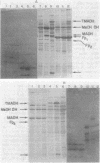
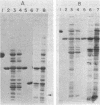
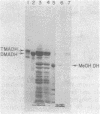
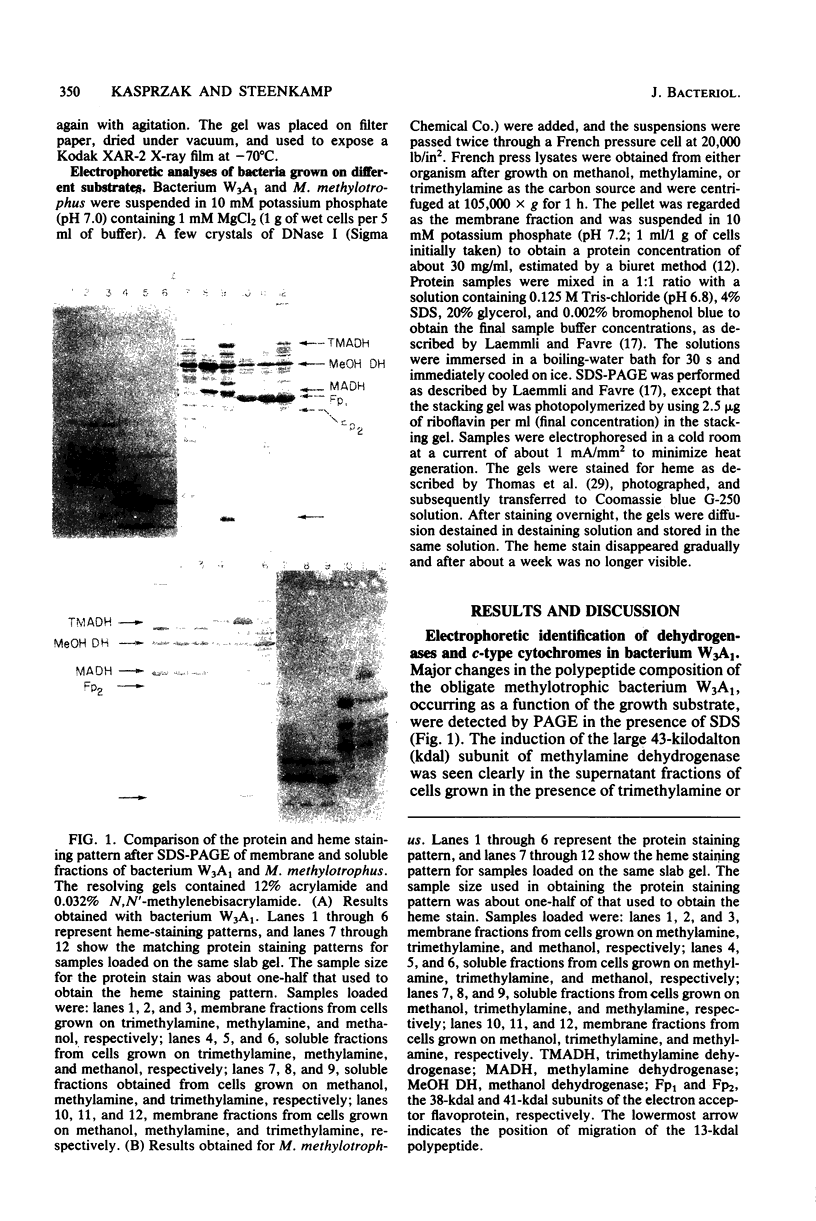
Abstract
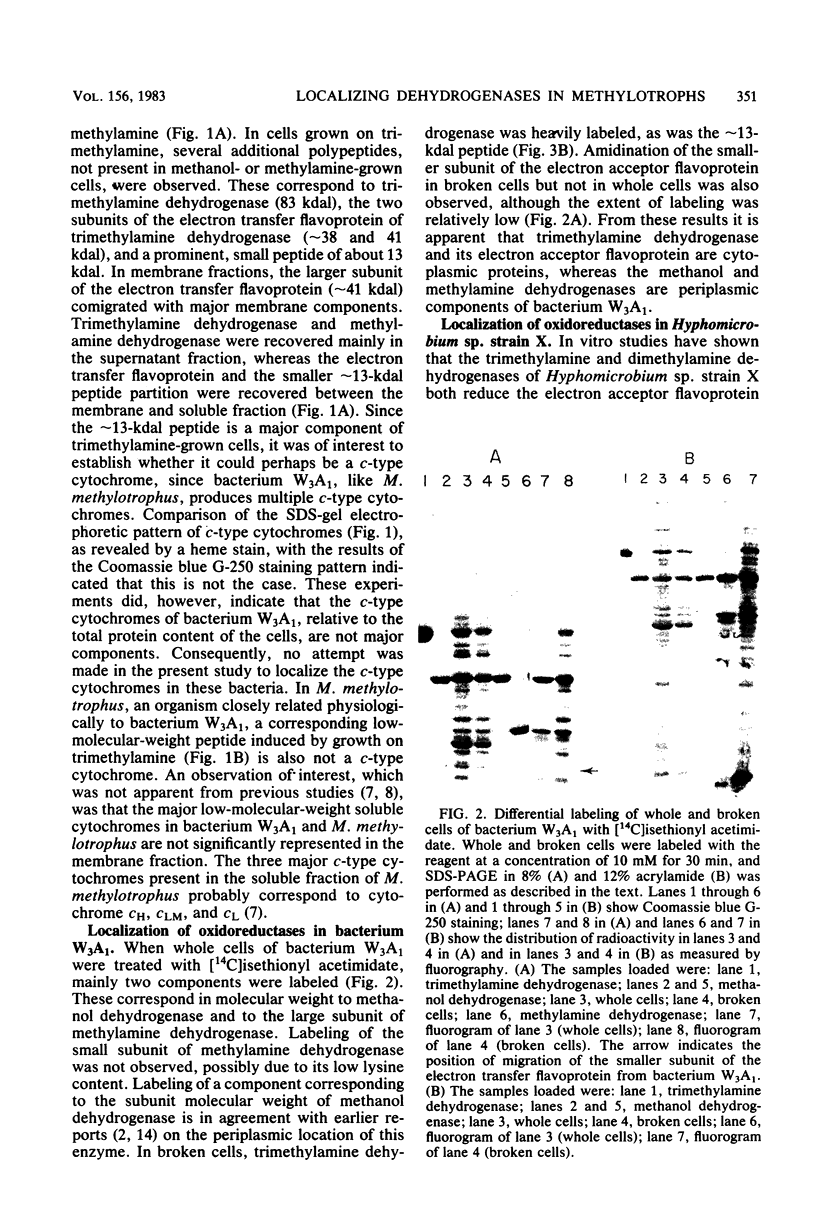
The localization of prominent proteins in intact cells of two methylotrophic bacteria, Hyphomicrobium sp. strain X and bacterium W3A1, was investigated by radiochemical labeling with [14C]isethionyl acetimidate. In bacterium W3A1, trimethylamine dehydrogenase was not labeled by the reagent and is, therefore, an intracellular protein, whereas the periplasmic location of the methylamine and methanol dehydrogenases was evidenced by being readily labeled in intact cells. Similarly, an intracellular location of the trimethylamine and dimethylamine dehydrogenases in Hyphomicrobium sp. strain X was indicated, whereas methanol dehydrogenase was periplasmic.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alefounder P. R., Ferguson S. J. A periplasmic location for methanol dehydrogenase from Paracoccus denitrificans: implications for proton pumping by cytochrome aa3. Biochem Biophys Res Commun. 1981 Feb 12;98(3):778–784. doi: 10.1016/0006-291x(81)91179-7. [DOI] [PubMed] [Google Scholar]
- Alefounder P. R., Ferguson S. J. The location of dissimilatory nitrite reductase and the control of dissimilatory nitrate reductase by oxygen in Paracoccus denitrificans. Biochem J. 1980 Oct 15;192(1):231–240. doi: 10.1042/bj1920231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre A., Galiazzo F., Lehninger A. L. On the location of the H+-extruding steps in site 2 of the mitochondrial electron transport chain. J Biol Chem. 1980 Nov 25;255(22):10721–10730. [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. The prosthetic group of the alcohol dehydrogenase of Pseudomonas sp. M27: a new oxidoreductase prosthetic group. Biochem J. 1967 Sep;104(3):960–969. doi: 10.1042/bj1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. R., LeGall L., Peck H. D. Evidence for the periplasmic location of hydrogenase in Desulfovibrio gigas. J Bacteriol. 1974 Nov;120(2):994–997. doi: 10.1128/jb.120.2.994-997.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain R. C., Marinetti G. V. Phospholipid topology of the inner mitochondrial membrane of rat liver. Biochemistry. 1979 May 29;18(11):2407–2414. doi: 10.1021/bi00578a041. [DOI] [PubMed] [Google Scholar]
- Cross A. B., Anthony C. The electron-transport chains of the obligate methylotroph Methylophilus methylotrophus. Biochem J. 1980 Nov 15;192(2):429–439. doi: 10.1042/bj1920429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Anthony C. The purification and properties of the soluble cytochromes c of the obligate methylotroph Methylophilus methylotrophus. Biochem J. 1980 Nov 15;192(2):421–427. doi: 10.1042/bj1920421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Westerling J. Purification and properties of methanol dehydrogenase from Hyphomicrobium x. Biochim Biophys Acta. 1978 Jun 9;524(2):277–287. doi: 10.1016/0005-2744(78)90164-x. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Microbial oxidation of amines. Spectral and kinetic properties of the primary amine dehydrogenase of Pseudomonas AM1. Biochem J. 1971 Aug;123(5):757–771. doi: 10.1042/bj1230757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough G. F., Jr, Vallee B. L. Characteristic extrinsic Cotton effects of azo proteins. Biochemistry. 1970 Oct 13;9(21):4087–4094. doi: 10.1021/bi00823a009. [DOI] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Orientation of succinate dehydrogenase and cytochrome b558 in the Bacillus subtilis cytoplasmic membrane. J Bacteriol. 1983 Jan;153(1):57–65. doi: 10.1128/jb.153.1.57-65.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Effects of permeability barriers imposed by the cytoplasmic membrane. Biochem J. 1977 Apr 15;164(1):199–211. doi: 10.1042/bj1640199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. The proton-consuming site of the respiratory nitrate reductase of Escherichia coli is on the cytoplasmic aspect of the cytoplasmic membrane [proceedings]. Biochem Soc Trans. 1978;6(2):416–418. doi: 10.1042/bst0060416. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Nemes P. P., Miljanich G. P., White D. L., Dratz E. A. Covalent modification of rhodopsin with imidoesters: evidence for transmembrane arragnement of rhodopsin in rod outer segment disk membranes. Biochemistry. 1980 May 13;19(10):2067–2074. doi: 10.1021/bi00551a010. [DOI] [PubMed] [Google Scholar]
- Owens J. D., Keddie R. M. The nitrogen nutrition of soil and herbage coryneform bacteria. J Appl Bacteriol. 1969 Sep;32(3):338–347. doi: 10.1111/j.1365-2672.1969.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Rothman J. E., Kennedy E. P. Asymmetrical distribution of phospholipids in the membrane of Bacillus megaterium. J Mol Biol. 1977 Mar 5;110(3):603–618. doi: 10.1016/s0022-2836(77)80114-9. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Extracellular labeling of growing secreted polypeptide chains in Bacillus subtilis with diazoiodosulfanilic acid. Biochemistry. 1979 Jan 9;18(1):198–202. doi: 10.1021/bi00568a030. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp D. J., Gallup M. The natural flavorprotein electron acceptor of trimethylamine dehydrogenase. J Biol Chem. 1978 Jun 25;253(12):4086–4089. [PubMed] [Google Scholar]
- Steenkamp D. J., Mallinson J. Trimethylamine dehydrogenase from a methylotrophic bacterium. I. Isolation and steady-state kinetics. Biochim Biophys Acta. 1976 May 13;429(3):705–719. doi: 10.1016/0005-2744(76)90319-3. [DOI] [PubMed] [Google Scholar]
- Steenkamp D. J., Peck H. D., Jr Proton translocation associated with nitrite respiration in Desulfovibrio desulfuricans. J Biol Chem. 1981 Jun 10;256(11):5450–5458. [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Utting J. M., Kohn L. D. Structural, kinetic, and renaturation properties of an induced hydroxypyruvate reductase from Pseudomonas acidovorans. J Biol Chem. 1975 Jul 10;250(13):5233–5242. [PubMed] [Google Scholar]
- Whiteley N. M., Berg H. C. Amidination of the outer and inner surfaces of the human erythrocyte membrane. J Mol Biol. 1974 Aug 15;87(3):541–561. doi: 10.1016/0022-2836(74)90103-x. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Periplasmic location of the terminal reductase in nitrite respiration. FEBS Lett. 1978 Aug 15;92(2):214–218. doi: 10.1016/0014-5793(78)80757-1. [DOI] [PubMed] [Google Scholar]