Abstract
Mutant mice lacking the integrin α8 subunit exhibit variable defects in kidney development with most mutants missing both kidneys. Several lines of evidence indicate that the known extracellular matrix ligands for integrin α8β1 are either dispensable for or not involved in α8β1 signaling during kidney development. This suggests the presence of an unknown ligand. A novel α8β1 ligand, nephronectin, has now been identified. Nephronectin is a new extracellular matrix protein associated with the Wolffian duct and the ureteric bud, epithelial structures with well-defined roles in kidney development.
Congenital abnormalities of the kidney and urinary tract are relatively common in the human population, affecting ∼0.5% of pregnancies. The most severe congenital defects, such as bilateral renal agenesis (absence of both kidneys), result in death shortly after birth, whereas the more common milder anomalies result in dysplasia, cysts, stone formation, vesicoureteral reflux, and sometimes renal failure (Pope et al., 1999; Glassock and Massry, 2001). A better understanding of the mechanisms which regulate both kidney and ureter development at the molecular level is essential for effective treatment and prevention of these congenital disorders. In this issue, Brandenberger et al. (2001) report the identification of nephronectin, a novel extracellular matrix molecule likely to have an important role in kidney development. Understanding the biology of nephronectin should improve prospects for elucidating the causes of congenital kidney malformations.
The developing kidney continues to be an excellent model system for investigating the inter- and intracellular signals involved in branching morphogenesis, mesenchyme to epithelium transitions, vasculogenesis, and cell type-specific gene expression. In the mouse, kidney development begins on the eleventh day of gestation when the ureteric bud, an outgrowth of the Wolffian duct, is induced to grow towards and invade the metanephric mesenchyme (MM)* (Fig. 1) . The MM is a distinct population of loosely arranged mesoderm-derived cells that are determined to form nephrons, the individual filtration units of the definitive (metanephric) kidney. When the ureteric bud invades the MM, it induces condensation, epithelialization, and nephron formation in a subset of the MM. In turn, signals from the MM induce the ureteric bud to branch and grow (Fig. 1), whereupon a new population of MM is induced to form nephrons. This cycle of reciprocal interactions between the ureteric bud and the MM continues throughout gestation and for several weeks postnatally to eventually form ∼1,500 nephrons per kidney in the mouse (Saxen, 1987; for review see Davies and Bard, 1998).
Figure 1.
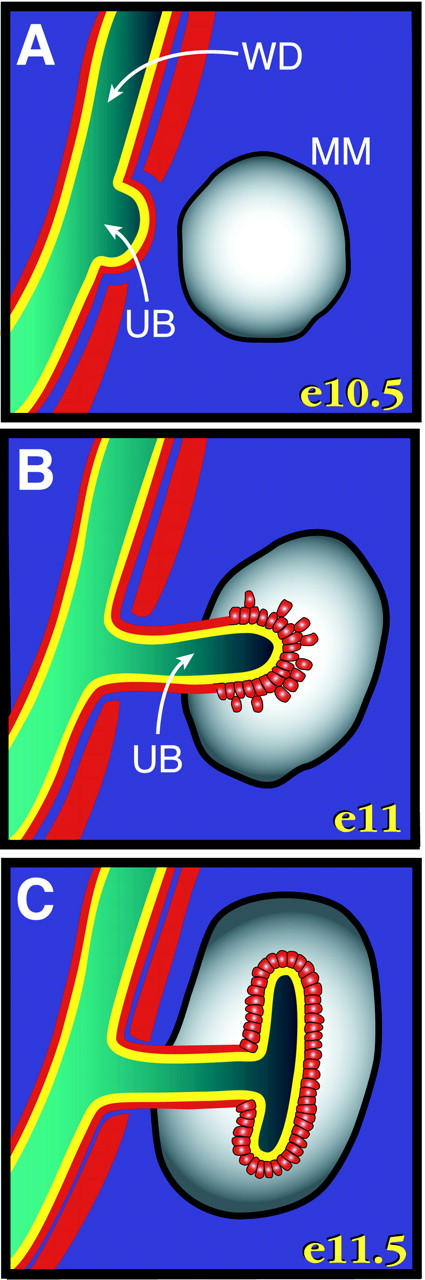
Schematic representation of early kidney development with known and hypothesized locations of nephronectin (yellow) and integrin α8 (red) indicated. The interaction of integrin α8-expressing mesenchymal cells with nephronectin in the ureteric bud basement membrane is critical for normal ureteric bud outgrowth and branching. (A) Embryonic day (e) 10.5. The ureteric bud (UB) has just begun to bud from the Wolffian duct (WD). Nephronectin is associated with the Wolffian duct and ureteric bud basement membranes. Integrin α8 is present on the mesenchymal cells immediately adjacent to basement membranes and elsewhere in the urogenital ridge but not in the MM. (B) e11. The ureteric bud has invaded the MM. Integrin α8 now becomes detectable on the condensing cells in the MM near the tip of the bud. (C) e11.5. The MM has induced the ureteric bud to branch. Nephronectin is found throughout the ureteric bud basement membrane, and mesenchymal cells expressing integrin α8 are found both along the proximal stalk of the ureteric bud and adjacent to the branched ureteric bud.
The generation and analysis of mice bearing targeted mutations has been especially instrumental in defining the repertoire of proteins that play important roles in kidney development. Members of many well-known gene families involved in diverse biological pathways have been shown to be required for normal kidney development (for review see Kuure et al., 2000). These include the Wilms' tumor suppressor protein (WT-1), the receptor tyrosine kinase c-ret, its ligand, glial cell line–derived neurotrophic factor, Pax-2, Eya-1, Emx-2, BF-2, Pod1, Lmx1b, p53, BMPs, FGFs, Wnts, Hox proteins, cadherins, integrins, and laminins. In some cases, the mutated gene of interest was not known or predicted to be involved in kidney development. In one example, mice with a mutation in the gene encoding heparan sulfate 2–sulfotransferase, an enzyme involved in proteoglycan synthesis, exhibit renal agenesis due to failure of ureteric bud outgrowth (Bullock et al., 1998). This indicates an important role for one or more sulfated proteoglycans either in the extracellular matrix or on the cell surface. Similar scenarios will likely recur frequently as large scale mouse mutagenesis screens come to fruition.
One such scenario transpired when a targeted mutation in the integrin α8 chain gene (Itga8) was generated (Muller et al., 1997). At the time, integrin α8 was known to be expressed on the surface of sensory, motor, and other neurons, especially at sites of apposition of their axons to basement membranes. Because α8β1 bound several matrix components, including fibronectin, vitronectin, and tenascin-C (Muller et al., 1995; Schnapp et al., 1995; Varnum-Finney et al., 1995), the expectation was that mutants would have defects in the nervous system, perhaps in outgrowth or pathfinding. Surprisingly, most Itga8 −/− mice lacked kidneys and died shortly after birth. (Kidney function is not required in utero but is required postnatally.) However, ∼32% of homozygotes had at least one kidney, which was smaller than those observed in wild-type mice but nevertheless was functional in a subset of cases. This resulted in a cohort of Itga8 −/− mice with one kidney that were viable and fertile. No neuronal defects have been reported in these mutants, but significant defects were found in a subset of vestibular hair cells (Littlewood Evans and Muller, 2000). Despite the presence of functional kidneys in some Itga8 mutants, these data showed that integrin α8 plays an important role in kidney development (Muller et al., 1997).
In an attempt to define the role of integrin α8 in kidney development, its expression patterns at early stages of metanephrogenesis in wild-type kidney were determined by immunohistochemistry (Muller et al., 1997). Integrin α8 was clearly expressed on the surface of the induced condensing MM and was also concentrated on the mesenchymal cells adjacent to proximal segments of the ureteric bud (Fig. 1, B and C). Once the condensed MM epithelialized, integrin α8 was no longer detectable. In addition, there was no evidence that the ureteric bud epithelial cells expressed integrin α8. The straightforward interpretation of these results is that binding of integrin α8 on the surface of cells in the MM to a ligand synthesized by the ureteric bud is critical for normal kidney development. The interaction may be necessary to induce or maintain signaling from the MM to the ureteric bud. Alternatively, the interaction itself may result in a conformational change in the ligand, perhaps altering its functional properties. This could affect ureteric bud outgrowth either directly via a different receptor or indirectly by increasing the concentration or accessibility of critical factors secreted by the MM such as glial cell line–derived neurotrophic factor.
Although it is relatively simple to explain how an integrin on cells in the MM might influence ureteric bud branching and outgrowth within the metanephros, it is more difficult to understand how it could promote initial bud outgrowth from the Wolffian duct towards the MM, a process which is slowed in the Itga8 mutants. This difficulty arises because: (a) the MM is not in direct contact with the Wolffian duct and (b) uninduced MM does not even express integrin α8 (Muller et al., 1997). However, expression of integrin α8 by mesenchymal cells directly adjacent to the Wolffian duct and the nascent ureteric bud could promote bud outgrowth by the same mechanisms discussed above, assuming the ligand is present at early stages of bud outgrowth (and it appears to be; see below). Whatever the mechanism, in order to determine how integrin α8 acts the identity of the ligand needed to be determined.
Could it be one of the known α8β1 ligands? Three of the known ligands, fibronectin, vitronectin, and tenascin-C, were not deposited appropriately in the developing kidney to be considered likely candidates (Muller et al., 1997). A fourth ligand, osteopontin, was expressed in the developing kidney, but mutation of its gene showed that it was not required for kidney development in vivo (Denda et al., 1998; Liaw et al., 1998). By using a soluble alkaline phosphatase-tagged α8β1 dimer (α8β1-AP), which presumably bound to the same ligand(s) as the endogenous α8β1, the putative ligand was localized in the developing kidney adjacent to the ureteric bud in a pattern consistent with its being present in the ureteric bud basement membrane (Muller et al., 1997). The putative ligand was therefore present exactly where it needed to be in order to interact with integrin α8β1 on the surface of mesenchymal cells. Furthermore, on a blot the soluble α8β1-AP bound in an arginine-glycine-aspartic acid (RGD)–dependent fashion to several proteins from embryonic kidney, including a major band at 60–90 kD, which did not correspond to the known ligands (Muller et al., 1997). This suggested that a novel ligand for α8β1 existed in developing kidney.
In this issue, Brandenberger et al. (2001) report the identification and characterization of this novel ligand, nephronectin. By using the soluble α8β1-AP as a specific probe, cDNAs encoding nephronectin were isolated from a bacteriophage expression library. Based on the deduced amino acid sequence, nephronectin is a novel secreted protein, which contains an RGD sequence, five epidermal growth factor–like repeats, and a COOH-terminal domain with homology to MAM repeats. Both epidermal growth factor–like and MAM repeats are thought to mediate protein–protein interactions, so nephronectin likely interacts with itself or with other proteins in the extracellular matrix.
By generating antisera to nephronectin, Brandenberger et al. (2001) show that it is deposited in the developing kidney in a pattern similar to that revealed by the α8β1-AP probe, primarily in the ureteric bud basement membrane but also in the Wolffian duct basement membrane (Fig. 1) and at low levels in primitive nephron structures. Surprisingly, nephronectin was detected strongly in the glomerular basement membrane of maturing glomeruli. The glomerulus is that part of the nephron where filtration occurs, and the glomerular basement membrane is one important component of the filtration barrier. Therefore, nephronectin may play a role in establishing or maintaining the filtration barrier. However, integrin α8 is not associated with glomerular cells at this stage (Muller et al., 1997). Thus, there may be a receptor other than integrin α8 on glomerular cells that mediates interactions with nephronectin. One possibility is integrin α3, which is expressed by podocytes (glomerular epithelial cells) and required for formation of the glomerular filtration barrier (Kreidberg et al., 1996). However, integrin α3β1-expressing cells did not adhere to an RGD-containing fragment of nephronectin (Brandenberger et al., 2001). Nevertheless, its binding site may be elsewhere in nephronectin, as α3β1 is not a classic RGD-binding integrin. Cells expressing αV integrins did bind to the nephronectin fragment (Brandenberger et al., 2001), so it is possible that αV integrins on glomerular endothelial cells mediate their binding to nephronectin in the glomerular basement membrane.
The expression pattern of nephronectin in the ureteric bud and Wolffian duct is similar to that of laminin-10, composed of the laminin α5, β1, and γ1 chains (Miner et al., 1997; Sorokin et al., 1997). Knockout mice lacking the laminin α5 chain have multiple defects in kidney development, including uni- or bilateral renal agenesis in ∼20% of mutant embryos. This was associated with defective ureteric bud outgrowth and branching (Miner and Li, 2000). As nephronectin and laminin-10 appear to be colocalized in the Wolffian duct and ureteric bud basement membranes, it is possible that they may interact with each other and/or cooperate to promote efficient ureteric bud outgrowth and branching, either through parallel or common pathways. Alternatively, they may work synergistically with the other matrix molecules and receptors known to be involved in kidney development (for review see Muller and Brandli, 1999).
Despite its nephro-centric moniker, nephronectin expression is not restricted to the kidney. Immunohistochemical assays demonstrate that it is widely expressed during development, in most cases associated with basement membranes (Brandenberger et al., 2001). Might nephronectin have a role in the development of other organs? A genetic analysis of nephronectin function will hopefully answer this question and further elucidate the role of the integrin α8β1–nephronectin interaction in ureteric bud outgrowth and nephrogenesis.
Acknowledgments
I thank Michael Rauchman and Joshua Sanes for helpful discussions and comments on the article.
This work was supported by a George M. O'Brien Kidney Research Center grant from the National Institutes of Health (P50 DK45181).
Footnotes
Abbreviations used in this paper: AP, alkaline phosphatase; MM, metanephric mesenchyme; RGD, arginine-glycine-aspartic acid.
References
- Brandenberger, R., A. Schmidt, J. Linton, D. Wang, C. Backus, S. Denda, U. Muller, and L.F. Reichardt. 2001. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin α8β1 in the embryonic kidney. J. Cell Biol. 154:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock, S.L., J.M. Fletcher, R.S. Beddington, and V.A. Wilson. 1998. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 12:1894–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, J.A., and J.B.L. Bard. 1998. The development of the kidney. Curr. Topics Dev. Biol. 39:245–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denda, S., L.F. Reichardt, and U. Muller. 1998. Identification of osteopontin as a novel ligand for the integrin alpha8 beta1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol. Biol. Cell. 9:1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassock, R.J., and S.G. Massry. 2001. Other congenital disorders. Massry and Glassock's Textbook of Nephrology. S.G. Massry and R.J. Glassock, editors. Lippincott Williams & Wilkins, Philadelphia, PA. 865–868.
- Kreidberg, J.A., M.J. Donovan, S.L. Goldstein, H. Rennke, K. Shepherd, R.C. Jones, and R. Jaenisch. 1996. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 122:3537–3547. [DOI] [PubMed] [Google Scholar]
- Kuure, S., R. Vuolteenaho, and S. Vainio. 2000. Kidney morphogenesis: cellular and molecular regulation. Mech. Dev. 92:31–45. [DOI] [PubMed] [Google Scholar]
- Liaw, L., D.E. Birk, C.B. Ballas, J.S. Whitsitt, J.M. Davidson, and B.L. Hogan. 1998. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J. Clin. Invest. 101:1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood Evans, A., and U. Muller. 2000. Stereocilia defects in the sensory hair cells of the inner ear in mice deficient in integrin alpha8beta1. Nat. Genet. 24:424–428. [DOI] [PubMed] [Google Scholar]
- Miner, J.H., and C. Li. 2000. Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev. Biol. 217:278–289. [DOI] [PubMed] [Google Scholar]
- Miner, J.H., B.L. Patton, S.I. Lentz, D.J. Gilbert, W.D. Snider, N.A. Jenkins, N.G. Copeland, and J.R. Sanes. 1997. The laminin α chains: expression, developmental transitions, and chromosomal locations of α1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J. Cell Biol. 137:685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, U., and A.W. Brandli. 1999. Cell adhesion molecules and extracellular-matrix constituents in kidney development and disease. J. Cell Sci. 112:3855–3867. [DOI] [PubMed] [Google Scholar]
- Muller, U., B. Bossy, K. Venstrom, and L.F. Reichardt. 1995. Integrin alpha 8 beta 1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol. Biol. Cell. 6:433–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, U., D. Wang, S. Denda, J.J. Meneses, R.A. Pedersen, and L.F. Reichardt. 1997. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 88:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope, J.C., IV, J.W. Brock III, M.C. Adams, F.D. Stephens, and I. Ichikawa. 1999. How they begin and how they end: classic and new theories for the development and deterioration of congenital anomalies of the kidney and urinary tract, CAKUT. J. Am. Soc. Nephrol. 10:2018–2028. [DOI] [PubMed] [Google Scholar]
- Saxen, L. 1987. Organogenesis of the kidney. Cambridge University Press, Cambridge, UK. 173 pp.
- Schnapp, L.M., N. Hatch, D.M. Ramos, I.V. Klimanskaya, D. Sheppard, and R. Pytela. 1995. The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J. Biol. Chem. 270:23196–23202. [DOI] [PubMed] [Google Scholar]
- Sorokin, L.M., F. Pausch, M. Durbeej, and P. Ekblom. 1997. Differential expression of five laminin α (1-5) chains in developing and adult mouse kidney. Dev. Dyn. 210:446–462. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney, B., K. Venstrom, U. Muller, R. Kypta, C. Backus, M. Chiquet, and L.F. Reichardt. 1995. The integrin receptor alpha 8 beta 1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron. 14:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]


