Abstract
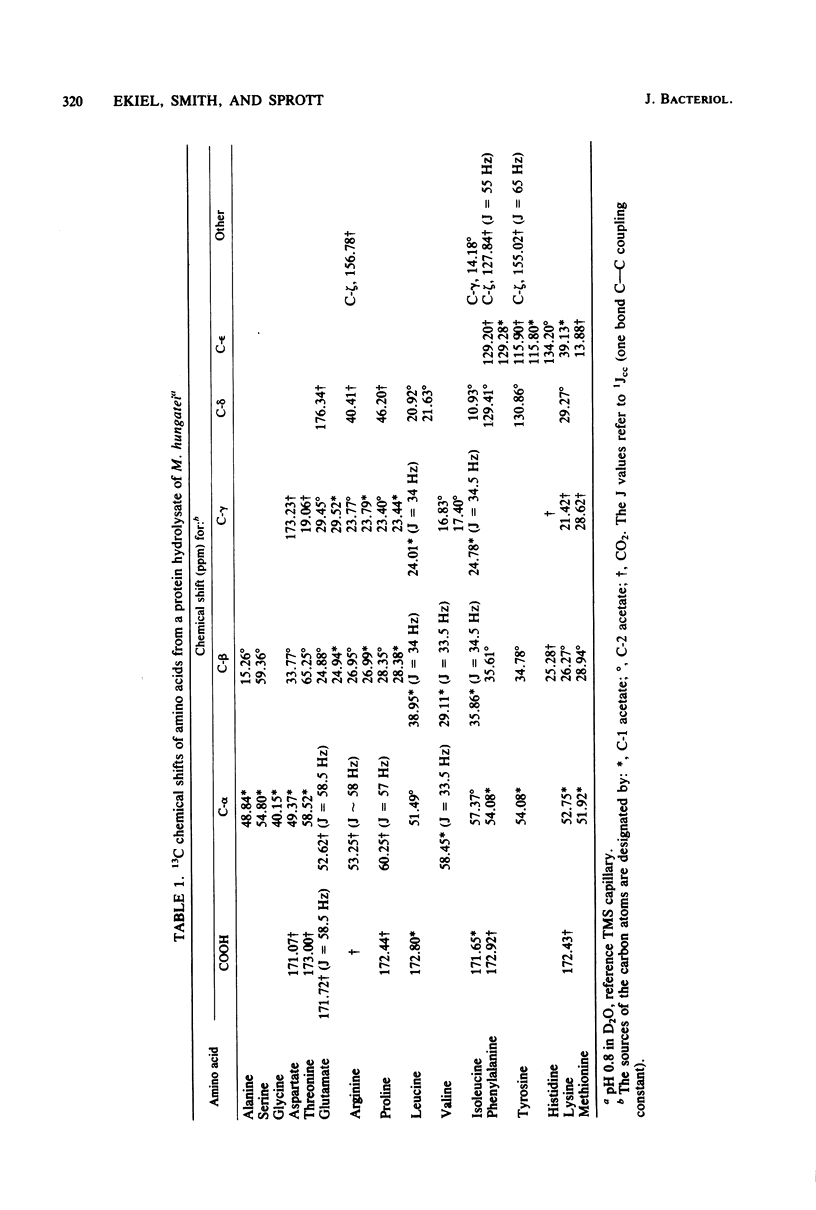
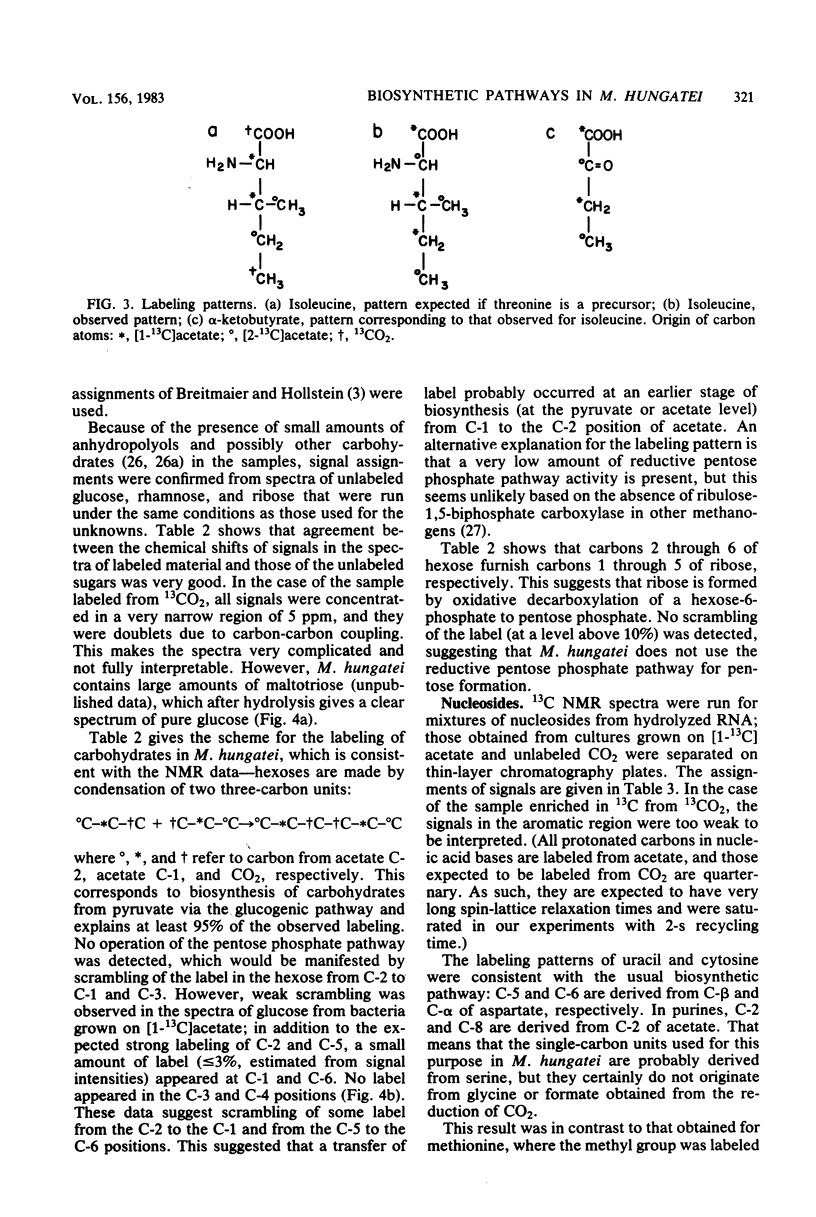
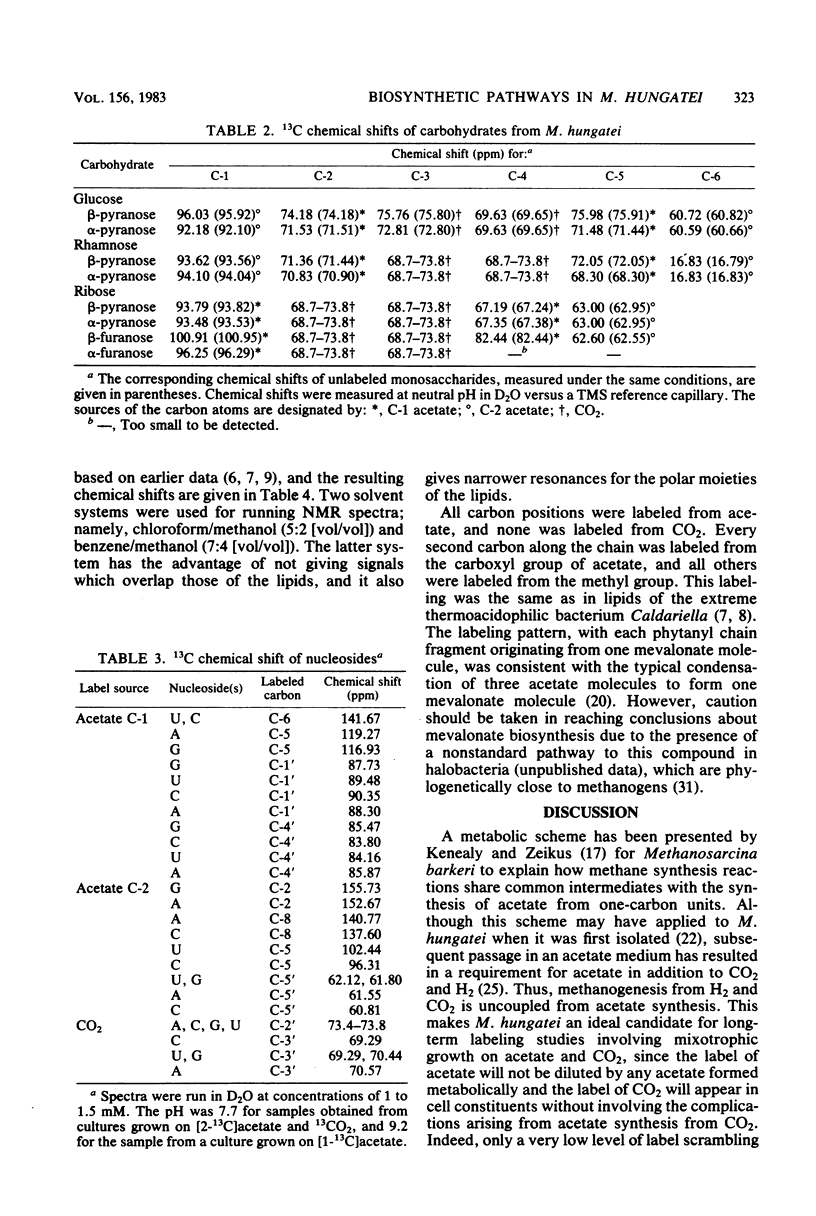
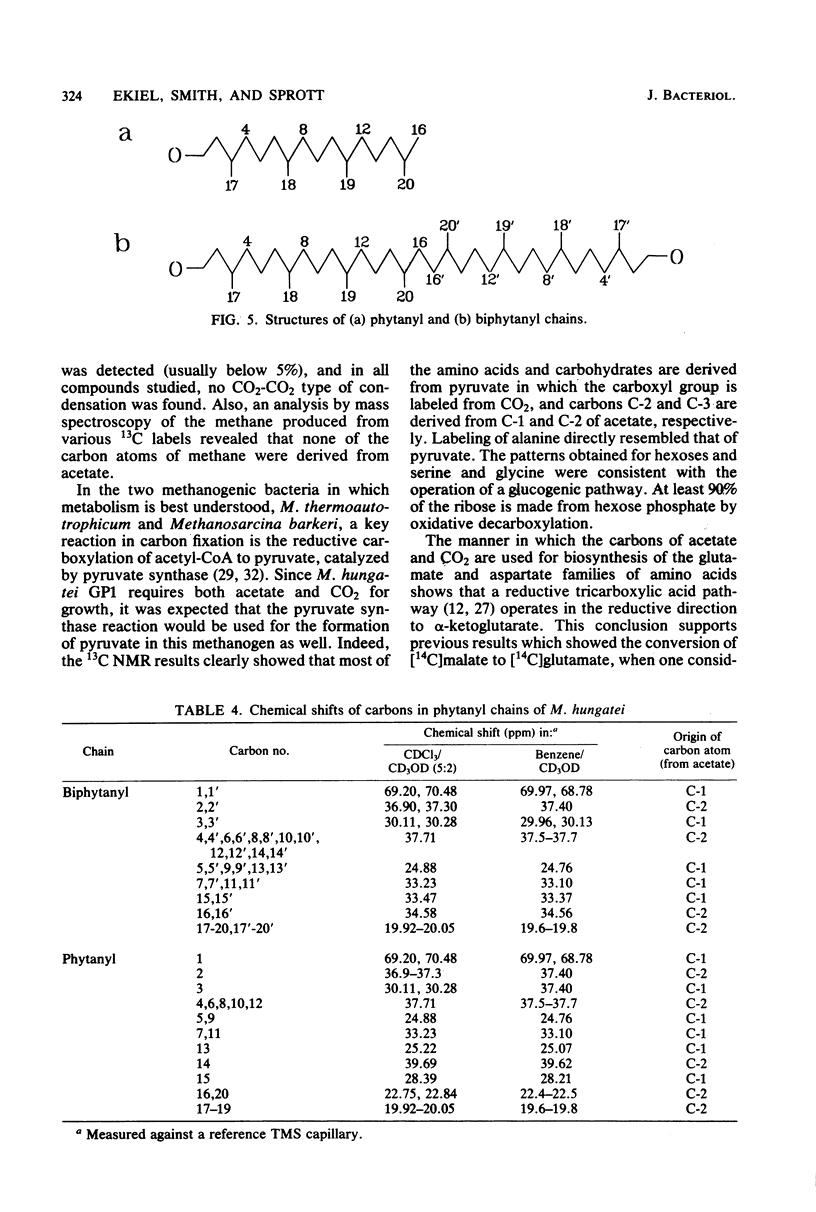
The main metabolic pathways in Methanospirillum hungatei GP1 were followed by using 13C nuclear magnetic resonance, with 13C-labeled acetate and CO2 as carbon sources. The labeling patterns found in carbohydrates, amino acids, lipids, and nucleosides were consistent with the formation of pyruvate from acetate and CO2 as the first step in biosynthesis. Carbohydrates are formed by the glucogenic pathway, and no scrambling of label was observed, indicating that the oxidative or reductive pentose phosphate pathways are not functioning at significant rates. The pathways for amino acid biosynthesis are the usual ones, with the exception of that for isoleucine. The tricarboxylic acid pathway is incomplete and operates in a reductive direction to form alpha-ketoglutarate. The phytanyl chains of lipids are synthesized from acetate via mevalonic acid.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Charon N. W., Johnson R. C., Peterson D. Amino acid biosynthesis in the spirochete Leptospira: evidence for a novel pathway of isoleucine biosynthesis. J Bacteriol. 1974 Jan;117(1):203–211. doi: 10.1128/jb.117.1.203-211.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degani H., Danon A., Caplan S. R. Proton and carbon-13 nuclear magnetic resonance studies of the polar lipids of Halobacterium halobium. Biochemistry. 1980 Apr 15;19(8):1626–1631. doi: 10.1021/bi00549a016. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Fuchs G., Stupperich E. Evidence for an incomplete reductive carboxylic acid cycle in Methanobacterium thermoautotrophicum. Arch Microbiol. 1978 Jul;118(1):121–125. doi: 10.1007/BF00406084. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Colvin J. R., Sprott G. D. Spontaneous protoplast formation in Methanobacterium bryantii. J Bacteriol. 1982 Jan;149(1):346–353. doi: 10.1128/jb.149.1.346-353.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy W. R., Zeikus J. G. One-carbon metabolism in methanogens: evidence for synthesis of a two-carbon cellular intermediate and unification of catabolism and anabolism in Methanosarcina barkeri. J Bacteriol. 1982 Aug;151(2):932–941. doi: 10.1128/jb.151.2.932-941.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Pathway for isoleucine formation form pyruvate by leucine biosynthetic enzymes in leucine-accumulating isoleucine revertants of Serratia marcescens. J Biochem. 1977 Jul;82(1):95–103. doi: 10.1093/oxfordjournals.jbchem.a131698. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Sprott G. D., Smith I. C. Novel polar lipids from the methanogen Methanospirillum hungatei GP1. Biochim Biophys Acta. 1981 Apr 23;664(1):156–173. doi: 10.1016/0005-2760(81)90038-2. [DOI] [PubMed] [Google Scholar]
- Patel G. B., Roth L. A., van den Berg L., Clark D. S. Characterization of a strain of Methanospirillum hungatti. Can J Microbiol. 1976 Sep;22(9):1404–1410. doi: 10.1139/m76-208. [DOI] [PubMed] [Google Scholar]
- Scott A. I., Baxter R. L. Applications of 13C NMR to metabolic studies. Annu Rev Biophys Bioeng. 1981;10:151–174. doi: 10.1146/annurev.bb.10.060181.001055. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Jarrell K. F. K+, Na+, and Mg2+ content and permeability of Methanospirillum hungatei and Methanobacterium thermoautotrophicum. Can J Microbiol. 1981 Apr;27(4):444–451. doi: 10.1139/m81-067. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., McKellar R. C. Composition and properties of the cell wall of Methanospirillum hungatii. Can J Microbiol. 1980 Feb;26(2):115–120. doi: 10.1139/m80-017. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Shaw K. M., Jarrell K. F. Isolation and chemical composition of the cytoplasmic membrane of the archaebacterium Methanospirillum hungatei. J Biol Chem. 1983 Mar 25;258(6):4026–4031. [PubMed] [Google Scholar]
- Vollbrecht D. Three pathways of isoleucine biosynthesis in mutant strains of Saccharomyces cerevisiae. Biochim Biophys Acta. 1974 Sep 5;362(2):382–389. doi: 10.1016/0304-4165(74)90231-1. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate assimilation pathway of Methanosarcina barkeri. J Bacteriol. 1979 Jan;137(1):332–339. doi: 10.1128/jb.137.1.332-339.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Fox G. E. Archaebacteria. J Mol Evol. 1978 Aug 2;11(3):245–251. doi: 10.1007/BF01734485. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


