Abstract
The axon initial segment is an excitable membrane highly enriched in voltage-gated sodium channels that integrates neuronal inputs and initiates action potentials. This study identifies Nav1.6 as the voltage-gated sodium channel isoform at mature Purkinje neuron initial segments and reports an essential role for ankyrin-G in coordinating the physiological assembly of Nav1.6, βIV spectrin, and the L1 cell adhesion molecules (L1 CAMs) neurofascin and NrCAM at initial segments of cerebellar Purkinje neurons. Ankyrin-G and βIV spectrin appear at axon initial segments by postnatal day 2, whereas L1 CAMs and Nav1.6 are not fully assembled at continuous high density along axon initial segments until postnatal day 9. L1 CAMs and Nav1.6 therefore do not initiate protein assembly at initial segments. βIV spectrin, Nav1.6, and L1 CAMs are not clustered in adult Purkinje neuron initial segments of mice lacking cerebellar ankyrin-G. These results support the conclusion that ankyrin-G coordinates the physiological assembly of a protein complex containing transmembrane adhesion molecules, voltage-gated sodium channels, and the spectrin membrane skeleton at axon initial segments.
Keywords: βIV spectrin; sodium channel Nav1.6; neurofascin; NrCAM; axon hillock
Introduction
Axon initial segments, nodes of Ranvier, and postsynaptic folds of the neuromuscular junction contain high densities of voltage-gated sodium channels crucial for generating sufficient local current to initiate a self-regenerating action potential. The mechanism for targeting and restricting sodium channels to these excitable membranes has been proposed to require one or more of the proteins that colocalize with sodium channels including βIV spectrin (Berghs et al., 2000), ankyrin-G (ankyrin-3) (Kordeli et al., 1995; Zhou et al., 1998), and the L1 cell adhesion molecules (L1 CAMs)* neurofascin and NrCAM (Lambert et al., 1997; Ratcliffe et al., 2001).
Spectrins are a family of extended molecules comprised of α and β subunits that assemble with actin to form a subplasmalemmal lattice required for mechanical support of plasma membranes of metazoan cells (Moorthy et al., 2000; for review see Bennett and Baines, 2001). Spectrins are linked to the plasma membrane through ankyrins and through ankyrin-independent interactions with several molecules including membrane lipids, the NMDA receptor, the α subunit of the epithelial sodium channel, and the Na+/H+ exchanger (Bennett and Baines, 2001). The recent identification of a novel β spectrin isoform, βIV spectrin, that is targeted specifically to nodes of Ranvier and axon initial segments has led to the proposal that βIV spectrin is involved in targeting and/or stabilizing the other proteins at these sites (Berghs et al., 2000).
Ankyrins are a family of adaptor proteins that link the spectrin-based membrane skeleton to a number of integral membrane proteins including the anion exchanger, the Na+/Ca++ exchanger, the Na+/K+ ATPase, members of the L1 CAM family, and voltage-gated sodium channels (Bennett and Baines, 2001). The membrane-binding domain of ankyrin contains at least two distinct binding sites for the anion exchanger and L1 CAMs (Michaely and Bennett, 1995). Thus, ankyrins potentially can form homo- and heterocomplexes with multiple transmembrane ankyrin-binding proteins and couple these proteins to the spectrin skeleton.
Knockout of ankyrin-G expression in Purkinje neurons results in an abnormal distribution of the L1 CAM neurofascin and an impaired ability to generate action potentials, suggesting an abnormal sodium channel distribution (Zhou et al., 1998). Additionally, the absence of ankyrin-G disrupts localization of voltage-gated sodium channels in initial segments of unmyelinated cerebellar granule cell axons. However, abnormal localization of sodium channels in Purkinje neurons of ankyrin-G mutant mice has not been directly evaluated due to lack of appropriate sodium channel antibodies (Zhou et al., 1998).
In contrast to axon initial segments, initiation of protein clustering at nodes of Ranvier has been hypothesized to depend on the L1 CAMs neurofascin and/or NrCAM (Lambert et al., 1997; Ratcliffe et al., 2001). L1 CAM clusters that do not contain ankyrin-G or sodium channels have been described during sciatic nerve development (Lambert et al., 1997). Moreover, neurofascin binds directly to the voltage-gated sodium channel β1 and β3 subunits (Ratcliffe et al., 2001), suggesting that L1 CAMs could recruit sodium channels to developing nodes. L1 CAMs are comprised of variable extracellular domains that can contribute to both homophilic and heterophilic binding, and a relatively conserved cytoplasmic domain containing the conserved sequence QFNEDGSFIGQY that mediates an association with ankyrin when the FIGQY tyrosine is dephosphorylated (Davis et al., 1993; Zhang et al., 1998; Hortsch, 2000). FIGQY-dephosphorylated neurofascin and NrCAM are present in sciatic nerve at mature nodes of Ranvier (Jenkins et al., 2001), suggesting that an unphosphorylated FIGQY tyrosine may mediate assembly of L1 CAM–ankyrin-G complexes.
Although βIV spectrin, ankyrin-G, and L1 CAMs have each been hypothesized to be involved in targeting other proteins to nodes of Ranvier and/or axon initial segments, the role of these proteins relative to each other as well as to specific voltage-gated sodium channel isoforms is unknown. This work used Purkinje neuron initial segments during development and in ankyrin-G mutant mice to evaluate the potential role of each initial segment component in protein targeting.
Results and discussion
Voltage-gated sodium channel 1.6 (Nav1.6) is present at adult Purkinje neuron initial segments
Voltage-gated sodium channel 1.6 (Nav1.6), the major sodium channel isoform in mature nodes of Ranvier (Caldwell et al., 2000; Boiko et al., 2001; Kaplan et al., 2001), was identified at rat Purkinje neuron initial segments (Fig. 1). Purkinje neurons in sections of adult rat cerebellum were distinguished by labeling with an antibody against calbindin, a marker of Purkinje neurons. Purkinje neuron initial segments were identified by the presence of ankyrin-G (green) overlapping a calbindin-positive process emanating from a Purkinje cell body (blue) (Fig. 1, arrowheads). The calbindin-positive axon is not visible in the overlaid image due to the much brighter ankyrin-G signal. However, a calbindin-positive process extending beyond the initial segment can be seen (see Fig. 4 G, yellow arrowhead). Fig. 1, A–C, shows a field triple labeled for calbindin (blue), ankyrin-G (Fig. 1 A, green), and Nav1.6 (Fig. 1 B, red). The merged image showing overlap between all three channels is shown in Fig. 1 C. The Nav1.6 signal at initial segments was completely displaced by preincubating the antibody with the immunizing peptide (unpublished data). Adult initial segments did not contain voltage-gated sodium channel 1.2 (Nav1.2) as indicated by an isoform-specific antibody (unpublished data).
Figure 1.

Nav1.6, ankyrin-G, βIV spectrin, neurofascin, and NrCAM are targeted to axon initial segments of cerebellar Purkinje neurons. Sections of adult rat cerebellum were triple labeled with antibodies against calbindin (blue), ankyrin-G (green), and either Nav1.6 (B), βIV spectrin (E), neurofascin (H), or NrCAM (K) (red). Composite images are shown in C, F, I, and L. Arrowheads indicate Purkinje cell initial segments. Bars, 10 μm.
Figure 4.
Targeting of βIV spectrin, Nav1.6, NrCAM, and neurofascin to Purkinje neuron initial segments is disrupted in ankyrin-G cerebellum- specific knockout mice. Cerebellar sections from wild-type (A, D, G, J, and M) or cerebellar ankyrin-G knockout mice (B, C, E, F, H, I, K, L, N, and O) were double labeled with antibodies against calbindin (red in A–F; green in G–L) and either ankyrin-G (green in A–C), βIV spectrin (green in D–F), Nav1.6 (red in G–I), NrCAM (red in J–L), or neurofascin (M–O). All images except M–O are composites. Bars: (A, B, and E) 5 μm; (C, D, F, G, I, and J–O) 10 μm; and (H) 25 μm.
βIV spectrin (Fig. 1 E, red) overlaps with ankyrin-G (Fig. 1 D, green) at Purkinje cell initial segments (arrowheads in Fig. 1 D–F, see F for composite). βIV spectrin also overlaps with ankyrin-G at other sites in the cerebellum in the molecular layer (ML) and in the granule cell layer (GCL) (Fig. 1, D–F).
The L1 CAMs neurofascin (Fig. 1, G–I) and NrCAM (Fig. 1, J–L) are concentrated at Purkinje neuron initial segments (arrowheads). Neurofascin is also distributed around the Purkinje cell body at lower levels than at the initial segment (Fig. 1 H, red). Triple-labeled composite images are shown in Fig. 1, I and L.
Ankyrin-G and βIV spectrin precede Nav1.6 and L1 CAMs at developing Purkinje neuron initial segments
We next examined protein clustering at Purkinje neuron initial segments during development. Before birth, Purkinje axons could not be distinguished from axons of extracerebellar origin also containing ankyrin-G, βIV spectrin, and L1 CAMs that permeate the embryonic Purkinje cell layer (unpublished data). We therefore restricted our analysis to developmental times when individual calbindin-positive Purkinje neurons (Figs. 2 and 3, blue) could be distinguished and individual axons emanating from Purkinje neurons could be identified. Purkinje neuron initial segments already contain a full complement of ankyrin-G at postnatal day 2 (Fig. 2 A, green, arrowheads). All ankyrin-positive initial segments also contain βIV spectrin (Fig. 2 B, red, C for overlap).
Figure 2.
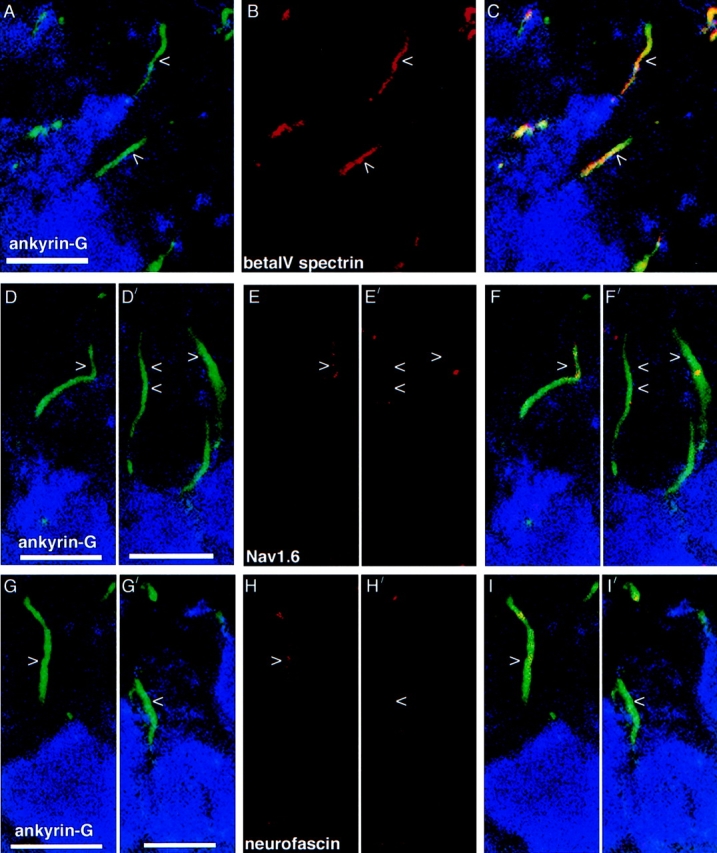
Targeting of ankyrin-G and βIV spectrin to Purkinje neuron initial segments precedes Nav1.6 and neurofascin during development. Sections of P2 rat cerebellum were triple labeled with the same antibodies as described in the legend to Fig. 1. D-I show initial segments containing punctate Nav1.6 (E) or neurofascin (H). D′–I′ show initial segments that do not contain Nav1.6 (E′) or neurofascin (H′). Arrowheads mark Purkinje neuron axon initial segments. Bars, 10 μm.
Figure 3.

Nav1.6 and neurofascin are clustered throughout the entire axon initial segment of Purkinje neurons by postnatal day 9. Sections of P9 rat cerebellum were triple labeled with the same antibodies as described in the legend to Fig. 1. Arrowheads indicate Purkinje cell initial segments. Bars, 10 μm.
No Nav1.6 clustering was observed in ∼37% (30/81) of P2 Purkinje neuron initial segments marked by ankyrin labeling (Fig. 2 D′, green; Fig. 2 E′, arrowheads, F′ for composite). When Nav1.6 clustering was evident in initial segments, it was generally punctate, not covering the entire ankyrin-G–positive region, and the signal at P2 was typically less intense than that in fully developed initial segments (Fig. 2 E, arrowheads, F for composite).
∼34% (25/73) of P2 Purkinje neuron initial segments (Fig. 2 G′, green) contained no neurofascin (Fig. 2 H′, I′ for composite). As with Nav1.6, when neurofascin clustering was present at P2 initial segments, it was generally punctate, not covering the entire ankyrin-G–positive region, and the signal at P2 was often less intense than that in adult initial segments (Fig. 2 H, I for composite). We were unable to determine whether neurofascin and Nav1.6 overlapped in P2 initial segments because both antibodies were made in rabbit. Neurofascin labeling was not seen in the periphery of the Purkinje cell body at P2. NrCAM clustering was minimal in P2 Purkinje neuron initial segments (unpublished data).
As at P2, P9 Purkinje neuron initial segments contained both ankryrin-G (Fig. 3 A, green, arrowheads) and βIV spectrin (Fig. 3 B, red, C for composite). ∼88% (35/40) of the P9 Purkinje neuron initial segments examined (Fig. 3, D and G, green) contained Nav1.6 (Fig. 3 E, red) and 96% (23/24) contained neurofascin (Fig. 3 H, red). Nav1.6 and neurofascin cover the majority of the initial segment, whereas the distribution is still somewhat punctate. See Fig. 3, F and I, for triple label composite images. Neurofascin is not distributed around the Purkinje cell body at this stage (Fig. 3, H and I).
These results imply the existence of an as yet undiscovered ankyrin-G receptor that recruits ankyrin and/or βIV spectrin to developing initial segments. At nodes of Ranvier, this ankyrin receptor has been hypothesized to be an L1 CAM because clusters of neurofascin and NrCAM are present in the absence of ankyrin-G or sodium channels during sciatic nerve development (Lambert et al., 1997). A receptor role for L1 CAMs at initial segments is unlikely because targeting of neurofascin and NrCAM occurs after ankyrin-G and βIV spectrin during development and because these L1 CAMs depend on ankyrin-G for their normal localization at initial segments (see below). Resolution of this discrepancy will require experimental manipulation of ankyrin and/or L1 CAMs in a model of node development. The presence of ankyrin-G at Purkinje neuron nodes of Ranvier in mutant mice prevented evaluation of the role of ankyrin-G in node formation.
Nav1.2 precedes Nav1.6 clustering at nodes of Ranvier (Boiko et al., 2001; Kaplan et al., 2001). However, ankyrin clustering precedes sodium channels at nodes (Rasband et al., 1999; unpublished data), demonstrating that Nav1.2 is not the ankyrin receptor. Nav1.2 also precedes Nav1.6 at Purkinje neuron initial segments but is not maintained in adult initial segments, although it is present in the adult granule and molecular layers (unpublished data). Nav1.2 is not maintained at initial segments in adult ankyrin-G mutant mice (unpublished data).
The proposed ankyrin-G receptor may cluster at developing nodes of Ranvier of CNS axons in response to a soluble factor secreted by oligodendrocytes (Kaplan et al., 1997, 2001). Restriction of ankyrin-G to initial segments in cultured dorsal root ganglia neurons requires multiple domains (Zhang and Bennett, 1998), suggesting that restriction to initial segments under physiological conditions is not mediated only by the ankyrin membrane–binding domain.
Localization of Nav1.6, βIV spectrin, and L1 CAMs at Purkinje neuron initial segments is ankyrin-G dependent
We next examined protein clustering at ankyrin-G–deficient Purkinje neuron initial segments in cerebellum-specific ankyrin-G exon 1B knockout mice (Zhou et al., 1998). Fig. 4 shows double-labeled composite images of calbindin (red in A–F, green in G–L) and each initial segment protein in wild-type and mutant mice. Ankyrin-G was prominently expressed at initial segments of wild-type mice (Fig. 4 A, green, arrowhead), but not in mutant mice (Fig. 4, B and C). In Fig. 4, B and C, the calbindin-positive Purkinje cell axon is clearly visible, whereas there is no detectable ankyrin-G along its length (Fig. 4, B and C, white arrowheads). In contrast to initial segments, ankyrin-G was still clustered at nodes of Ranvier in Purkinje neuron axons of ankyrin-G mutants (unpublished data). Thus, the isoform(s) of ankyrin-G at nodes of Ranvier is distinct from those encoded by the major exon 1b-containing transcripts in Purkinje neurons. No obvious change in ankyrin-B levels or expression pattern was seen in mutant mice (unpublished data).
βIV spectrin is also prominently localized to initial segments of wild-type mice (Fig. 4 D green, initial segment marked by arrowheads) but was not concentrated in Purkinje neuron initial segments of ankyrin-G knockout mice (Fig. 4, E and F). The Purkinje cell axon is clearly visible in these images (Fig. 4, E and F, red, arrowheads), but the initial segment does not contain detectable βIV spectrin. βIV spectrin is clustered in mutant Purkinje neuron nodes of Ranvier along with ankyrin-G (unpublished data). Therefore, the lack of βIV spectrin at Purkinje neuron initial segments in mutant mice is likely due to a failure of βIV spectrin targeting rather than a lack of βIV spectrin expression or its general destabilization although we cannot rule out these explanations. An antibody against total brain spectrin did not reveal any general changes in spectrin levels or distribution (unpublished data).
βIV spectrin has been proposed to interact with ankyrin-G via a spectrin domain similar to ankyrin-binding domains in other spectrin isoforms (Berghs et al., 2000). Our results demonstrate that ankyrin-G expression is necessary for appropriate assembly of the spectrin-based membrane skeleton at Purkinje neuron initial segments and imply that targeting βIV spectrin to nodes of Ranvier will also require ankyrin-G. Our results do not rule out the possibility that βIV spectrin will reciprocally be required for ankyrin-G clustering. A βIV spectrin knockout mouse has recently been generated, and the results with this animal should clarify the role of βIV spectrin in domain assembly (Komada, M., and P. Soriano, personal communication). Truncation of βIV spectrin, resulting in the quivering phenotype in mice, results in the abnormal localization of voltage-gated potassium channels (Parkinson et al., 2001). The effect, if any, on ankyrin or voltage-gated sodium channels has not been reported.
Nav1.6 also is present at initial segments in wild-type mice (Fig. 4 G, red, white arrowheads) but not at initial segments in ankyrin-G mutant mice (Fig. 4, H and I), despite the clear presence of Purkinje cell axons (Fig. 4, H and I, green, white arrowheads). As with βIV spectrin, Nav1.6 is present at Purkinje neuron nodes of Ranvier (unpublished data), suggesting its absence at initial segments is due to lack of targeting rather than decreased expression or stability.
The ankyrin-G dependence of Nav1.6 targeting to initial segments is the first example of a specific sodium channel isoform that requires an ankyrin for its correct localization. Ankyrin-dependent localization may be a general feature of sodium channels given the absence of clustering of voltage-gated sodium channels in ankyrin-G−/− granule cell initial segments (Zhou et al., 1998). Targeting of sodium channels likely requires the accessory β subunits, which contain an extracellular domain that interacts with neurofascin and an intracellular domain that interacts with ankyrin (Malhotra et al., 2000; Ratcliffe et al., 2001).
Absence of Nav1.6 decreases Purkinje neuron firing (Raman et al., 1997). A missense mutation in Nav1.6 in mice causes the jolting phenotype characterized by cerebellar ataxia, decreased Purkinje neuron spontaneous firing, and Purkinje neuron degeneration (Dick et al., 1986; Kohrman et al., 1996). Ankyrin-G exon 1B knockout mice exhibit tremor, impaired Purkinje neuron action potential generation, and Purkinje neuron degeneration (Zhou et al., 1998). The phenotype seen in ankyrin-G knockout animals is likely due to the loss of Nav1.6 at axon initial segments, emphasizing the importance of the axon initial segment in motor function.
Localization of NrCAM and neurofascin are also disrupted in ankyrin-G knockout mice. NrCAM is present in Purkinje neuron initial segments of wild-type (Fig. 4 J, red, arrowhead) but not mutant (Fig. 4, K and L) mice. Neurofascin is present at initial segments and at lower levels around the cell body of Purkinje cells in wild-type animals (Fig. 4 M). Calbindin reactivity was omitted from Fig. 4, M–O, because it obscured the neurofascin reactivity around the cell body. Neurofascin expression in initial segments of mutant mice (Fig. 4, N and O) was more variable than the other initial segment components examined. Consistent with previous findings (Zhou et al., 1998), neurofascin levels were generally decreased at Purkinje cell initial segments of mutant mice and often showed a uniform distribution around the initial segment and cell body (Fig. 4, N and O). Fig. 4, M–O, were prepared with identical microscope and Adobe® Photoshop™ settings.
Loss of ankyrin-G may also disrupt the localization of other spectrin-binding proteins and membrane lipids that bind to the βIV spectrin PH domain or to the palmitoylated membrane-spanning domains of L1 CAMs. The axon initial segment has been proposed to act as a diffusion barrier restricting the lateral mobility of membrane-bound proteins and establishing an asymmetric protein distribution (Winckler et al., 1999). It will be important to determine whether a diffusion barrier persists in the absence of ankyrin-G at initial segments.
Our results imply the existence of a physiological ankyrin-G–dependent multiprotein complex containing transmembrane adhesion molecules, voltage-gated sodium channels, and the βIV spectrin membrane skeleton. The presence of ankyrin-G and βIV spectrin at initial segments before the L1 CAMs and Nav1.6 suggests that neither L1 CAMs nor Nav1.6 initiate protein clustering during initial segment morphogenesis. βIV spectrin, Nav1.6, and NrCAM are absent from ankyrin-G–deficient Purkinje neuron initial segments, and neurofascin localization is abnormal in the absence of ankyrin-G.
These results demonstrate the importance of ankyrin-G in targeting βIV spectrin and Nav1.6, as well as L1 CAMs, to initial segments of Purkinje neurons. It will be of interest to determine if these observations apply to other domains containing ankyrin-G such as nodes of Ranvier, the neuromuscular junction, and basolateral domains of epithelial cells. Ankyrin-G–dependent targeting of ion channels and membrane-skeleton components might be a widely employed mechanism throughout biology for assembly of microdomains.
Materials and methods
Immunocytochemistry
Tissue sections were prepared as described (Lambert et al., 1997) from adult (postnatal day 30–40) rats and mice that were perfused with 3.0 mM Na EDTA in PBS followed by 2.0% paraformaldehyde. Antibodies used include a mouse monoclonal antibody against the ankyrin-G spectrin–binding domain (Jenkins et al., 2001); affinity-purified rabbit polyclonal antibodies against neurofascin (Davis et al., 1996), NrCAM (Davis et al., 1996) and the peptide CIANHTGVDIHRNGDFQKNG corresponding to residues 1042–1061 of mouse or rat Nav1.6 (Alomone Labs); a goat polyclonal antibody against calbindin (Santa Cruz Biotech), and a chicken polyclonal antibody against the βIV spectrin unique domain that has been adsorbed against brain lysate from a βIV spectrin knockout mouse (gift of Dr. M. Komada, Tokyo Institute of Technology, Tokyo, Japan). Secondary antibodies were donkey anti–mouse, –rabbit, –goat, and –chicken labeled with rhodamine, fluorescein, or Cy-5.
Cerebellum-specific ankyrin-G knockout mice
Mice with a targeted disruption of ankyrin-G exon1b have been described previously (Zhou et al., 1998). Mutant mice were identified by the presence of tremor by 3 wk of age. Genotypes were confirmed by PCR analysis using the following primers: 5′-GGAGATCACCTTTCTCAGAG-3′ and 5′-GCCATGGCACACATAGCTACC-3′ which resulted in PCR products of ∼1,200 bp for knockouts and 300 bp for wild-type animals (unpublished data).
Acknowledgments
We would like to thank Dr. M. Komada for the anti–βIV spectrin polyclonal antibody.
This work was supported by the Howard Hughes Medical Institute.
Footnotes
Abbreviations used in this paper: L1 CAM, L1 cell adhesion molecule; Nav1.6, voltage-gated sodium channel 1.6; Nav1.2, voltage-gated sodium channel 1.2.
References
- Bennett, V., and A.J. Baines. 2001. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81:1353–1392. [DOI] [PubMed] [Google Scholar]
- Berghs, S., D. Aggujaro, R. Dirkx, Jr., E. Maksimova, P. Stabach, J.M. Hermel, J.P. Zhang, W. Philbrick, V. Slepnev, T. Ort, and M. Solimena. 2000. βIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J. Cell Biol. 151:985–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko, T., M.N. Rasband, S.R. Levinson, J.H. Caldwell, G. Mandel, J.S. Trimmer, and G. Matthews. 2001. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 30:91–104. [DOI] [PubMed] [Google Scholar]
- Caldwell, J.H., K.L. Schaller, R.S. Lasher, E. Peles, and S.R. Levinson. 2000. Sodium channel Nav1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc. Natl. Acad. Sci. 97:5616–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J.Q., S. Lambert, and V. Bennett. 1996. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. J. Cell Biol. 135:1355–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J.Q., T. McLaughlin, and V. Bennett. 1993. Ankyrin-binding proteins related to nervous system cell adhesion molecules: candidates to provide transmembrane and intercellular connections in adult brain. J. Cell Biol. 121:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, D.J., R.J. Boakes, J.M. Candy, J.B. Harris, and M.J. Cullen. 1986. Cerebellar structure and function in the murine mutant jolting. J. Neurol. Sci. 76:255–267. [DOI] [PubMed] [Google Scholar]
- Hortsch, M. 2000. Structural and functional evolution of the L1 family: are four adhesion molecules better than one? Mol. Cell Neurosci. 15:1–10. [DOI] [PubMed] [Google Scholar]
- Jenkins, S.M., K. Kizhatil, N.R. Kramarcy, A. Sen, R. Sealock, and V. Bennett. 2001. FIGQY-phosphorylation defines discrete populations of L1 cell adhesion molecules at sites of cell-cell contact and in migrating neurons. J. Cell Sci. In press. [DOI] [PubMed] [Google Scholar]
- Kaplan, M.R., M.H. Cho, E.M. Ullian, L.L. Isom, S.R. Levinson, and B.A. Barres. 2001. Differential control of clustering of the sodium channels Nav1.2 and Nav1.6 at developing CNS nodes of Ranvier. Neuron. 30:105–119. [DOI] [PubMed] [Google Scholar]
- Kaplan, M.R., A. Meyer-Franke, S. Lambert, V. Bennett, I.D. Duncan, S.R. Levinson, and B.A. Barres. 1997. Induction of sodium channel clustering by oligodendrocytes. Nature. 386:724–728. [DOI] [PubMed] [Google Scholar]
- Kohrman, D.C., M.R. Smith, A.L. Goldin, J. Harris, and M.H. Meisler. 1996. A missense mutation in the sodium channel Scn8a is responsible for cerebellar ataxia in the mutant mouse jolting. J. Neurosci. 16:5993–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli, E., S. Lambert, and V. Bennett. 1995. Ankyrin G: a new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of ranvier. J. Biol. Chem. 270:2352–2359. [DOI] [PubMed] [Google Scholar]
- Lambert, S., J.Q. Davis, and V. Bennett. 1997. Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J. Neurosci. 17:7025–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, J.D., K. Kazen-Gillespie, M. Hortsch, and L.L. Isom. 2000. Sodium channel β subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J. Biol. Chem. 275:11383–11388. [DOI] [PubMed] [Google Scholar]
- Michaely, P., and V. Bennett. 1995. Mechanism for binding site diversity on ankyrin. Comparison of binding sites on ankyrin for neurofascin and the Cl−/HCO3 − anion exchanger. J. Biol. Chem. 270:31298–31302. [DOI] [PubMed] [Google Scholar]
- Moorthy, S., L. Chen, and V. Bennett. 2000. Caenorhabditis elegans β–G spectrin is dispensible for establishment of epithelial polarity, but essential for muscular and neuronal function. J. Cell Biol. 149:915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, N.J., C.L. Olsson, J.L. Hallows, J. Mckee-Johnson, B.P. Keogh, K. Noben-Trauth, S.G. Kujawa, and B.L. Tempel. 2001. Mutant β-spectrin 4 causes auditory and motor neuropathies in quivering mice. Nature Genetics. 29:61–65. [DOI] [PubMed] [Google Scholar]
- Raman, I.M., L.K. Sprunger, M.H. Meisler, and B.P. Bean. 1997. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 19:881–891. [DOI] [PubMed] [Google Scholar]
- Rasband, M.N., E. Peles, J.S. Trimmer, R. Levinson, S.E. Lux, and P. Shrager. 1999. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J. Neurosci. 19:7516–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe, C.F., R.E. Westenbroek, R. Curtis, and W.A. Catterall. 2001. Sodium channel β1 and β3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J. Cell Biol. 154:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler, B., P. Forscher, and I. Mellman. 1999. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 397:698–701. [DOI] [PubMed] [Google Scholar]
- Zhang, X., and V. Bennett. 1998. Restriction of 480/270-kD ankyrinG to axon proximal segments requires multiple ankyrinG-specific domains. J. Cell Biol. 142:1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., J.Q. Davis, S. Carpenter, and V. Bennett. 1998. Structural requirements for association of neurofascin with ankyrin. J. Biol. Chem. 273:30785–30794. [DOI] [PubMed] [Google Scholar]
- Zhou, D., S. Lambert, P.L. Malen, S. Carpenter, L.M. Boland, and V. Bennett. 1998. Ankyrin G is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 143:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]



