scheiffele, P., J. Fan, J. Choih, R. Fetter, and T. Serafini. 2000. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 101:657–669. [DOI] [PubMed] [Google Scholar]
- Serpinskaya, A.S., G. Feng, J.R. Sanes, and A.M. Craig. 1999. Synapse formation by hippocampal neurons from agrin-deficient mice. Dev. Biol. 205:65–78. [DOI] [PubMed] [Google Scholar]
- Song, J.-Y., K. Ichtchenko, T.C. Südhof, and N. Brose. 1999. Neuroligin 1 is a postsynaptic cell-adhesion moleculr of excitatory synapses. Proc. Natl. Acad. Sci. USA. 96:1100–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita, S., K. Ichtchenko, M. Khvotchev, and T.C. Südhof. 1998. α-Latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein linked receptors. J. Biol. Chem. 273:32715–32724. [DOI] [PubMed] [Google Scholar]
- Sugita, S., M. Khvochtev, and T.C. Südhof. 1999. Neurexins are functional α-latrotoxin receptors. Neuron. 22:489–496. [DOI] [PubMed] [Google Scholar]
- Talts, J.F., Z. Andac, W. Gohring, A. Brancaccio, and R. Timpl. 1999. Binding of the G domains of laminin α1 and α2 chains and perlecan to heparin, sulfatides, α-dystroglycan and several extracellular matrix proteins. EMBO J. 18:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, M., C. Jacobson, S.H. Gee, K.P. Campbell, S. Carbonetto, and M. Jucker. 1996. Dystroglycan in the cerebellum is a laminin α2-chain binding protein at the glial-vascular interface and is expressed in Purkinje cells. Eur. J. Neurosci. 8:2739–2747. [DOI] [PubMed] [Google Scholar]
- Timpl, R., D. Tisi, J.F. Talts, Z. Andac, T. Sasaki, and E. Hohenester. 2000. Structure and function of laminin LG modules. Matrix Biol. 19:309–317. [DOI] [PubMed] [Google Scholar]
- Tisi, D., J.F. Talts, R. Timpl, and E. Hohenester. 2000. Structure of the C-terminal laminin G-like domain pair of the laminin α2 chain harbouring binding sites for α-dystroglycan and heparin. EMBO J. 19:1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich, B., Y.A. Ushkaryov, and T.C. Südhof. 1995. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 14:497–507. [DOI] [PubMed] [Google Scholar]
- Ushkaryov, Y.A., and T.C. Südhof. 1993. Neurexin IIIα: extensive alternative splicing generates membrane-bound and soluble forms in a novel neurexin. Proc. Natl. Acad. Sci. USA. 90:6410–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushkaryov, Y.A., A.G. Petrenko, M. Geppert, and T.C. Südhof. 1992. Neurexins: synaptic cell surface proteins related to the α-latrotoxin receptor and laminin. Science. 257:50–56. [DOI] [PubMed] [Google Scholar]
- Ushkaryov, Y.A., Y. Hata, K. Ichtchenko, C. Moomaw, S. Afendis, C.A. Slaughter, and T.C. Südhof. 1994. Conserved domain structure of α-neurexins. J. Biol. Chem. 269:11987–11992. [PubMed] [Google Scholar]