Abstract
The epithelial–mesenchymal interactions required for kidney organogenesis are disrupted in mice lacking the integrin α8β1. None of this integrin's known ligands, however, appears to account for this phenotype. To identify a more relevant ligand, a soluble integrin α8β1 heterodimer fused to alkaline phosphatase (AP) has been used to probe blots and cDNA libraries. In newborn mouse kidney extracts, α8β1-AP detects a novel ligand of 70–90 kD. This protein, named nephronectin, is an extracellular matrix protein with five EGF-like repeats, a mucin region containing a RGD sequence, and a COOH-terminal MAM domain. Integrin α8β1 and several additional RGD-binding integrins bind nephronectin. Nephronectin mRNA is expressed in the ureteric bud epithelium, whereas α8β1 is expressed in the metanephric mesenchyme. Nephronectin is localized in the extracellular matrix in the same distribution as the ligand detected by α8β1-AP and forms a complex with α8β1 in vivo. Thus, these results strongly suggest that nephronectin is a relevant ligand mediating α8β1 function in the kidney. Nephronectin is expressed at numerous sites outside the kidney, so it may also have wider roles in development. The approaches used here should be generally useful for characterizing the interactions of novel extracellular matrix proteins identified through genomic sequencing projects.
Keywords: integrin; nephronectin; extracellular matrix; organogenesis; kidney
Introduction
Integrins are a large family of heterodimeric receptors important in development, wound healing, immunity, and malignant transformation (Hynes, 1996; Fässler et al., 1996). The integrin α8β1 has recently been shown to play a crucial role during early steps of kidney morphogenesis (Müller et al., 1997). This integrin is expressed in the metanephric mesenchyme, but not in the ureteric bud, and is downregulated as the mesenchymal cells undergo epithelialization. In mice homozygous for a mutation in the α8 gene, initial growth and branching of the ureter are severely impaired. Thus the presence of α8β1 promotes the development of the ureteric bud in a non–cell-autonomous manner. Using a soluble α8β1 heterodimer fused to alkaline phosphatase (AP),* we identified a potential new ligand that is colocalized with α8β1 at the interface between the ureter and the surrounding mesenchyme (Müller et al., 1997). Fibronectin (FN), vitronectin (VN), tenascin-C (TN-C), and osteopontin (OPN) are ligands for this integrin (Müller et al., 1995; Schnapp et al., 1995b; Varnum-Finney et al., 1995; Denda et al., 1998b). OPN is expressed at the interface between the ureter and the mesenchyme, and inhibition of OPN function impairs kidney development in organ culture (Rogers et al., 1997), but mice lacking OPN develop normal kidneys (Liaw et al., 1998). Thus, OPN alone cannot be mediating α8β1 function. TN-C and VN are not expressed in the correct spatiotemporal pattern in the kidney to be essential ligands for this integrin (Aufderheide et al., 1987; Seiffert et al., 1991). Moreover, mice lacking TN-C or VN develop without kidney abnormalities (Saga et al., 1992; Zheng et al., 1995). Mice lacking FN die before the onset of kidney development (George et al., 1993), but FN is not expressed in the same pattern in the embryonic kidney as the ligand detected by α8β1-AP (Ekblom, 1981; unpublished data). Thus, none of these ligands appears to be a strong candidate to mediate the essential functions of α8β1 in the developing kidney.
To understand the mechanisms of α8β1 function, we have sought to identify a ligand that mediates its function during kidney morphogenesis. In blots, α8β1-AP detects several proteins in embryonic kidney extracts, most prominent of which are protein bands of 70–90 kD (Müller et al., 1997). In this paper, we have used α8β1-AP in an expression cloning strategy to identify novel ligands for this integrin. This strategy has yielded cDNAs encoding a novel extracellular matrix protein. Its distribution indicates that it is an extracellular matrix protein, synthesized by the ureteric bud epithelium that is localized with α8β1 at the interface between the ureteric bud and the metanephric mesenchyme. Because of its localization in the kidney extracellular matrix, we have named it nephronectin. Nephronectin binds to integrin α8β1 in an RGD-sensitive fashion and is the 70–90-kD protein recognized by α8β1-AP in protein blots. Nephronectin can be coimmunoprecipitated with α8β1 from kidney extracts, indicating that this integrin and ligand exist in a complex in the kidney in vivo. Based on these findings, we suggest that nephronectin is a ligand in the kidney that mediates α8β1 function during development.
Results
Strategy to identify a novel ligand for integrin α8β1
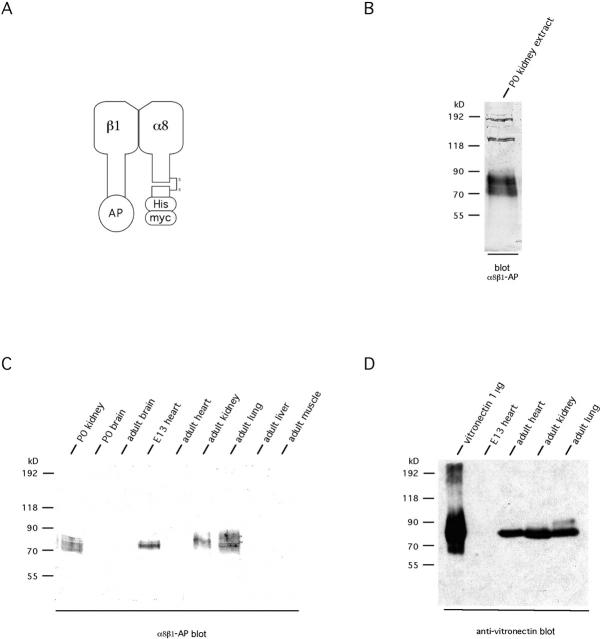
To identify additional ligands for the integrin α8β1, we used α8β1-AP, which consists of a heterodimer of the extracellular domain of α8 and the extracellular domain β1 fused to AP (Fig. 1 A; Denda et al., 1998a). In previous work, this protein complex has been shown to recognize each of the known ligands for integrin α8β1 and has been used successfully in histochemistry and protein blots (Fig. 1 B; Müller et al., 1997; Denda et al., 1998a,b). In blots using newborn mouse kidney extracts, α8β1-AP recognizes a prominent group of proteins with molecular masses between 70 and 90 kD (Fig. 1 B). At least two bands can be distinguished within that range plus additional bands with molecular masses >100 kD (Fig. 1 B). Of the higher molecular mass bands, the 200-kD band is likely to be FN (Müller et al., 1997), whereas the other higher molecular weight bands do not comigrate with any of the known ligands for α8β1. Among the known ligands for integrin α8β1, OPN (Denda et al., 1998b) and VN (Müller et al., 1995) are expected to comigrate with the 70–90-kD proteins and could be responsible for the signals obtained in α8β1-AP blots. VN, however, is present in adult heart extracts (Fig. 1 D), but the 70–90-kD bands do not show up in a blot using this tissue (Fig. 1 C). In contrast, embryonic day 13 heart extract does not contain any detectable VN (Fig. 1 D). Nonetheless, α8β1-AP recognizes a 70–90-kD ligand in a far Western blot of this extract (Fig. 1 C). Similarly, kidney extracts from mice lacking OPN show unchanged levels of the bands between 70 and 90 kD in blots (not shown). Thus, the protein bands at 70–90-kD and at least some of the higher molecular weight bands appear to contain additional ligands for integrin α8β1.
Figure 1.
Strategy to identify a novel ligand for integrin α8β1. (A) Schematic representation of soluble integrin α8β1-AP heterodimer. α8β1-AP protein was expressed as described (Denda et al., 1998a). The soluble α8β1-AP has the same binding characteristics as the transmembrane protein (Denda et al., 1998a,b). AP, secreted AP; His, polyhistidine tag; myc, c-myc epitope tag. (B) The soluble α8β1-AP recognizes several different proteins in kidney extracts. Extracts were prepared from postnatal day 0 (P0) mouse kidneys, separated on 7% SDS-PAGE, transferred to nitrocellulose, and probed with α8β1-AP in the presence of 2 mM Mn2+. The most prominent potential ligands for α8β1-AP have molecular masses of 70–90 kD. (C) Screening of mouse tissues for the presence of a 70–90-kD ligand(s) for α8β1-AP. Embryonic day 13 (E13) heart, adult kidney, and lung contain a 70–90-kD ligand(s) similar to newborn (P0) kidney. (D) Embryonic day 13 heart tissue does not contain detectable amounts of VN, a known ligand for α8β1, as compared with other tissues positive for the 70–90-kD ligand. Therefore, E13 heart was chosen as the tissue to screen for a novel ligand for α8β1-AP.
To identify additional ligands for this integrin, we performed an expression cloning screen using a lambda UNIZAP® library. The lambda phage contain inserts whose expression is under the control of an isopropyl-β-d-thiogalactopyranoside–inducible promoter and can therefore be used to screen for expressed proteins in infected bacteria. To select a library for this purpose, tissues for which commercial UNIZAP® libraries are available were screened for the presence of 70–90-kD ligand(s) for α8β1, using α8β1-AP (Fig. 1 C). Western blots of those expressing such ligands were used to test for the presence of the FN and VN, known α8β1 ligands with molecular masses in the 70–90-kD range. All the tissues tested contained FN (data not shown), but embryonic day 13 (E13) heart was the only tissue that did not contain detectable amounts of VN (Fig. 1 D). Thus, E13 heart was chosen as the starting tissue for the expression screen. 2 × 106 plaques were screened with the α8β1-AP, and 18 different phage were recovered. Among these, the cDNA inserts of 15 encoded FN, whereas the other 3 encoded for a novel protein that we have named nephronectin, based on its potential role in kidney development (see below). The longest cDNA of 3.4 kb contains an ORF of 1,683 bp (561 amino acids) (Fig. 2 A). The ORF extends from nucleotide 124–1,810 (corresponding to 1–1,683 of the ORF) of the 3.4-kb cDNA. The first stop codon is followed by several stop codons in multiple reading frames that were confirmed by sequencing a second cDNA. The cDNA contains an AUUAAA motif as a potential polyadenylation signal. This is followed by a GU-rich region ∼25 nucleotides downstream from the AUUAAA site. ∼50 nucleotides downstream from the AUUAAA site is another region rich in GU. This pattern of motifs is consistent with the overall structure of described polyadenylation sites (Zhao et al., 1999).
Figure 2.
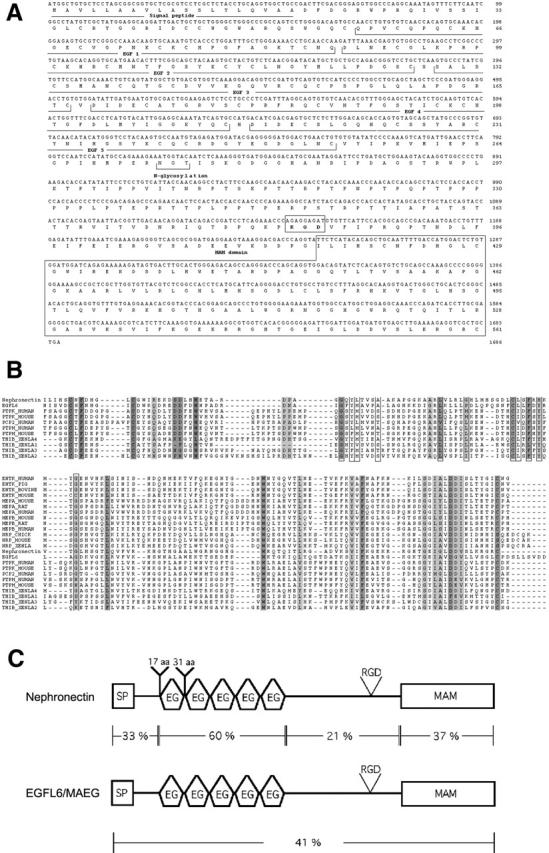
Nephronectin, a novel extracellular matrix protein. (A) Mouse nephronectin cDNA nucleotide sequence and deduced amino acid sequence. The putative signal peptide, the EGF repeats, the RGD motif, N-glycosylation sites, and the MAM homology region are indicated. (B) Alignment of the MAM domain. MAM domains were aligned using ClustalW 1.8. The boxed amino acids are the consensus residues of the prosite signature. Shaded amino acids are residues conserved in ≥70% of the proteins. (C) Overall homology of nephronectin to EGFL6 (Yeung et al., 1999). The two proteins share the same overall organization and sequence of domains. The least conserved domain is the one containing the RGD motif.
A putative signal peptide is located at the NH2 terminus of the ORF of nephronectin, suggesting that the protein is secreted. Nephronectin also contains five EGF–like repeats between amino acids 57–250 and a region at the COOH terminus that shares homology with MAM repeats (amino acids 417–561) (Fig. 2 B). The protein also contains a RGD sequence at amino acids 382–384 and a potential N-glycosylation site at amino acid 273. When comparing nephronectin to the nonredundant EMBL/GenBank/DDBJ database using BLASTP, a protein with a similar domain organization called EGFL6 was found (Yeung et al., 1999) (Fig. 2 C). A murine homologue of EGFL6/MAEG has been identified (Buchner et al., 2000). If the two proteins are aligned with the best-fit program, an overall sequence identity of 41% is obtained. Interestingly, the EGF repeat region is the most highly conserved (60%), whereas the region between the EGF repeats and the MAM domain is the least conserved. This region contains the RGD sequence in both nephronectin and EGFL6/MAEG (amino acids 382–384 in nephronectin, and amino acids 362–364 in EGFL6). In nephronectin, the region from amino acids 251–386 is encoded by a single exon (data not shown) and is very rich in proline, serine, and threonine (62 of 131 amino acids). This is a feature found in mucin-like proteins (Lan et al., 1990). Mucins are glycoproteins that are highly O-glycosylated on serine and threonine residues. Indeed, a high degree of glycosylation is predicted for this region of nephronectin by the neural network based O-glycosylation prediction program Net-O-glyc (http://www.cbs.dtu.dk/ services/NetOGlyc/; Hansen et al., 1998). No transmembrane region was detected, suggesting that nephronectin is an extracellular matrix protein. Nephronectin also contains two alternatively spliced exons, one encoding 17 amino acids that is NH2-terminal to the first EGF repeat, and a second one encoding 31 amino acids between the first and the second EGF repeats. All possible combinations of splice variants have been detected by RT-PCR (data not shown). The DNA and protein sequences have been deposited in EMBL/GenBank/DDBJ (under accession nos. AY035898 and AY035899).
Integrin α8β1 binds to nephronectin in the region containing an RGD sequence in an RGD-dependent manner
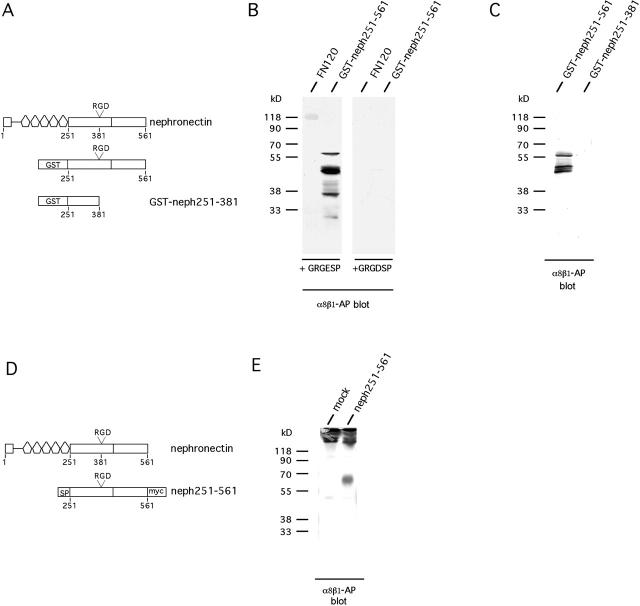
Integrin α8β1 is a member of the RGD-dependent subfamily of integrins (Bossy et al., 1991; Schnapp et al., 1995a,b). To test whether the binding of integrin α8β1 to nephronectin is RGD sensitive, fragments of nephronectin were expressed in Escherichia coli as glutathione S-transferase (GST) fusion proteins (Fig. 3 A). GST-neph251-561 contains the fragment from after EGF repeat 5 to the COOH terminus of the protein (amino acids 251–561), GST-neph251-381 from after EGF 5 to immediately before the RGD sequence (amino acids 251–381). Both fusion proteins were expressed in E. coli and were purified from the soluble fraction. Some degradation was apparent. Nevertheless, strong binding of α8β1-AP to GST-neph251-561 was observed (Fig. 3 B). α8β1-AP binds to the intact fusion protein of ∼60 kD as well as to two degradation products ∼50 kD and one ∼38 kD. This experiment also shows that integrin α8β1-AP binds to nephronectin in the region COOH-terminal to the EGF repeats. Most likely, the binding site includes the RGD sequence because addition of 50 μg/ml GRGDSP peptide abolished the binding, whereas addition of the same concentration of GRGESP peptide had no effect (Fig. 3 B). To explore this further, GST-neph251-381, a GST fusion protein lacking the RGD and sequences COOH-terminal to the RGD, was used as a substrate and, as expected, did not bind to GST-neph251-381 (Fig. 3 C). Thus, the binding site appears to be contained in amino acids 382–561 and is most likely to include the RGD sequence, but this has not been definitely proven by mutation of this sequence.
Figure 3.
The RGD-containing region in nephronectin is required for binding by α8β1. (A) Schematic representation of E. coli fusion proteins. GST-neph251-561 contains the COOH-terminal part of nephronectin from immediately after the EGF repeats to the end of the protein, whereas GST-neph251-381 contains the fragment from the end of the EGF repeats to just in front of the RGD motif. α8β1-AP binds in an RGD-inhibitable manner to nephronectin. 2 μg of FN, GST-neph251-561, or GST-neph251-381 were separated on a 12% SDS-PAGE, transferred to nitrocellulose, and probed with α8β1-AP in the presence of 2 mM Mg2+. (B) GRGDSP or GRGESP peptides were added at 50 μg/ml. Note that, under the conditions used, binding of α8β1-AP to FN is weaker than to GST-neph251-561. This blot was developed longer than the blot in C to show the FN binding. Therefore, more degradation products of GST-neph251-561 are seen than in C. (C) The RGD region of nephronectin is required for binding of α8β1-AP to nephronectin. A GST fusion protein lacking the RGD-containing region and more COOH-terminal sequences does not bind the α8β1-AP. (D) Schematic representation of fusion protein expressed in COS7 cells. Neph251-561 contains an IgG signal peptide, a fragment of nephronectin extending from the COOH terminus of the EGF repeats to the end of the protein with a myc/His tag at the COOH terminus. SP, Ig κ signal peptide; myc, myc/his tag. (E) Nephronectin fragment expressed in eukaryotic cells binds to α8β1-AP. Neph251-561 protein was transiently expressed in COS-7 cells. Then, the supernatant was harvested and immunoprecipitated with anti-myc antibody. Immunoprecipitates were separated by 12% nonreducing SDS-PAGE, transferred to nitrocellulose, and probed with α8β1-AP. mock, empty plasmid negative control. The strong band at the top of the gel represents nonreduced 9E10 Ig.
To determine whether improper folding, absence of disulfide bridges, or absence of normal glycosylation unmasks an integrin binding site that is not present in the native protein, the nephronectin fragment neph251-561 was transiently expressed in COS-7 cells with an NH2-terminal signal peptide and a COOH-terminal myc/His6 tag (Fig. 3 D). Secreted neph251-561 protein was harvested by immunoprecipitation with anti–c-myc antibody 9E10 from conditioned medium. It was then analyzed in blots using gels run in nonreducing conditions (Fig. 3 E). As can be seen in Fig. 3 E, α8β1-AP binds to this fragment of nephronectin under these conditions. A single band of 60 kD is detected. The same band is recognized by anti–c-myc antibody. Interestingly, the predicted molecular mass for neph251-561 is 41 kD. This suggests the presence of a considerable amount of glycosylation, probably in the mucin-like region of nephronectin.
Characterization of integrin interactions with nephronectin
In an effort to determine whether α8β1 and additional RGD-binding integrins mediate attachment of cells to nephronectin, we conducted adhesion assays using K562 cells expressing different integrin heterodimers. Parental K562 cells express high levels of α5β1 and very low or undetectable levels of other integrins (Blystone et al., 1994). Results in Fig. 4 A demonstrate that, even in the presence of Mn2+, which is an activator of all integrins, K562 cells do not bind to the RGD-containing COOH-terminal portion of nephronectin, neph251-561, which was purified from COS-7 cell supernatant. As expected, K562 cells bind efficiently in a dose-dependent manner to FN in the presence of Mn2+, a cation known to activate integrins. In contrast, K562 cells expressing the α8β1 heterodimer bind efficiently to both FN and nephronectin in dose-dependent manners. Although amounts of the two proteins actually bound to substrata have not be quantitated, the binding curves suggest that α8β1 binds to lower amounts of nephronectin than FN.
Figure 4.
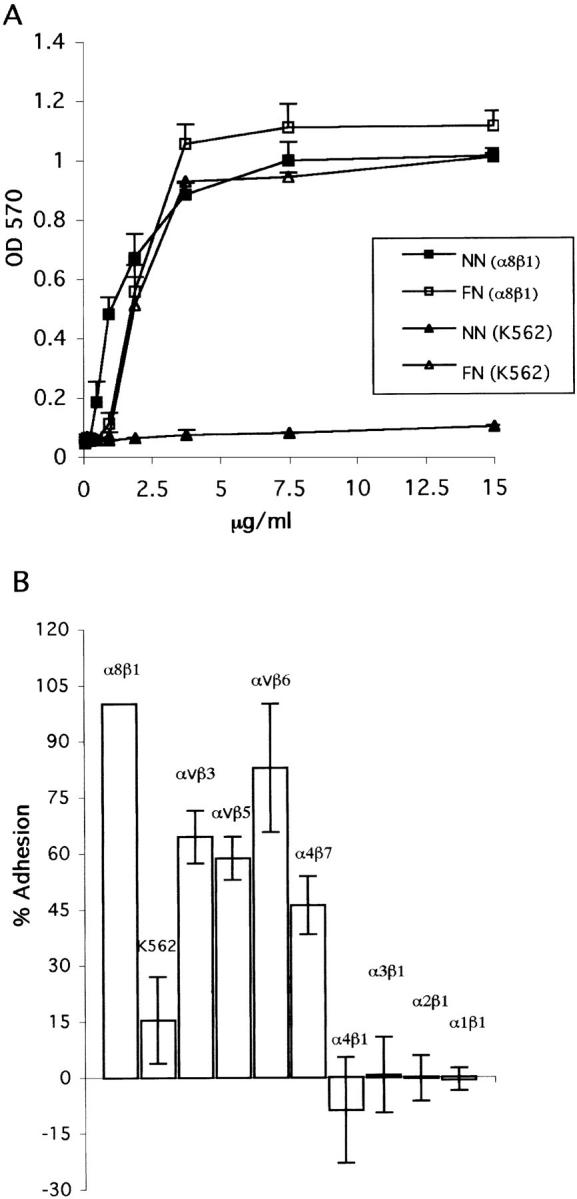
Adhesion of K562 cells, and K562 expressing specific additional integrins, to nephronectin. (A) K562 and K562 expressing α8β1 were allowed to adhere to increasing concentrations, 0.06–15.0 μg/ml, of either FN or amino acids 251–561 of nephronectin (NN) purified from CHO cell–conditioned medium. Experiments were carried out in the presence of 1 mM Mn2+. (B) Adhesion of K562 cells expressing indicated additional integrin heterodimers to 2 μg/ml of a GST fusion protein containing amino acids 251–561 of nephronectin in the presence of 1 mM Mn2+ and the anti-α5 mAb BIIG2 to inhibit α5β1-mediated adhesion. Adhesion of the parental K562 cells that express α5β1 to these two substrates was measured in the absence of the anti-α5 mAb. For all lines, OD values for wells coated with GST alone were subtracted. Adhesion of α8β1-expressing K562 cells was defined as 100% and adhesion of other cells is expressed as a percentage of that value.
In studies presented later in this paper (see Fig. 9) , we have observed expression of nephronectin in numerous locations within developing embryos, suggesting that this protein may be a ligand for additional integrins. To explore this possibility, we have examined the abilities of several K562 cell lines expressing various additional integrin heterodimers to bind to GST-neph251-561. Results presented in Fig. 4 B show that K562 cells expressing α8β1, αVβ3, αVβ5, αVβ6, and α4β7 bind strongly to this protein in the presence of Mn2+. None of these integrins bound detectably to GST-neph251-381 (not shown), so each could potentially bind to the RGD sequence. In contrast, significant binding was not observed using the parental K562 cells or K562 cells expressing α1β1, α2β1, α3β1, or α4β1. Each cell line that failed to bind to nephronectin was shown to bind to a characterized ligand for the integrin that it expressed. α1β1- and α2β1-expressing cells were shown to adhere efficiently to collagen III. α3β1-expressing cells adhered efficiently to laminin-5 (kalinin), and α4β1-expressing cells adhered to FN in the presence of a function-blocking antibody to α5β1. Thus, nephronectin is a ligand for several activated integrins, including many but not all of the RGD-binding integrins (Yang et al., 1998).
Figure 9.
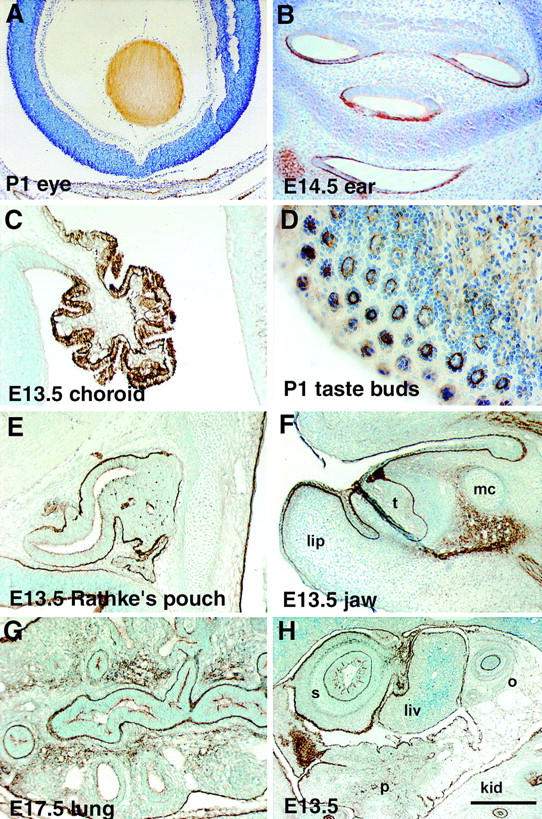
Expression of nephronectin in various tissues and organs. (A) Eye of a P1 animal. Note uniform expression of nephronectin in the lens. (B) Ear of an E14.5 embryo. Note expression of nephronectin in basal lamina surrounding epithelium and on apical surface of epithelia. (C) Choroid plexus of an E13.5 embryo. Nephronectin is localized in basal lamina and is strongly expressed by epithelial cells in this organ. (D) Tongue of a P1 animal. Nephronectin is expressed in developing taste buds. Lighter expression is also present in muscle. (E) Rathke's pouch at E13.5. Nephronectin is present in the basal lamina surrounding this organ that develops into the pituitary gland. (F) Jaw of E13.5 animal. In cross section, extensive expression of nephronectin can be observed in the basal laminae underlying the skin and oral epithelium. Nephronectin also surrounds a developing incisor (t). Lip and Meckel's cartilage (mc) are indicated. (G) E13.5 lung. Nephronectin is expressed in the basal lamina surrounding the branching epithelia in this organ. In contrast to embryonic kidney, nephronectin is also present in the surrounding mesenchyme. (H) Expression of nephronectin in various organs of E13.5 embryo. Nephronectin expression is prominent in the developing stomach (s) and oesophagus (o). In contrast, very little expression is present in a lobe of the liver (liv). Light expression is observed in the pancreas (p). Expression in kidney (k) epithelia is obvious at bottom right. Bar: (F) 267 μm; (A and H) 200 μm; (C, E, and G) 100 μm; (B) 90 μm; (D) 50 μm.
Identification of nephronectin as the 70–90-kD ligand for α8β1 previously identified in kidney extracts
To determine whether nephronectin is indeed the 70–90-kD protein in kidney extracts identified as a potential novel ligand for integrin α8β1, immunodepletions of kidney extracts were performed using an antibody prepared to the recombinant nephronectin fragment GST-neph251-381. The depleted extract and the immunoprecipitate were then analyzed by blotting (Fig. 5) . In comparison to the control, virtually all the 70–90-kD ligand(s) for α8β1 were lost from the depleted extract. In addition, a 70–90-kD ligand for α8β1 was present in the antinephronectin immunoprecipitate. The same doublet of bands was also recognized by the antinephronectin antibody in Western blots of kidney extracts (data not shown). Thus, the 70–90-kD α8β1 ligand(s) in kidney extracts appear to mainly consist of nephronectin.
Figure 5.
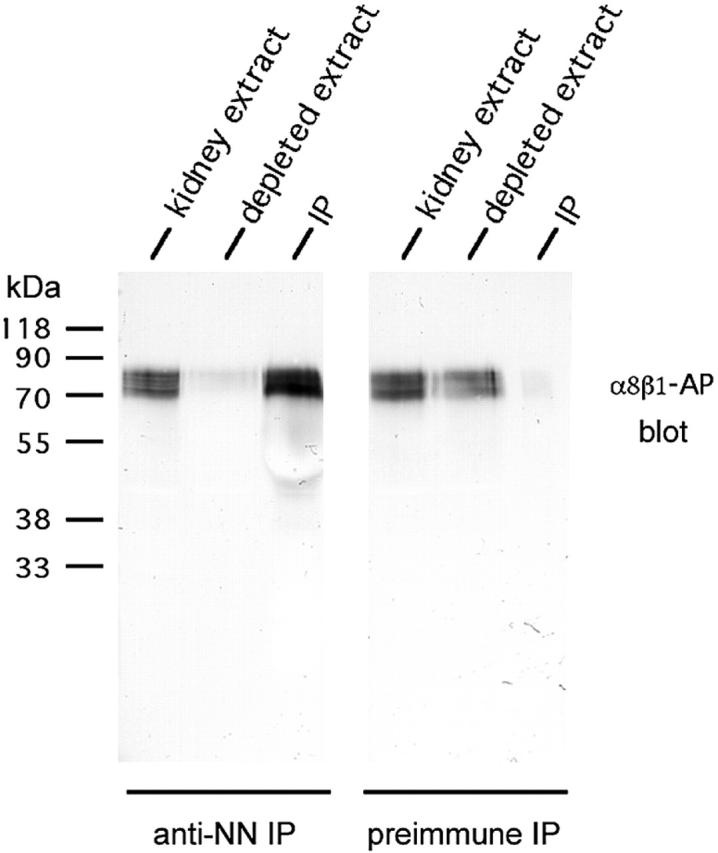
Nephronectin is the protein(s) of 70–90 kD recognized by α8β1-AP in kidney extracts. Newborn mouse kidney extracts were immunoprecipitated with antinephronectin antiserum or preimmune serum. The extract, depleted extract, and the immunoprecipitate were analyzed in blots using α8β1-AP. Antinephronectin depleted virtually all α8β1-AP binding activity from the kidney extract, whereas the preimmune serum did not reduce the activity at 70–90 kD responsible for α8β1-AP binding.
Nephronectin and α8β1 interact in the kidney in vivo
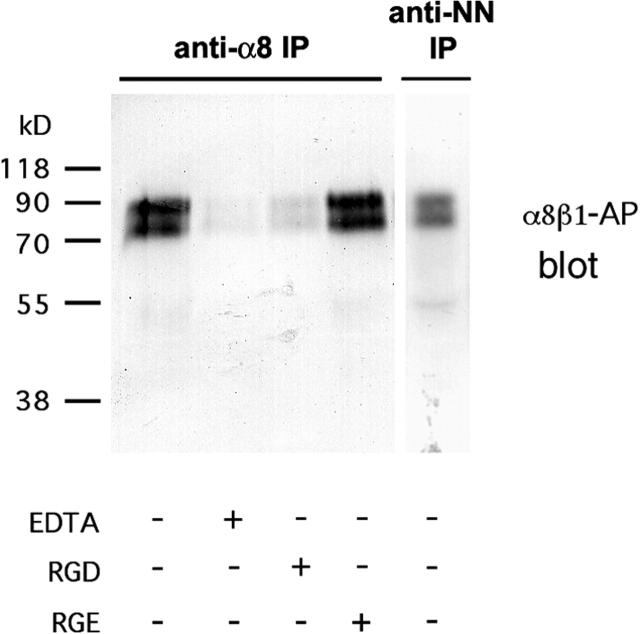
We have shown that nephronectin, a novel extracellular protein, is a ligand for α8β1 in blots and cell adhesion assays. To determine whether nephronectin and α8β1 interact in vivo, we examined whether they could be coimmunoprecipitated from kidney extracts (Fig. 6) . Extracts were prepared from the kidneys of newborn mice and were immunoprecipitated with anti–integrin α8. The immunoprecipitates were then assayed for the presence of nephronectin using α8β1-AP (Fig. 6). In the anti-α8 immunoprecipitate, α8β1-AP detects a doublet of bands identical to those precipitated by antinephronectin. This strongly suggests that integrin α8β1 and nephronectin are associated with each other in vivo. To test the specificity of this interaction, extracts were prepared from newborn kidneys in the presence of either 10 mM EDTA or GRGDSP peptide to disrupt α8β1 integrin interactions with its ligands. As a control for the GRGDSP peptide, the experiment was also performed in the presence of GRGESP peptide. The inclusion of either EDTA or GRGDSP, but not GRGESP in the extract and immunoprecipitation buffers greatly reduced the presence of nephronectin in the anti–α8 immunoprecipitate. Thus, the integrin α8β1 and nephronectin are associated in a complex with each other in the developing kidney. This provides strong evidence that nephronectin is an important functional ligand for integrin α8β1 in the kidney.
Figure 6.
Coimmunoprecipitation of α8β1 with nephronectin. Newborn mouse kidney extracts were immunoprecipitated with antiintegrin α8 serum or antinephronectin serum in the presence or absence of 10 mM EDTA, with 200 μg/ml GRGDSP or GRGESP peptides, as indicated. Precipitates were separated by 12% SDS-PAGE, were transferred to nitrocellulose, and were probed with α8β1-AP. The band(s) corresponding to nephronectin (compare with the antinephronectin immunoprecipitation) are present in anti-α8 immunoprecipitates. Addition of EDTA leads to the disappearance of the nephronectin from the α8 immunoprecipitates. Inclusion of GRGDSP but not GRGESP strongly reduces the amount of nephronectin in the anti-α8 immunoprecipitates.
Nephronectin is expressed throughout kidney development
To determine whether the expression pattern of nephronectin during kidney development is consistent with the possibility that it is the endogenous ligand detected by α8β1-AP, the antinephronectin antibody was first affinity purified on the GST-neph251-561 fusion protein coupled to a Sepharose column and was then passed over a GST–Sepharose column to remove antibodies specific for GST (see Materials and methods).
Nephronectin expression can be detected at E10.5 in the urogenital ridge (Fig. 7, A and B) and at E11.5 in the Wolffian and ureteric bud (Fig. 7 C, inset). At E13.5, nephronectin is localized at the interface between epithelial cells of the ureter and the surrounding metanephric mesenchyme (Fig. 7 C). Nephronectin is also localized between epithelial cells of the ureteric bud and the surrounding mesenchyme. Expression is present at the tips of the ureter branches, but expression levels seem higher around more mature regions of ureteric epithelia (Fig. 7, C and E). In contrast, α8 immunoreactivity is present in the metanephric mesenchyme (Fig. 7, D and F). In addition, α8 also is localized at the interface between the metanephric mesenchyme and the ureteric bud epithelium (Fig. 7 F), as would be anticipated for a receptor to nephronectin.
Figure 7.
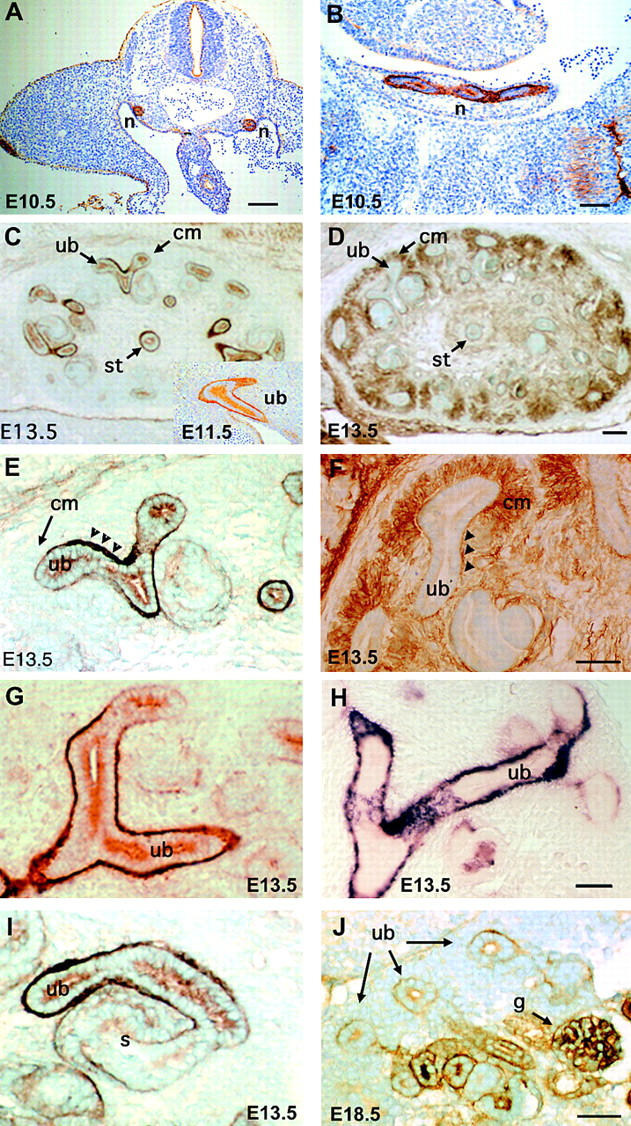
Expression of nephronectin, α8β1, and the α8β1-AP ligand. (A and B) Expression of nephronectin in E10.5 embryo. In coronal section (A), prominent expression is observed in the mesonephric duct within the urogenital ridge and in ectoderm surrounding the embryo. A sagittal section (B) illustrates expression in the developing mesonephric duct. (C) Expression of nephronectin in E13.5 kidney, as detected using antinephronectin. Note prominent expression of nephronectin at the interfaces between ureteric bud epithelial cells and surrounding mesenchyme. Expression of nephronectin is also strong in the stalk of the ureter. An insert in lower right corner of C illustrates nephronectin expression in the ureteric bud at E11.5. (D) Expression of the integrin α8 in E13.5 kidney visualized with anti-α8. α8 is expressed in the mesenchyme surrounding developing branches of the ureteric bud. α8 also is concentrated at the interface between the metanephric mesenchyme and ureteric epithelial cells. α8 is not expressed at detectable levels in the ureteric epithelial cells. (E) Higher resolution pattern of expression of nephronectin in E13.5 kidney. Note the strong expression in the basal lamina between the ureteric bud and metanephric mesenchyme. A condensing comma-shaped body of mesenchyme expresses little or no nephronectin. (F) Higher resolution illustration of expression of α8 in E13.5 kidney. Note strong expression of α8 in the metanephric mesenchyme and the absence of expression in the ureteric bud epithelial cells. (G and H) Comparison of expression patterns of nephronectin (G) and the ligand-bound by α8β1-AP (H) in E13.5 kidney. Note that both have similar patterns of expression in branching ureteric buds. (I) Expression of nephronectin continues to be high in the ureteric bud–derived portion of a tubule created by fusion of condensing mesenchyme with a ureteric bud in E13.5 kidney. Expression appears to be initiating in the S-shaped portion of this tubule derived from the condensing mesenchyme. (J) In the E18.5 kidney, nephronectin is expressed in maturing tubules and in differentiating glomeruli. n, mesonephric duct; cm, condensing mesenchyme; ub, ureteric bud; st, ureteric stalk; c, comma-shaped body; s, S-shaped body; g, glomerulus. Bars: (C and D) 250 μm; (A and E–J) 100 μm; (B) 50 μm.
The localization of nephronectin has been directly compared with that of the ligand detected by α8β1-AP, and representative results are illustrated in Fig. 7, G and H. In Fig. 7 G, nephronectin is visualized in a branching ureteric bud. The distribution of the ligand detected by α8β1-AP is illustrated in Fig. 7 H. Comparison of the two panels indicates that the distributions of these two ligands are very similar, compatible with their being the same protein.
As kidney development proceeds, the mesenchyme around the tips of the ureteric buds condenses and differentiates into epithelial structures that later fuse with the ureteric buds to form the proximal tubule and glomerulus of the mature kidney. Upon epithelialization of the mesenchyme, very low levels of nephronectin expression become apparent in comma-shaped bodies derived from the mesenchyme (Fig. 7 E). A somewhat later stage of maturation in which the epithelial structure formed by condensing mesenchyme has fused with a ureteric bud is illustrated in Fig. 7 I. Here, expression of nephronectin appears robust in the portion of the tubule derived from the ureteric bud. Expression is low, but significant in the S-shaped body derived from the metanephric mesenchyme. At E18.5, high levels of nephronectin are present in maturing glomeruli (Fig. 7 J).
As described above, the integrin α8β1 is expressed in the cells of the metanephric mesenchyme. Nephronectin mRNA was detected in RNA blots of kidney throughout metanephric development (not shown). To determine which cells express nephronectin, in situ analyses were performed using antisense and sense cRNA probes. In analyses using the antisense probe, presented in Fig. 8, A, C, and D , strong expression of mRNA encoding nephronectin is detected in tubular epithelial cells in the E15.5 and P0 kidney. Little or no mRNA is present in the surrounding mesenchyme. Consistent with the localization of nephronectin protein, many condensing mesenchymal structures can be observed that are expressing very low or undetectable levels of nephronectin mRNA. No mRNA is detected at either age using a sense cRNA probe as a control (Fig. 8 B and not shown). A similar but weaker pattern of expression using an antisense probe was observed at E13.5 (not shown). Thus, nephronectin and its receptor α8β1 appear to be synthesized by complementary cell types during kidney development.
Figure 8.
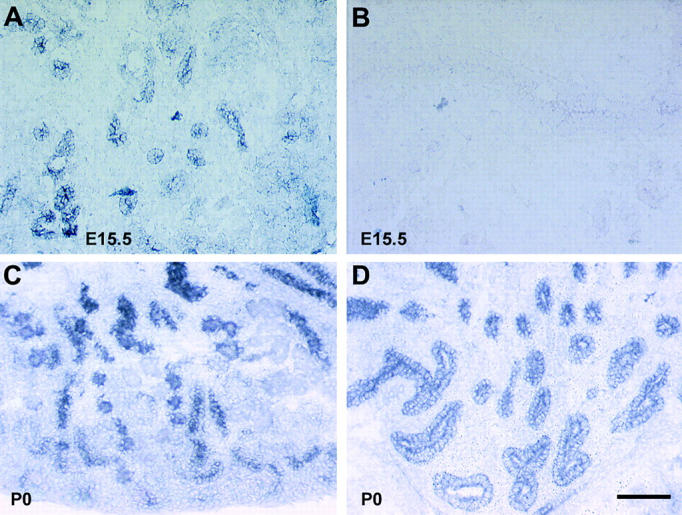
In situ analysis of nephronectin mRNA in E15.5 and P0 kidney. Nephronectin mRNA is present in branching epithelia of the developing kidney at E15.5 (A) and P0 (C and D). A view of the P0 kidney cortex is shown in C. Note the many condensing mesenchymal structures not expressing nephronectin mRNA. A view of the P0 pelvic region of the kidney is shown in D. Incubation of sections with a sense probe as a control at E15.5 (B) or P1 (not shown) resulted in virtually no reaction product. Bar, 100 μm.
As illustrated in Fig. 7 A, nephronectin is expressed early during embryogenesis, before initiation of formation of the metanephric kidney. At this stage, expression could be observed under the ectoderm, in the developing urogenital system, and in the digestive tract. Nephronectin was also present at interfaces between differentiating somites (Fig. 7 B). As illustrated in Fig. 9, nephronectin is also present in several organs and tissues of the embryo and neonate. At E13.5, nephronectin is observed in the choroid plexus (Fig. 9 C) and Rathke's pouch (Fig. 9 E). Within the embryonic jaw, prominent expression is observed underneath the ectoderm and surrounding developing teeth (Fig. 9 F). Nephronectin is also observed in several basal laminae in the embryonic lung (Fig. 9 G), stomach, and oesophagus (Fig. 9 H). Nephronectin is also expressed by epithelial cells in the otic system (Fig. 9 B). In neonates, expression can be observed in many tissues and organs, including the lens (Fig. 9 A) and taste buds (Fig. 9 D). Expression of nephronectin appears particularly prominent at epithelial–mesenchymal interfaces in tissues undergoing morphogenesis.
Discussion
In this paper, we identify and characterize a novel ligand for the integrin α8β1. We have named this protein nephronectin, because the studies that led to its discovery were initiated in the developing kidney. We have shown that this protein is associated with the extracellular matrix in the kidney where it is synthesized by ureteric epithelial cells and is deposited in the extracellular matrix at the interface between these cells and the metanephric mesenchyme. The integrin α8β1 is able to bind nephronectin in physiological salt conditions and is associated with nephronectin within the developing kidney in vivo. As an endogenous ligand for this integrin, it seems likely to be important for regulating development of the kidney. As nephronectin is expressed in other tissues and is recognized by other integrins, it may play a wider role in development.
Expression cloning using soluble integrin extracellular domains as probes seems likely to be generally useful for identifying novel ligands for these receptors. This may be particularly relevant since many constituents of the extracellular matrix are not well characterized. For example, ∼4% of the genes identified in the Drosophila genome sequencing project are candidates for involvement in cell adhesion, and many of these seem likely to be involved in cellular interactions with the extracellular matrix (Hynes and Zhao, 2000). With completion of the C. elegans genome project, it has also become clear that this organism expresses a very large number of extracellular matrix–associated molecules, many of which appear to be species specific (Hutter et al., 2000). It seems almost certain that the completion of vertebrate genome sequencing projects will reveal many additional constituents of the extracellular matrix, many of which are likely to play important roles in organogenesis. The approach described in this paper provides a method for identifying the proteins present in any tissue that mediate cell adhesion. Our methods can also be used to rapidly examine the receptors capable of binding any individual protein. As such, these procedures should be useful in sorting out the functional properties of the diversity of extracellular matrix proteins being identified as part of genomic sequencing projects.
As described in the Results, examination of the EMBL/GenBank/DDBJ database identified a homologue to nephronectin named EGLF6 (human) or MAEG (mouse) (Yeung et al., 1999; Buchner et al., 2000). The sequence of murine nephronectin predicts an ORF of 561 amino acids including a signal sequence of 19 amino acids, whereas that of murine EGFL6/MAEG predicts an ORF of 550 amino acids, including a signal sequence of 18 amino acids. In each protein the signal sequence is followed by five EGF repeats. Interestingly, the sequences of repeats 2, 3, and 4 in EGFL6/MAEG and of repeats 2, 4, and 5 in nephronectin indicate that they are Ca2+-binding EGF domains. This subclass of EGF repeat is found in the extracellular domains of several cell surface–associated and extracellular matrix proteins and has been shown to mediate protein–protein interactions (Rao et al., 1995). Each protein also contains a MAM domain in its COOH-terminal region. MAM domains have also been shown to mediate protein–protein interactions, so it seems likely that nephronectin and EGFL-6 interact with other proteins or with each other in the extracellular matrix. These are the only genes described to date that contain both Ca2+-binding EGF repeats and MAM domains (Buchner et al., 2000). In the region between the EGF repeats and the MAM domains, both proteins have regions containing an RGD sequence as a potential integrin-binding site and sequences predicted to be modified by N-linked and O-linked glycosylation. Thus, these two proteins share homologies in each of their domains and may form a new subfamily of proteins. Results in this paper have shown directly that nephronectin is localized to the extracellular matrix region in the developing kidney. It seems very likely that EGFL6/MAEG is also a constituent of the extracellular matrix. It will be surprising if it does not prove to be a ligand for some of the RGD-binding integrins.
In previous work, the integrin α8β1 has been shown to bind many, but not all of the RGD-containing ligands for integrins (Müller et al., 1995; Schnapp et al., 1995b; Varnum-Finney et al., 1995; Denda et al., 1998a,b). These include FN, VN, OPN, and the cell-binding domain of TN-C. α8β1, however, does not bind detectably to other RGD-containing proteins, such as thrombospondin and entactin (Denda et al., 1998a). Fibronectin has been shown previously to be expressed in the metanephric mesenchyme before invasion by the ureteric bud (Ekblom, 1981; Aufderheide et al., 1987). α8β1-AP does not exhibit detectable binding to regions of mesenchyme not in contact with the ureteric bud, indicating that it is not binding efficiently to FN (Müller et al., 1997). Even though VN is a well-characterized ligand for this integrin, binding to VN is not observed by α8β1-ΑP blots of adult heart, which clearly contains significant amounts of this protein as assessed using anti-VN in Western blots (unpublished data). Thus, the binding of this integrin to VN also appears to be less avid than its binding to nephronectin. Expression of tenascin is restricted to stromal cells in early stages of differentiation (Aufderheide et al., 1987). We have not observed binding to these cells by cytochemistry using α8β1-AP. After incubations with α8β1-AP in probes of tissue sections or blots, it is likely that only unusually avid interactions between α8β1-AP and its ligands are maintained during stringent washes. Consistent with this, α8β1 and nephronectin remain associated in the presence of detergent in immunoprecipitates of kidney extracts (Fig. 6).
Although RGD peptides have been shown to inhibit binding to other ligands by each of the integrins shown to bind to nephronectin in this paper (Yang et al., 1998), it is less certain that the RGD sequence in nephronectin forms the attachment site for each integrin. In our expression studies, the integrin binding site(s) were mapped roughly to a region of 180 amino acids that includes the RGD site. In particular, the integrin α4 sequence is not in the same subgroup of integrin α sequences as those of αV-, α5-, and α8-, and α4-containing integrins have been shown to bind to sequences not containing an RGD site (Moyano et al., 1997).
As described above, the distribution of nephronectin in the developing kidney appears to be essentially identical to that of the ligand(s) detected by α8β1-AP, and nephronectin is clearly the major protein detected in blots of embryonic kidney extracts using α8β1-AP. It is unusual for an integrin to remain associated with its ligands after immunoprecipitation, so the association of nephronectin with α8β1 in immunoprecipitates suggests that these two proteins are associated in vivo. For this reason, it seems very likely to be a critical ligand in the signaling pathway in early metanephric kidney development that was revealed by the phenotype of mice lacking α8β1. Although this ligand is very likely to be functionally important, we can not exclude roles for other matrix proteins. As summarized in the Introduction, with the exception of OPN, none of the other known ligands for α8β1 has a distribution appropriate to mediate the essential interactions with α8β1 revealed by the α8 mutant. OPN has been shown to be a ligand for α8β1 (Denda et al., 1998b). OPN has also been shown to be localized in the extracellular matrix between the ureteric bud epithelium and metanephric mesenchyme in approximately the same distribution as the ligand detected by α8β1-AP and antibodies to OPN inhibit development of organ-cultured embryonic kidneys (Rogers et al., 1997). Notably, though, mice lacking OPN develop normal kidneys (Liaw et al., 1998), and we have shown that these mice continue to express a ligand in the extracellular matrix between the ureteric bud epithelium and metanephric mesenchyme that is detected by α8β1-AP (data not presented). These data leave open the possibility that OPN and nephronectin have overlapping roles in kidney development. mRNA encoding EGFL6/MAEG is also present in the embryonic kidney (Buchner et al., 2000), so this protein may also help regulate kidney development.
The identification and characterization of nephronectin should be useful in pursuing studies on the puzzling phenotype of the α8 mutant. As summarized in the Introduction, the earliest phenotype observed in the α8 mutant is a delay in growth of the ureteric bud into the metanephric mesenchyme (Müller et al., 1997). Since the integrin α8β1 is expressed in the mesenchyme and not in the ureteric bud epithelium, this observation suggests that the integrin functions in the mesenchyme to control expression or signaling efficiency of a factor necessary for ureteric bud growth. During the past few years, analyses of several mutants have identified a growth factor–mediated signaling pathway important in this process. Glial cell–derived neurotrophic factor (GDNF) is synthesized in the metanephric mesenchyme (Moore et al., 1996). Secreted GDNF has been shown to control ingrowth of the ureteric bud by binding to GFR-α1, which results in activation of the c-ret tyrosine kinase (for review see Sariola and Sainio, 1997). The early phenotypes of mice lacking GDNF, GFR-α1, or c-ret are very similar to that of mice lacking the α8β1 integrin, so it seems likely that this integrin is involved in this signaling pathway. Potentially, nephronectin acting through α8β1 could induce a signaling pathway that results in enhanced expression of GDNF. Alternatively, it could synergize with GDNF by promoting secretion of this factor or its localization in the extracellular matrix. Whatever the mechanism, further investigations using this ligand should advance our understanding of the puzzling function of α8β1 in kidney development.
Materials and methods
Lambda phage expression cloning
A mouse embryonic day 13 heart, Lambda UNIZAP® library (Stratagene) was screened with α8β1-AP as follows. Lambda phage was plated onto 15-cm NZY plates with host bacteria at a density of 50,000 plaque forming units, as recommended by the manufacturer. Plates were incubated for 3 h at 42°C until plaques were just visible. Nitrocellulose membranes (Stratagene) were wetted in 10 mM isopropyl-β-d-galactopyranoside and air dried. The membranes were placed on the plates and incubated overnight at 30°C. Membranes were removed, washed several times with TBS (20 mM Tris/HCl, pH 7.5, 150 mM NaCl), blocked with TBS containing 3% milk powder, probed with α8β1-AP, and developed as described above with the addition of MnCl2. Phage were recovered from positive plaques and rescreened until a single plaque could be picked. Excision of phage and plasmid recovery were done as described in the Stratagene manual.
Recombinant protein production
The nephronectin fragments neph251-561 (amino acids 251–561) and neph251-381 (amino acids 251–381) were expressed as NH2-terminal GST fusion proteins in E. coli. Both fragments were generated by PCR and cloned into the pGEX-4T-3 vector (Amersham Pharmacia Biotech). Constructs were verified by sequencing. Recombinant fusion proteins were expressed in E. coli BL21 cells. Bacteria were grown at 37°C in LB medium to OD600 = 0.8 and were then transferred to 30°C. Cells were induced with 1 mM isopropyl-β-d-thiogalactopyranoside and grown for an additional 2.5 h. Cells were collected by centrifugation, resuspended in lysis buffer (50 mM Tris-Cl, pH 8, 150 mM NaCl, 2 mM EDTA, 0.1% Triton X-100 containing 1 μg/ml PMSF, 2 μg/ml aprotinin, 0.7 μg/ml pepstatin A), and lysed by sonication. After centrifugation, the supernatant was incubated for 1 h at 4°C with glutathione–Sepharose (Amersham Pharmacia Biotech). Beads were washed with lysis buffer and with PBS and eluted with PBS containing 50 mM reduced glutathione.
For expression of the nephronectin fragment neph251-561 (amino acids 251–561) in COS7 cells, a PCR fragment was generated and cloned into the pSecTag2 vector (Invitrogen) containing an Ig κ chain signal peptide and a COOH-terminal myc/His6 tag. COS7 cells were grown in DME (GIBCO BRL) supplemented with 10% fetal bovine serum and penicillin/streptomycin. Cells were transiently transfected with LipofectAmine (GIBCO BRL). After transfection, medium was changed to DME supplemented with Nutridoma HU (Roche Biochemicals) and penicillin/streptomycin. Conditioned medium was collected every 2 d for 8 d.
To establish stably transfected cell lines, CHO cells were transfected with the pSecTag2-neph251-561 vector described above. 24 h after transfection, 500 micrograms/ml zeocin (Invitrogen) was added to the medium. After 2–3 wk selection, single colonies were picked and expanded. Expanded clones were screened by Western blot for expression of neph251-561. For expression of recombinant neph251-561, stably transfected CHO cells were adapted to serum-free CHO-S-SFMII medium (GIBCO BRL) according to the manufacturer's instructions and grown in spinner bottles in the presence of 250 μg/ml zeocin. Conditioned medium was harvested when cell density was >1–2 10 × 6 cells/ml. Cells and debris were removed by centrifugation. After the addition of 0.02% sodium azide and 1 μ/ml PMSF, the medium was filtered (number 1 filter; Whatman) and concentrated 10–20-fold using a S1Y30 spiral ultrafiltration cartridge (Amicon), followed by a second 10-fold concentration step through a 10-kD cut-off ultrafiltration membrane (Amicon).
As a preclearing step, the supernatant was passed twice over a protein G–Sepharose column (Amersham Pharmacia Biotech). The flow-through was adsorbed to a protein G–Sepharose column coupled with anti–c-myc antibody 9E10 (Developmental Studies Hybridoma Bank). The column was washed with 10-column volumes PBS and eluted with 100 mM triethylamine, pH 11, washed with PBS, and eluted again with 100 mM glycine, pH 2.5. Fractions from both elution steps containing purified nephronectin were identified by blotting with α8β1-AP and were dialyzed against PBS. Protein was quantified by standard protein assay (Bio-Rad Laboratories).
Recombinant α8β1-AP was expressed as described (Denda et al., 1998a). Conditioned medium was concentrated 100-fold. Then, Tris-Cl, pH 8, was added to a final concentration of 20 mM, and PMSF was added to 1 μg/ml. For use on blots, the conditioned medium was diluted 5–10-fold with blocking buffer (see below).
Antibodies and reagents
An antiserum to nephronectin was generated by immunizing rabbits with purified GST fusion protein, GST-neph251-381. For immunohistochemistry, the antibody was affinity purified against GST-neph251-381 coupled to CNBr-Sepharose (Amersham Pharmacia Biotech). The antiserum was passed over a Sepharose column coupled to GST to remove antibodies recognizing the GST. Specificity of the affinity-purified antibody was tested by Western blot with a maltose-binding protein–nephronectin fusion protein.
Antiserum 1526 against the integrin α8 extracellular domain used in this study has been described previously (Müller et al., 1997). Anti–mouse VN was a gift from D. Seiffert (Seiffert, 1996). Anti–c-myc 9E10 ascites was generated using the hybridoma cell line 9E10 developed by J.M. Bishop and was obtained from the Developmental Studied Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Human plasma FN, the 120-kD FN fragment (FN120), bovine plasma VN, and GRGDSP and GRGESP peptides were purchased from GIBCO BRL.
Protein extracts
Newborn mouse kidneys were homogenized in ice-cold TBS containing 1 mM PMSF, 10 μg/ml leupeptin, and 0.7 μg/ml pepstatin A at a 10:1 vol/wt ratio. After centrifugation at 15,000 g in a tabletop centrifuge for 30 min, the pellet was resuspended in extraction buffer (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 50 mM n-octylglucoside, 2 mM MgCl2, 1 μg/ml PMSF, 10 μg/ml leupeptin, 0.7 μg/ml pepstatin A). After a 10-min extraction, samples were centrifuged at 15,000 rpm for 30 min to remove debris. Protein concentrations were determined with the detergent compatible protein assay (Bio-Rad Laboratories), and 100 μg total protein was loaded on SDS-PAGE.
Protein blots
For antigen and α8β1-AP ligand blotting, protein extracts or purified proteins were boiled in SDS sample buffer, separated on 7 or 12% SDS-PAGE and transferred to nitrocellulose membranes. Nonspecific binding sites were blocked with blocking buffer (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 3% milk powder) at room temperature. For Western blots, the nitrocellulose was incubated with antibodies diluted in blocking buffer for 1 h at room temperature, washed three times for 5 min each with wash buffer (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 0.1% Tween-20), and incubated with secondary antibody (goat anti–rabbit IgG, AP conjugated; Sigma-Aldrich) for 1 h at room temperature. This was followed by three washes of 5 min each with wash buffer and two washes with a AP buffer (100 mM Tris-Cl, pH 9.5, 100 mM NaCl, 5 mM MgCl2). Nitrocellulose membranes were then developed with AP buffer containing 0.33 mg/ml NBT and 0.17 mg/ml BCIP. For blotting using α8β1-AP, blocked nitrocellulose membranes were incubated with α8β1-AP in blocking buffer containing 2 mM MgCl2 or MnCl2 for 2 h at room temperature. Membranes were washed three times for 5 min with TBS containing 2 mM MgCl2 or MnCl2, followed by two washes with AP buffer. For incubations with MnCl2, 0.5 mM MnCl2 was included in the AP buffer. Blots were developed with AP buffer containing 0.33 mg/ml nitro blue tetrazolium and 0.17 mg/ml 5-bromo-4-chloro-3-indolylphosphate.
Immunohistochemistry
Histological methods were carried out as described previously (Jones et al., 1994). Immunohistochemistry with antiintegrin α8 antiserum 1526 and with α8β1-AP have been described previously (Müller et al., 1997; Denda et al., 1998b). For staining with antinephronectin antibodies, sections were blocked with 1% BSA, 0.1% Triton X-100, 1% H2O2 in PBS; washed with 1% BSA, 0.1% Triton X-100 in PBS; incubated with affinity-purified antinephronectin antibody at 10 μg/ml in the same buffer; washed and detected with the ABC kit (Vector Laboratories); and counterstained with Nissl as described (Jones et al., 1994).
In situ hybridization
In situ hybridization was carried out on fresh frozen sections as described (Schaeren-Wiemers and Gerfin-Moser, 1993). For digoxygenin labeling of probes and detection of signal, the digoxygenin RNA labeling and detection kit (Roche Biochemicals) was used. A 0.9-kb nephronectin cDNA fragment was used as template.
Immunoprecipitation
For immunoprecipitation, antiintegrin α8 antibody was covalently coupled to protein A–Sepharose, that was added to 500 μl kidney extract for 2 h at 4°C. The beads were washed three times for 5 min with wash buffer (50 mM Tris-Cl, pH 7.5, 500 mM NaCl, 50 mM n-octylglucoside, 2 mM MgCl2, 1 μg/ml PMSF, 10 μg/ml leupeptin, 0.7 μg/ml pepstatin A). After addition of SDS sample buffer, the beads were boiled for 5 min and centrifuged. The supernatant was collected for blot analysis.
Coimmunoprecipitations
For coimmunoprecipitations, extracts and immunoprecipitations were done as above. As controls, 10 mM EDTA instead of MgCl2 or 600 μg/ml GRGDSP and GRGESP peptides were added to all the extraction buffers and wash buffers in the control samples.
Cell adhesion assays
For cell adhesion assays, substrate protein was diluted in PBS to the indicated concentrations. Linbro Titertek 96-well plates (Flow Laboratories) were then treated overnight at 4°C with a total volume of 100 μl of substrate solution per well. The wells were blocked with 10 mg/ml BSA in PBS for 1 h at 37°C. The cells were harvested with PBS containing 1 mM EDTA, washed once in TBS (24 mM Tris-Cl, pH 7.4, 137 mM NaCl, 2.7 mM KCl), counted, and then resuspended in TBS containing 0.1% BSA, 2 mM glucose, 1 mM MnCl2. The cells were counted once again, and a total of 2.0 × 105 cells were plated per well. The cells were incubated for 1.5 h at 37°C, washed four times with TBS containing 1 mM MnCl2, fixed for 15 min with 2% paraformaldehyde, and stained for 5 min with 2.5% crystal violet in 20% ethanol. Finally, each well was washed four times with water, and adherent cells were lysed with 1% SDS. Absorption values for each well were read at 570 nm using a microtiter plate reader and SOFTmax v2.35 (Molecular Devices). Final absorption values for wells coated with FN or nephronectin GST fusion proteins were determined by calculating the mean absorption value of duplicate or quadruplicate wells and subtracting the mean value from either BSA- or GST-treated control wells run in parallel. For antibody inhibition, cells were preincubated for 15 min on ice with the antibody before plating. Antibody was present throughout the adhesion assay. Antibody BIIG2 (anti-α5) was supplied as an ascites. This ascites blocked adhesion of the parental K562 cells to FN at a dilution of 1:20. K562 adhesion to FN was not affected by a control ascites when used at the same concentration.
Acknowledgments
We thank Dr. Lucy Liaw (Maine Medical Center Research Institute, Scarborough, ME) for providing OPN mutant mice; Dr. Eric Brown (University of California, San Francisco, San Francisco, CA) for providing K562 cells expressing αVβ3, αVβ5, αVβ6, α4β1, and α4β7; and Dr. Martin Hemler (Dana Farber Cancer Institute, Boston, MA) for providing K562 cells expressing α2β1 and α3β1. We thank Dr. Caroline Damsky (University of California, San Francisco) for anti-α5β1 mAb.
This work was supported by the Howard Hughes Medical Institute. R. Brandenberger was supported by fellowships from the Swiss National Science Foundation and the Human Frontiers Science Program. L.F. Reichardt is an investigator of the Howard Hughes Medical Institute.
Note added in proof: Since submision of this manuscript, Dr. Kenichi Tezuka and colleagues (Science University of Tokyo, Yamazaki, Noda, Chiba, Japan) have informed us that KA8 cells (the K562 cells expressing the integrin α8β1, used in our paper) bind to a novel protein named POEM (preosteoblast EGF-like repeat protein with MAM domain). Dr. Tezuka and colleagues sent us the sequence of the first 15 amino acids of POEM, and this sequence is identical to that of the corresponding residues in nephronectin. Also, POEM has the same number of EGF repeats and the same overall structure as nephronectin. Nephronectin and POEM thus are almost certainly the same protein. Dr. Tezuka and colleagues have submitted a manuscript on their work.
Ralph Brandenberger's present address is Celera Genomics, 850 Lincoln Center Dr., Foster City, CA 94404.
Sumiko Denda's present address is Shisheido Research Center 2, 2-12-1 Fukuura, Kanazawa-ku, Yokohama 236-8643, Japan.
Ullrich Müller's present address is Friedrich Miescher Institute, Maulbeerstrasse 66, CH-4058 Basel, Switzerland.
Footnotes
Abbreviations used in this paper: AP, alkaline phosphatase; FN, fibronectin; GDNF, glial cell–derived neurotrophic factor; GST, glutathione S-transferase; OPN, osteopontin; TN-C, tenascin-c; VN, vitronectin.
References
- Aufderheide, E., R. Chiquet-Ehrismann, and P. Ekblom. 1987. Epithelial–mesenchymal interactions in the developing kidney lead to expression of tenascin in the mesenchyme. J. Cell Biol. 105:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blystone, S.D., I.L. Graham, F.P. Lindberg, and E.J. Brown. 1994. Integrin αVβ3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor α5β1. J Cell Biol. 127:1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy, B., E. Bossy-Wetzel, and L.F. Reichardt. 1991. Characterization of the integrin α8 subunit: a new integrin β1 associated subunit, which is prominently expressed on axons and on cells in contact with basal laminae in chick embryos. EMBO J. 10:2375–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner G, U. Orfanelli, N. Quaderi, M.T. Bassi, G. Andolfi, A. Ballabio, and B. Franco. 2000. Identification of a new EGF-repeat-containing gene from human Xp22: a candidate for developmental disorders. Genomics. 65:16–23. [DOI] [PubMed] [Google Scholar]
- Denda, S., U. Müller, K.L. Crossin, H.P. Erickson, and L.F. Reichardt. 1998. a. Utilization of a soluble integrin-alkaline phosphatase chimera to characterize integrin α8β1 receptor interactions with tenascin: murine α8β1 binds to the RGD site in tenascin-c fragments, but not to native tenascin-c. Biochemistry. 37:5464–5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denda, S., L.F. Reichardt, and U. Müller. 1998. b. Identification of osteopontin as a novel ligand for the integrin α8β1 and potential roles for this integrin-ligend interaction in kidney morphogenesis. Mol. Biol. Cell. 9:1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom, P. 1981. Formation of basement membranes in the embryonic kidney: an immunohistochemical study. J. Cell Biol. 91:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fässler, R., E. Georges-Labouesse, and E. Hirsch. 1996. Genetic analysis of integrin function in mice. Curr. Opin. Cell Biol. 8:641–646. [DOI] [PubMed] [Google Scholar]
- George, E.L., E.N. Georges-Labouesse, R.S. Patel-King, H. Rayburn, and R.O. Hynes. 1993. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 119:1079–1091. [DOI] [PubMed] [Google Scholar]
- Hansen, J.E., O. Lund, N. Tolstrup, A.A. Gooley, K.L. Williams, and S. Brunak. 1998. NetOglyc: prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconjugate J. 15:115–130. [DOI] [PubMed] [Google Scholar]
- Hutter, H., B.E. Vogel, J.D. Plenefisch, C.R. Norris, R.B. Proenca, J. Spieth, C. Guo, S. Mastwal, X. Shu, J. Scheel, and E.M. Hedgecock. 2000. Conservation and novelty in the evolution of cell adhesion and extracellular matrix genes. Science. 287: 989–994. [DOI] [PubMed] [Google Scholar]
- Hynes, R.O. 1996. Targeted mutations in cell adhesion genes: what have we learned from them? Dev. Biol. 180:402–412. [DOI] [PubMed] [Google Scholar]
- Hynes, R.O., and Q. Zhao. 2000. The evolution of cell adhesion. J. Cell Biol. 150:F89–F95. [DOI] [PubMed] [Google Scholar]
- Jones, K.R, I. Fariñas, C. Backus, and L.F. Reichardt. 1994. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 76:989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan. M.S., S.K. Batra, W.N. Qi, R.S. Metzgar, and M.A. Hollingsworth. 1990. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J. Biol. Chem. 265:15294–15299. [PubMed] [Google Scholar]
- Liaw, L., D.E. Birk, C.B. Ballas, J.S. Whitsitt, J.M. Davidson, and B.L. Hogan. 1998. Altered wound healing in mice lacking a functional osteopontin gene (ssp1). J. Clin. Invest. 101:1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, M.W., R.D. Klein, I. Farinás, H. Eauer, M. Aronanini, H. Phillips, L.F. Reichardt, A.M. Ryan, K.C. Carver-Moore, and A. Rosenthal. 1996. Absence of kidneys and enteric nervous system but not of CNS dopaminergic or noradrenergic neurons in glial-derived neurotrophic factor deficient mice. Nature. 382:76–79. 8657308 [Google Scholar]
- Moyano, J.V., B. Carnemolla, C. Dominguez-Jimenez, M. Garcia-Gila, J.P. Albar, P. Sanchez-Aparicio, A. Leprini, G. Querze, L. Zardi, and A. Garcia-Pardo. 1997. Fibronectin type III5 repeat contains a novel cell adhesion sequence, KLDAPT, which binds activated α4β1 and α4β7 integrins. J. Biol. Chem. 272:24832–24836. [DOI] [PubMed] [Google Scholar]
- Müller, U., B. Bossy, K. Venstrom, and L.F. Reichardt. 1995. Integrin α8β1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol. Biol. Cell. 6:433–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, U., D. Wang, S. Denda, J.J. Meneses, R.A. Pedersen, and L.F. Reichardt. 1997. Integrin α8β1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 88:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, Z., P. Handford, M. Mayhew, V. Knott, G.G. Brownlee, and D. Stuart. 1995. The structure of a Ca2+-binding epidermal growth factor-like domain: its role in protein-protein interactions. Cell. 82:131–141. [DOI] [PubMed] [Google Scholar]
- Rogers, S. A., B.J. Padanilam, K.A. Hruska, C.M. Giachelli, and M.R. Hammerman. 1997. Metanephric osteopontin regulates nephrogenesis in vitro. Am. J. Physiol. 272:F469–F476. [DOI] [PubMed] [Google Scholar]
- Saga, Y., T. Yagi, Y. Ikawa, T. Sakakura, and S. Aizawa. 1992. Mice develop normally without tenascin. Genes Dev. 6:1821–1831. [DOI] [PubMed] [Google Scholar]
- Sariola, H., and K. Sainio. 1997. The tip-top branching ureter. Curr. Opin. Cell Biol. 9:877–884. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers, N., and A. Gerfin-Moser. 1993. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 100:431–440. [DOI] [PubMed] [Google Scholar]
- Schnapp, L.M., J.M. Breuss, D.M. Ramos, D. Sheppard, and R. Pytela. 1995. a. Sequence and tissue distribution of the human integrin α8 subunit: a β1-associated α subunit expressed in smooth muscle cells. J. Cell. Sci. 108:537–544. [DOI] [PubMed] [Google Scholar]
- Schnapp, L.M., N. Hatch, D.M. Ramos, I.V. Klimanskaya, D. Sheppard, and R. Pytela. 1995. b. The human integrin α8β1 functions as a receptor for tenascin, fibronectin, and vitronectin. J. Biol. Chem. 270:23196–23202. [DOI] [PubMed] [Google Scholar]
- Seiffert, D. 1996. Detection of vitronectin in mineralized bone matrix. J. Histochem. Cytochem. 44:275–280. [DOI] [PubMed] [Google Scholar]
- Seiffert, D., M. Keaton, T. Eguchi, M. Sawday, and D.J. Loskutoff. 1991. Detection of vitronectin mRNA in tissues and cells of the mouse. Proc. Natl. Acad. Sci. USA. 88:9402–9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum-Finney, B., K. Venstrom, U. Müller, R. Kypta, C. Backus, M. Chiquet, and L.F. Reichardt. 1995. The integrin receptor α8β1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron. 14:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, G., J.J. Mulero, R.P. Berntsen, D.B. Loeb, R. Drmanac, and J.E. Ford. 1999. Cloning of a novel epidermal growth factor repeat containing gene EGFL6: expressed in tumor and fetal tissues. Genomics. 62:304–307. [DOI] [PubMed] [Google Scholar]
- Yang, Y., P.M. Cardarelli, K. Lehnert, S. Rowland, and G.W. Krissansen,. 1998. LPAM-1 (integrin α4β7)-ligand binding: overlapping binding sites recognizing VCAM-1, MAdCAM-1 and CS-1 are blocked by fibrinogen, a fibronectin-like polymer and RGD-like cyclic peptides. Eur. J. Immunol. 28:995–1004. [DOI] [PubMed] [Google Scholar]
- Zhao, J., L. Hyman, and C. Moore. 1999. Formation of mRNA 3′ ends in eukaryotes: mechanisms, regulation, and interrelationships with other steps in mRNA synthesis. Microbiol. Mol. Biol. Rev. 63:405–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X., T.L. Saunders, S.A. Camper, L.C. Samuelson, and D. Ginsburg. 1995. Vitronectin is not essential for normal mammalian development and fertility. Proc. Natl. Acad. Sci. USA. 92:12426–12430. [DOI] [PMC free article] [PubMed] [Google Scholar]