Abstract
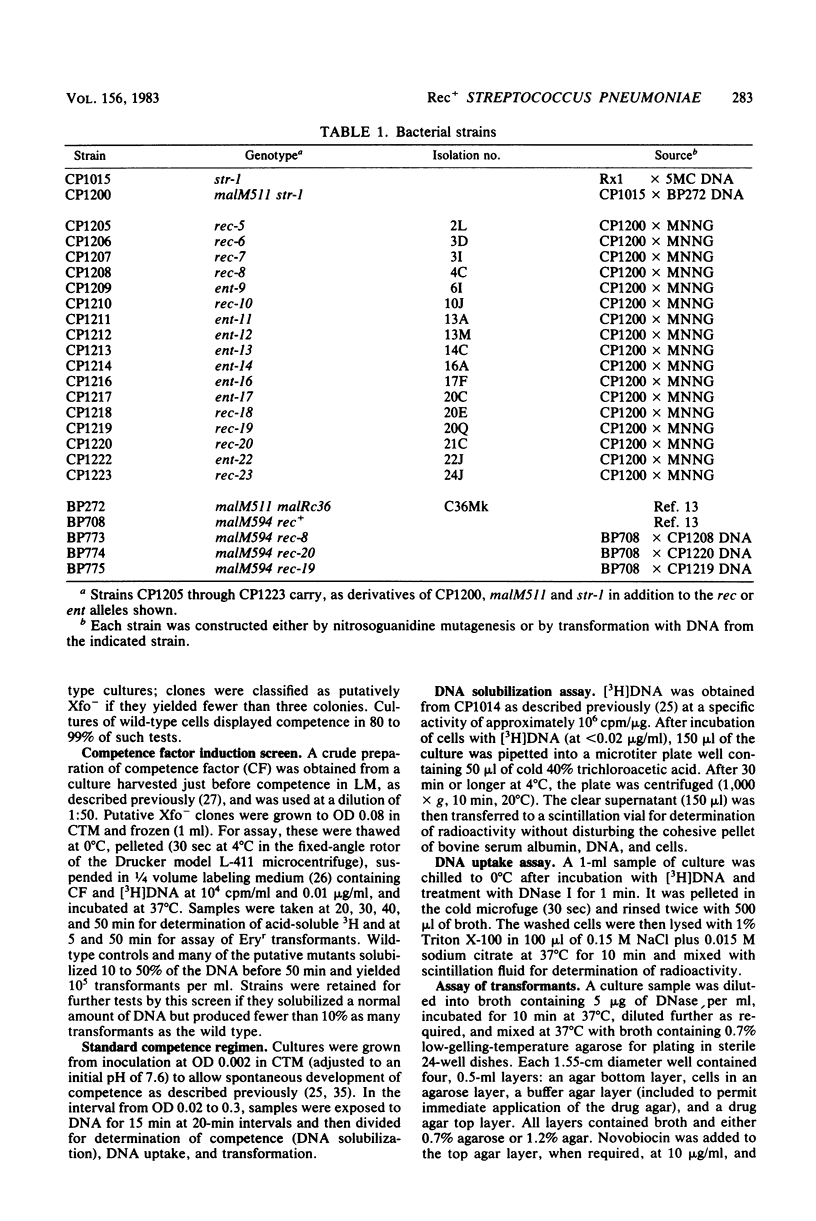
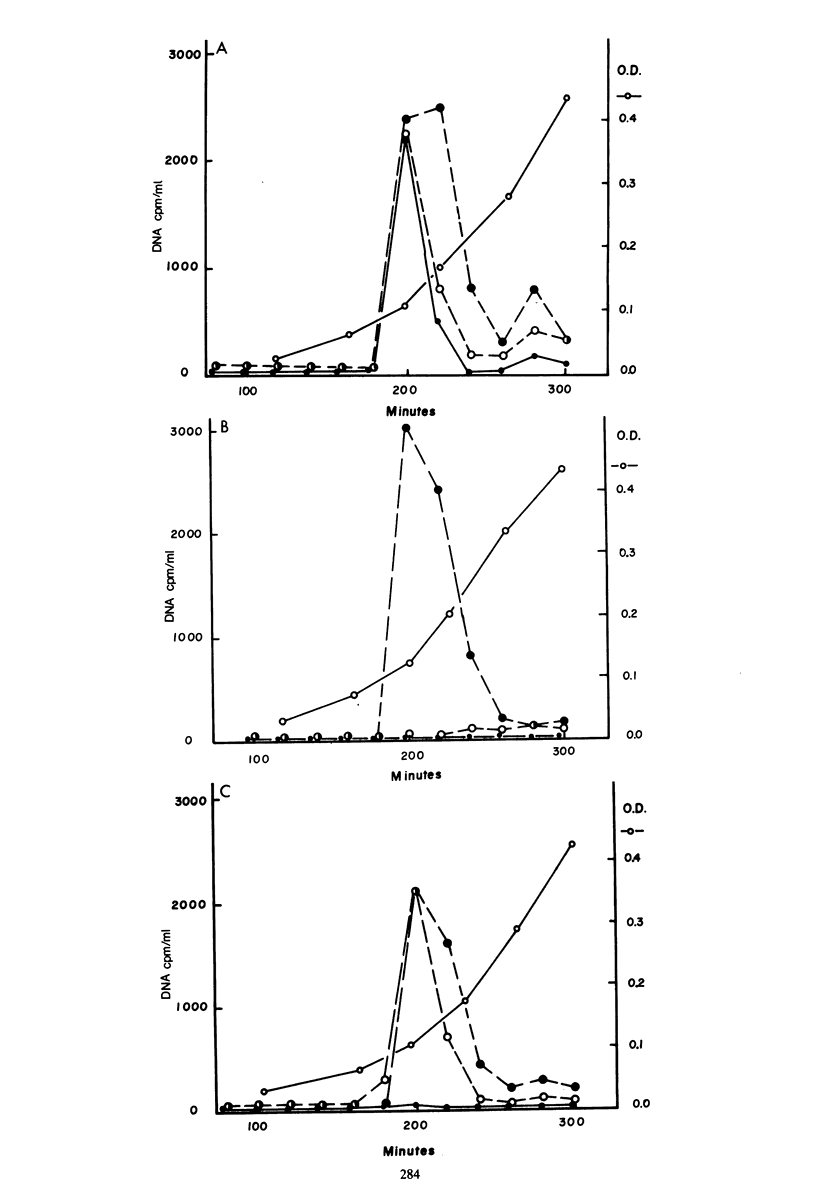
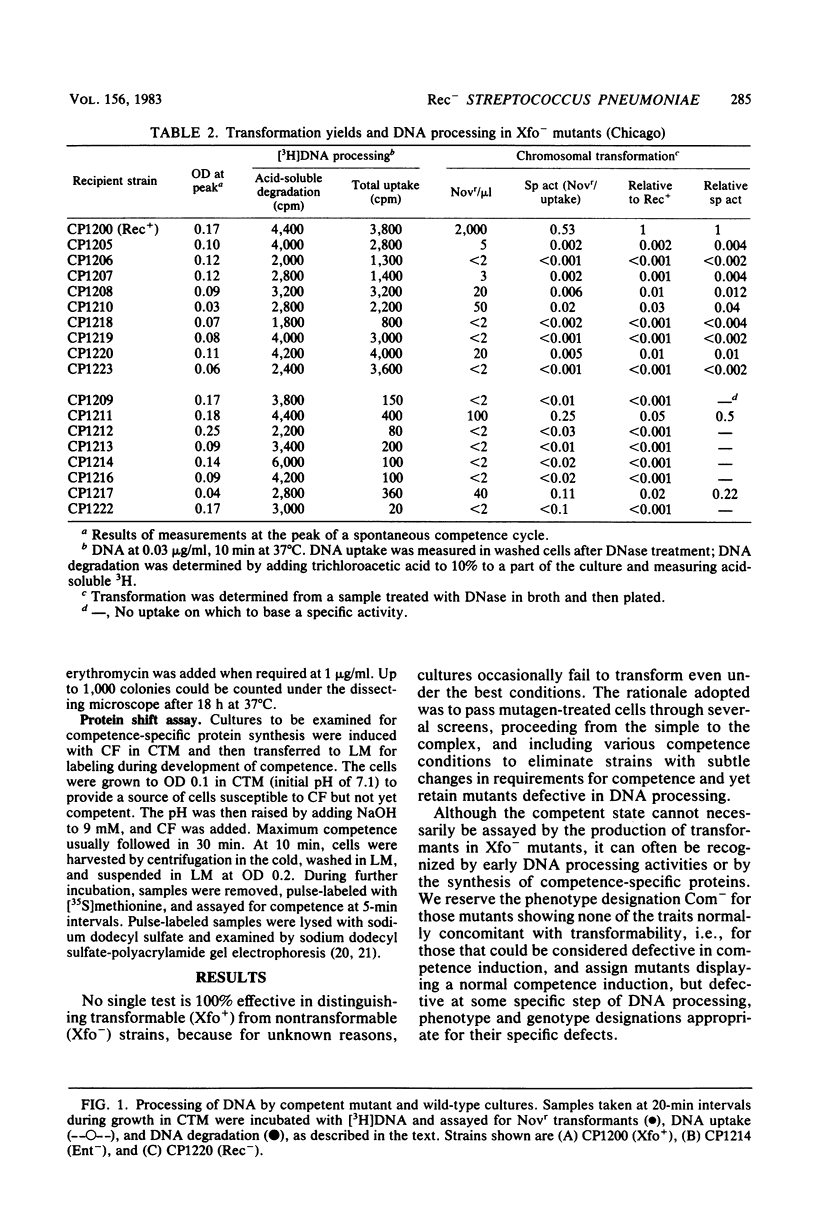
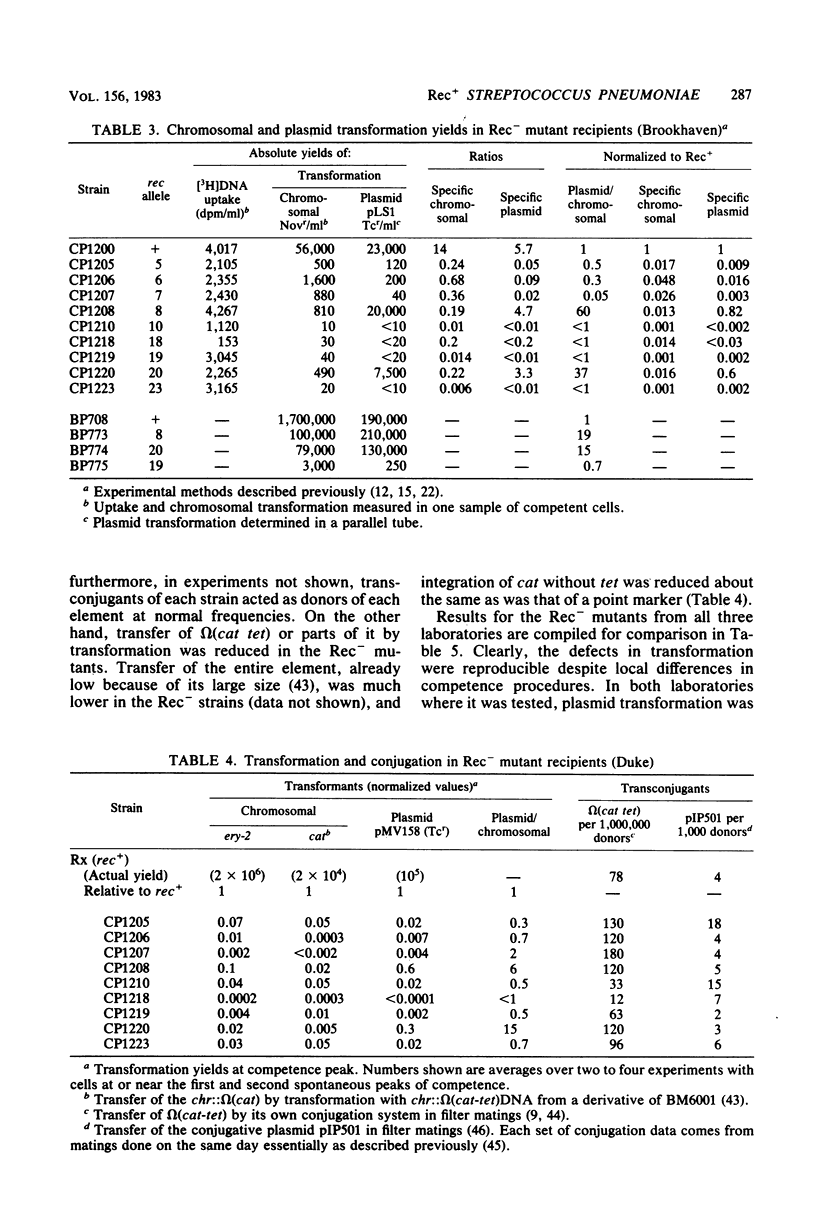
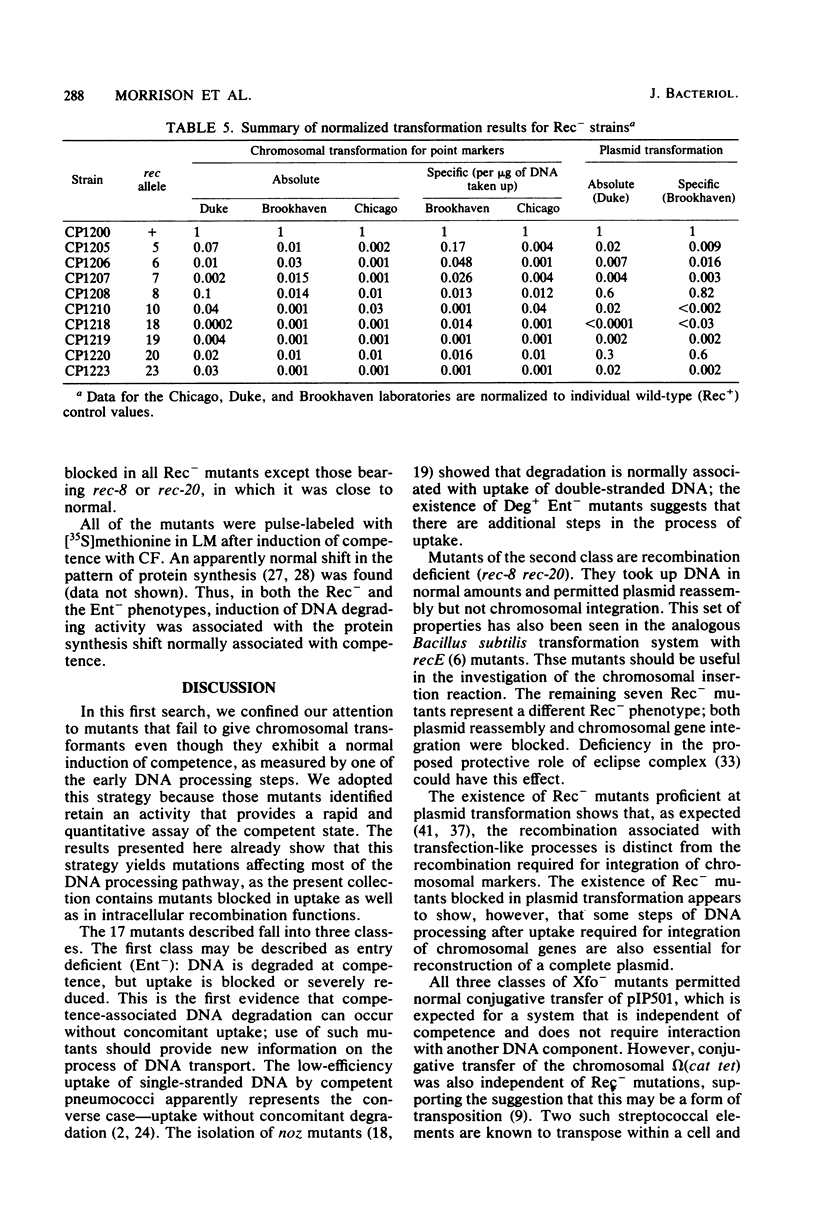
Transformation-deficient mutants of Streptococcus pneumoniae were isolated after nitrosoguanidine mutagenesis. Seventeen mutants developed normal peaks of competence, as tested by their ability to degrade one strand of donor DNA, but they yielded transformants for chromosomal point markers at efficiencies from less than 0.001 to 0.04 that of the wild type. Some of the mutants were defective in DNA uptake and are described as entry defective (Ent-). Others took up DNA in normal quantities, but they failed to give stable transformants and are described as recombination defective (Rec-). In two of the Rec- mutants, normal levels of transformation by plasmid DNA occurred; in the others, it was reduced as much as chromosomal transformation. Conjugative transfers of a chromosomal omega (cat tet) element and of the plasmid pIP501 occurred at normal levels both to and from Rec- mutants. Transfer of chloramphenicol resistance by transformation with omega (cat tet) donor DNA, however, was blocked in Rec- mutants to about the same extent as was transformation for point markers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cato A., Jr, Guild W. R. Transformation and DNA size. I. Activity of fragments of defined size and a fit to a random double cross-over model. J Mol Biol. 1968 Oct 14;37(1):157–178. doi: 10.1016/0022-2836(68)90080-6. [DOI] [PubMed] [Google Scholar]
- Claverys J. P., Lefevre J. C., Sicard A. M. Transformation of Streptococcus pneumoniae with S. pneumoniae-lambda phage hybrid DNA: induction of deletions. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3534–3538. doi: 10.1073/pnas.77.6.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverys J. P., Louarn J. M., Sicard A. M. Cloning of Streptococcus pneumoniae DNA: its use in pneumococcal transformation and in studies of mismatch repair. Gene. 1981 Jan-Feb;13(1):65–73. doi: 10.1016/0378-1119(81)90044-5. [DOI] [PubMed] [Google Scholar]
- Contente S., Dubnau D. Characterization of plasmid transformation in Bacillus subtilis: kinetic properties and the effect of DNA conformation. Mol Gen Genet. 1979 Jan 2;167(3):251–258. doi: 10.1007/BF00267416. [DOI] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney T., Jr, Fox M. S. Physical and genetic hybrids formed in bacterial transformation. J Mol Biol. 1968 Feb 28;32(1):83–100. doi: 10.1016/0022-2836(68)90147-2. [DOI] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- Lacks S. Genetic regulation of maltosaccharide utilization in Pneumococcus. Genetics. 1968 Dec;60(4):685–706. doi: 10.1093/genetics/60.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Competence for deoxyribonucleic acid uptake and deoxyribonuclease action external to cells in the genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1973 Apr;114(1):152–163. doi: 10.1128/jb.114.1.152-163.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Identification of a deoxyribonuclease implicated in genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1975 Jul;123(1):222–232. doi: 10.1128/jb.123.1.222-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Role of a deoxyribonuclease in the genetic transformation of Diplococcus pneumoniae. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2305–2309. doi: 10.1073/pnas.71.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Single-strand breakage on binding of DNA to cells in the genetic transformation of Diplococcus pneumoniae. J Mol Biol. 1976 Feb 25;101(2):255–275. doi: 10.1016/0022-2836(76)90376-4. [DOI] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Stassi D. L., Lacks S. A. Facilitation of plasmid transfer in Streptococcus pneumoniae by chromosomal homology. J Bacteriol. 1982 May;150(2):692–701. doi: 10.1128/jb.150.2.692-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Guild W. R. Competent Diplococcus pneumoniae accept both single- and double-stranded deoxyribonucleic acid. J Bacteriol. 1970 Feb;101(2):361–364. doi: 10.1128/jb.101.2.361-364.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Baker M. F. Competence for genetic transformation in pneumococcus depends on synthesis of a small set of proteins. Nature. 1979 Nov 8;282(5735):215–217. doi: 10.1038/282215a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Breakage prior to entry of donor DNA in Pneumococcus transformation. Biochim Biophys Acta. 1973 Apr 11;299(4):545–556. doi: 10.1016/0005-2787(73)90226-8. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Structure of deoxyribonucleic acid on the cell surface during uptake by pneumococcus. J Bacteriol. 1973 Sep;115(3):1055–1062. doi: 10.1128/jb.115.3.1055-1062.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Transformation and deoxyribonucleic acid size: extent of degradation on entry varies with size of donor. J Bacteriol. 1972 Dec;112(3):1157–1168. doi: 10.1128/jb.112.3.1157-1168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Mannarelli B. Transformation in pneumococcus: nuclease resistance of deoxyribonucleic acid in the eclipse complex. J Bacteriol. 1979 Nov;140(2):655–665. doi: 10.1128/jb.140.2.655-665.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in pneumococcus: existence and properties of a complex involving donor deoxyribonucleate single strands in eclipse. J Bacteriol. 1977 Nov;132(2):576–583. doi: 10.1128/jb.132.2.576-583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in pneumococcus: protein content of eclipse complex. J Bacteriol. 1978 Nov;136(2):548–557. doi: 10.1128/jb.136.2.548-557.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjean V., Claverys J. P., Vasseghi H., Sicard A. M. Rapid cloning of specific DNA fragments of Streptococcus pneumoniae by vector integration into the chromosome followed by endonucleolytic excision. Gene. 1981 Nov;15(2-3):289–293. doi: 10.1016/0378-1119(81)90139-6. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Characterization of some pneumococcal bacteriophages. J Virol. 1976 Aug;19(2):659–667. doi: 10.1128/jvi.19.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Number of transformable units per cell in Diplococcus pneumoniae. J Bacteriol. 1969 Mar;97(3):1033–1035. doi: 10.1128/jb.97.3.1033-1035.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Transfection in pneumococcus: single-strand intermediates in the formation of infective centers. J Virol. 1978 Jan;25(1):60–72. doi: 10.1128/jvi.25.1.60-72.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVIN A. W. Reciprocal capsular transformations of pneumococci. J Bacteriol. 1959 Mar;77(3):296–309. doi: 10.1128/jb.77.3.296-309.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina J. L., Macrina F. L. A competence specific inducible protein promotes in vivo recombination in Streptococcus sanguis. Mol Gen Genet. 1982;185(1):21–29. doi: 10.1007/BF00333785. [DOI] [PubMed] [Google Scholar]
- Raina J. L., Ravin A. W. Switches in macromolecular synthesis during induction of competence for transformation of Streptococcus sanguis. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6062–6066. doi: 10.1073/pnas.77.10.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C. W., Guild W. R. Pathway of plasmid transformation in Pneumococcus: open circular and linear molecules are active. J Bacteriol. 1981 May;146(2):517–526. doi: 10.1128/jb.146.2.517-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Guild W. R. Destruction of low efficiency markers is a slow process occurring at a heteroduplex stage of transformation. Mol Gen Genet. 1974;128(4):283–290. doi: 10.1007/BF00268516. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. DNase-resistant transfer of chromosomal cat and tet insertions by filter mating in Pneumococcus. Plasmid. 1980 Jan;3(1):80–87. doi: 10.1016/s0147-619x(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. Organization and transfer of heterologous chloramphenicol and tetracycline resistance genes in pneumococcus. J Bacteriol. 1979 Aug;139(2):432–441. doi: 10.1128/jb.139.2.432-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Guild W. R. Improved method for conjugative transfer by filter mating of Streptococcus pneumoniae. J Bacteriol. 1980 Oct;144(1):457–459. doi: 10.1128/jb.144.1.457-459.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Shoemaker N. B., Burdett V., Guild W. R. Transfer of plasmids by conjugation in Streptococcus pneumonias. Plasmid. 1980 Jan;3(1):70–79. doi: 10.1016/s0147-619x(80)90035-9. [DOI] [PubMed] [Google Scholar]
- Stassi D. L., Lopez P., Espinosa M., Lacks S. A. Cloning of chromosomal genes in Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7028–7032. doi: 10.1073/pnas.78.11.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., HOTCHKISS R. D. REGULATION OF THE TRANSFORMABILITY OF PHEUMOCOCCAL CULTURES BY MACROMOLECULAR CELL PRODUCTS. Proc Natl Acad Sci U S A. 1964 Mar;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby G., Claverys J. P., Sicard M. A. Integration efficiency in DNA-induced transformation of Pneumococcus. I. A method of transformation in solid medium and its use for isolation of transformation-deficient and recombination-modified mutants. Genetics. 1973 Sep;75(1):23–33. doi: 10.1093/genetics/75.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Mosser J. L. On the nature of the pneumococcal activator substance. Proc Natl Acad Sci U S A. 1966 Jan;55(1):58–66. doi: 10.1073/pnas.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]


