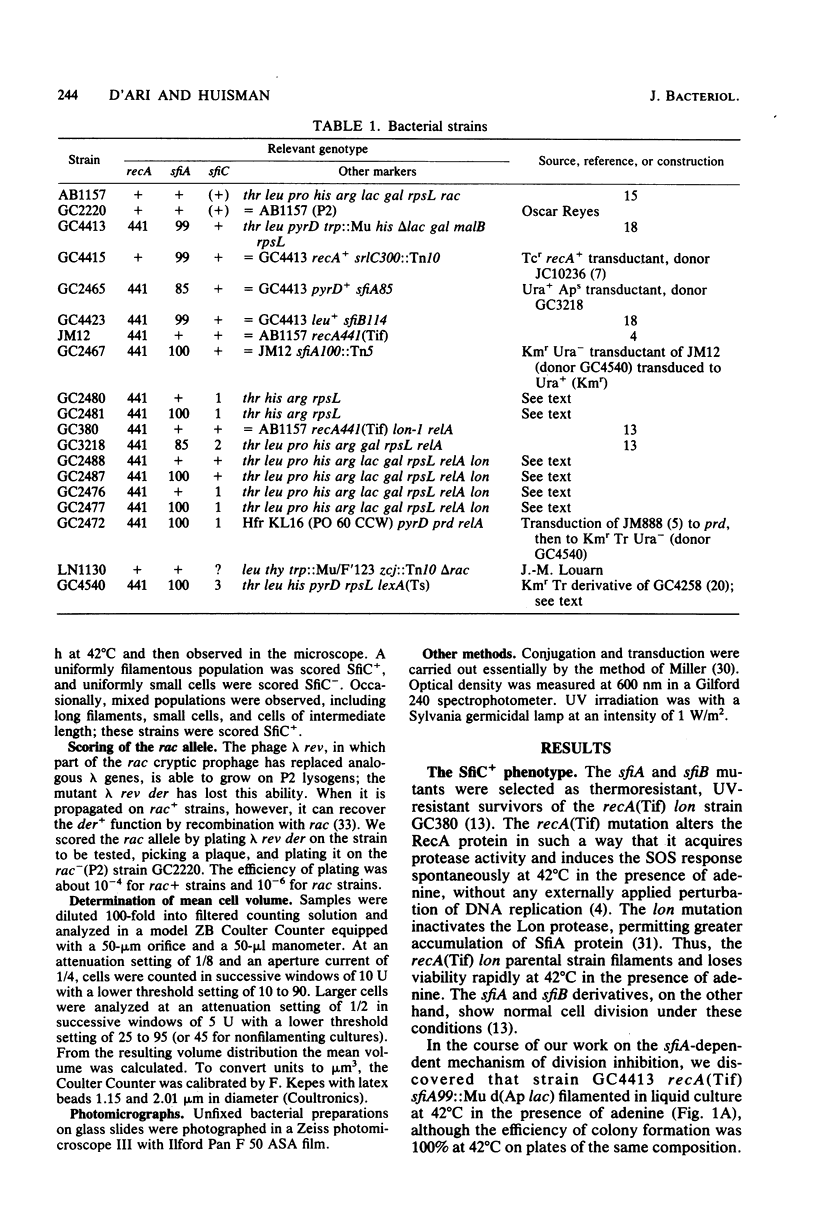
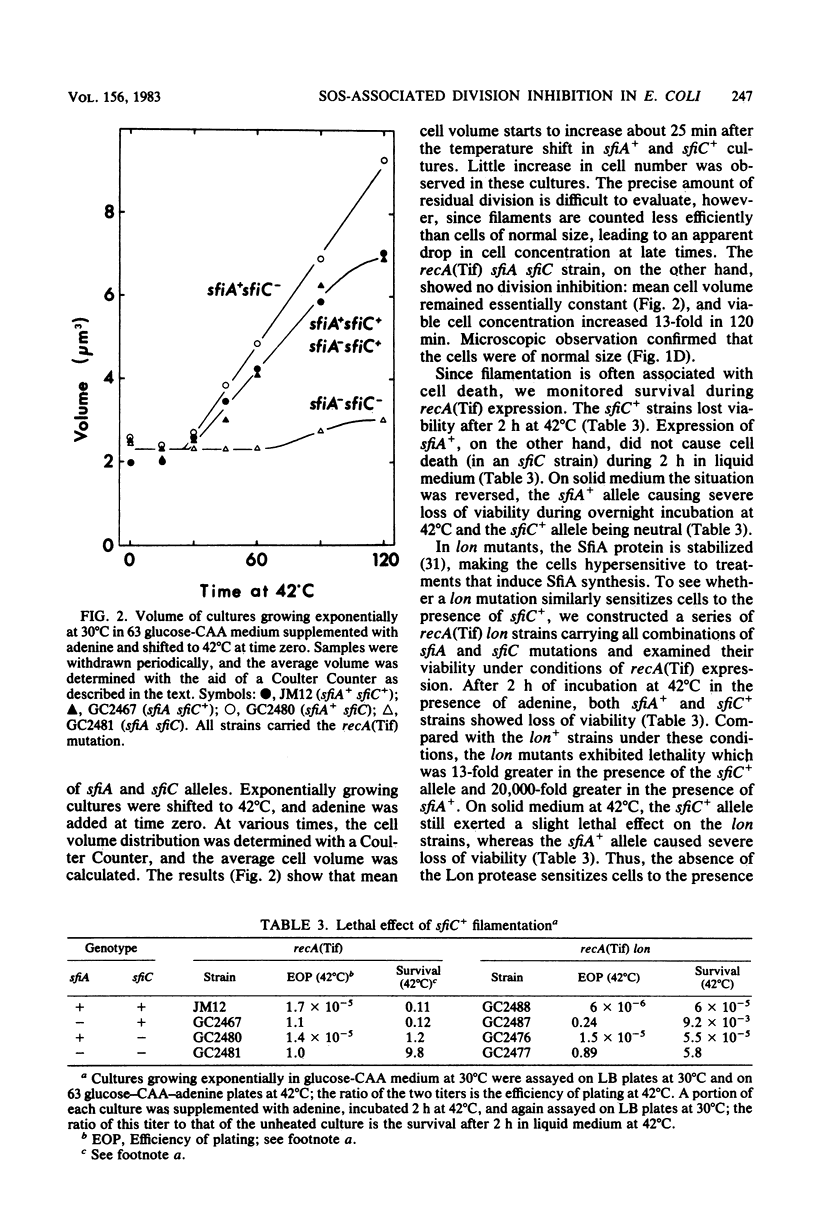
Abstract
Certain Escherichia coli strains were shown to possess a novel system of cell division inhibition, called the SfiC+ phenotype. SfiC+ filamentation had a number of properties similar to those of sfiA-dependent division inhibition previously described: (i) both are associated with the SOS response induced by expression of the recA(Tif) mutation, (ii) both are associated with cell death, (iii) both are amplified in mutants lacking the Lon protease, and (iv) both are suppressed by sfiB mutations. SfiC+ filamentation and sfiA-dependent division inhibition differed in (i) the physiological conditions under which loss of viability is observed, (ii) the extent of amplification in lon mutants, (iii) their genetic regulation (SfiC+ filamentation is not under direct negative control of the LexA repressor), and (iv) their genetic determinants (SfiC+ filamentation depends on a locus, sfiC+, near 28 min on the E. coli map and distinct from sfiA).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binding R., Romansky G., Bitner R., Kuempel P. Isolation and properties of Tn10 insertions in the rac locus of Escherichia coli. Mol Gen Genet. 1981;183(2):333–340. doi: 10.1007/BF00270637. [DOI] [PubMed] [Google Scholar]
- Burton P., Holland I. B. Two pathways of division inhibition in UV-irradiated E. coli. Mol Gen Genet. 1983;190(1):128–132. doi: 10.1007/BF00330334. [DOI] [PubMed] [Google Scholar]
- Casaregola S., D'Ari R., Huisman O. Quantitative evaluation of recA gene expression in Escherichia coli. Mol Gen Genet. 1982;185(3):430–439. doi: 10.1007/BF00334135. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., Morand P., George J., Buttin G. Prophage induction and cell division in E. coli. V. Dominance and complementation analysis in partial diploids with pleiotropic mutations (tif, recA, zab and lexB) at the recA locus. Mol Gen Genet. 1977 Jun 24;153(3):297–310. doi: 10.1007/BF00431595. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Construction of an Hfr strain useful for transferring recA mutations between Escherichia coli strains. J Bacteriol. 1980 Jul;143(1):529–530. doi: 10.1128/jb.143.1.529-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrouwere L., Van Montagu M., Schell J. The ral gene of phage lambda. III. Interference with E. coli ATP dependent functions. Mol Gen Genet. 1980;179(1):81–88. doi: 10.1007/BF00268449. [DOI] [PubMed] [Google Scholar]
- Fassler J. S., Tessman I. Suppression of polarity in the gal operon by ultraviolet irradiation. J Bacteriol. 1980 Dec;144(3):1205–1207. doi: 10.1128/jb.144.3.1205-1207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein S. I., Low K. B. Zygotic induction of the rac locus can cause cell death in E. coli. Mol Gen Genet. 1982;187(2):231–235. doi: 10.1007/BF00331122. [DOI] [PubMed] [Google Scholar]
- Gayda R. C., Yamamoto L. T., Markovitz A. Second-site mutations in capR (lon) strains of Escherichia coli K-12 that prevent radiation sensitivity and allow bacteriophage lambda to lysogenize. J Bacteriol. 1976 Sep;127(3):1208–1216. doi: 10.1128/jb.127.3.1208-1216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Greer H. The kil gene of bacteriophage lambda. Virology. 1975 Aug;66(2):589–604. doi: 10.1016/0042-6822(75)90231-7. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. THE EXCISION OF THYMINE DIMERS FROM DNA, FILAMENT FORMATION AND SENSITIVITY TO ULTRAVIOLET LIGHT IN ESCHERICHIA COLI K-12. Mutat Res. 1964 Oct;106:219–226. doi: 10.1016/0027-5107(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Howe W. E., Mount D. W. Production of cells without deoxyribonucleic acid during thymidine starvation of lexA- cultures of Escherichia coli K-12. J Bacteriol. 1975 Dec;124(3):1113–1121. doi: 10.1128/jb.124.3.1113-1121.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981 Apr 30;290(5809):797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., Casaregola S. How Escherichia coli sets different basal levels in SOS operons. Biochimie. 1982 Aug-Sep;64(8-9):709–712. doi: 10.1016/s0300-9084(82)80115-6. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. Effect of suppressors of SOS-mediated filamentation on sfiA operon expression in Escherichia coli. J Bacteriol. 1983 Jan;153(1):169–175. doi: 10.1128/jb.153.1.169-175.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., George J. Dissociation of tsl-tif-induced filamentation and recA protein synthesis in Escherichia coli K-12. J Bacteriol. 1980 Jun;142(3):819–828. doi: 10.1128/jb.142.3.819-828.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., George J. Further characterization of sfiA and sfiB mutations in Escherichia coli. J Bacteriol. 1980 Oct;144(1):185–191. doi: 10.1128/jb.144.1.185-191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., George J. Inducible sfi dependent division inhibition in Escherichia coli. Mol Gen Genet. 1980;177(4):629–636. doi: 10.1007/BF00272673. [DOI] [PubMed] [Google Scholar]
- Huisman O., Jacques M., D'ari R., Caro L. Role of the sfiA-dependent cell division regulation system in Escherichia coli. J Bacteriol. 1983 Feb;153(2):1072–1074. doi: 10.1128/jb.153.2.1072-1074.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Pleiotropic effect of the rec A gene of Escherichia coli: uncoupling of cell division from deoxyribonucleic acid replication. J Bacteriol. 1971 May;106(2):539–542. doi: 10.1128/jb.106.2.539-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F. Fine structure mapping and properties of mutations suppressing the lon mutation in Escherichia coli K-12 and B strains. Genet Res. 1977 Dec;30(3):273–286. doi: 10.1017/s0016672300017687. [DOI] [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Coupling of DNA replication and cell division: sulB is an allele of ftsZ. J Bacteriol. 1983 Jun;154(3):1339–1346. doi: 10.1128/jb.154.3.1339-1346.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa S., Gottesman S. Protein degradation in Escherichia coli: the lon gene controls the stability of sulA protein. Proc Natl Acad Sci U S A. 1983 Jan;80(2):358–362. doi: 10.1073/pnas.80.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji N., Iyehara-Ogawa H. Thermoresistant revertants of an Escherichia coli strain carrying tif-1 and ruv mutations: non-suppressibility of ruv by sfi. J Bacteriol. 1979 Apr;138(1):1–6. doi: 10.1128/jb.138.1.1-6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes O., Jedlicki E., Kusnierz J. P. Growth of lambda rev in phage P2 lysogenic hosts. Virology. 1981 Jul 30;112(2):651–661. doi: 10.1016/0042-6822(81)90310-x. [DOI] [PubMed] [Google Scholar]
- Shurvinton C. E., Lloyd R. G. Damage to DNA induces expression of the ruv gene of Escherichia coli. Mol Gen Genet. 1982;185(2):352–355. doi: 10.1007/BF00330811. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabeau M., Friedman S., Van Montagu M., Schell J. The ral gene of phage lambda. I. Identification of a non-essential gene that modulates restriction and modification in E. coli. Mol Gen Genet. 1980;179(1):63–73. doi: 10.1007/BF00268447. [DOI] [PubMed] [Google Scholar]



