Abstract
Membrane traffic between the endoplasmic reticulum (ER) and Golgi apparatus and through the Golgi apparatus is a highly regulated process controlled by members of the rab GTPase family. The GTP form of rab1 regulates ER to Golgi transport by interaction with the vesicle tethering factor p115 and the cis-Golgi matrix protein GM130, also part of a complex with GRASP65 important for the organization of cis-Golgi cisternae. Here, we find that a novel coiled-coil protein golgin-45 interacts with the medial-Golgi matrix protein GRASP55 and the GTP form of rab2 but not other Golgi rab proteins. Depletion of golgin-45 disrupts the Golgi apparatus and causes a block in secretory protein transport. These results demonstrate that GRASP55 and golgin-45 form a rab2 effector complex on medial-Golgi essential for normal protein transport and Golgi structure.
Keywords: Golgi apparatus; protein transport; rab GTPases; rab2; Golgi stacks
Introduction
The mammalian Golgi apparatus is composed of an ordered stack of cisternae often found in the perinuclear region of mammalian cells (Farquhar and Palade, 1998). This stacked cisternal structure is established by the action of a peripheral Golgi matrix or exoskeleton (Slusarewicz et al., 1994; Seemann et al., 2000a). The best characterized of these Golgi matrix proteins are the vesicle tethering protein p115, GM130, the integral membrane protein giantin, and the two GRASP proteins GRASP55 and GRASP65 (Linstedt and Hauri, 1993; Nakamura et al., 1995; Barr et al., 1997; Nakamura et al., 1997; Shorter et al., 1999; Allan et al., 2000). These factors have been shown to be important for the establishment of properly ordered cisternae in a cell-free assay, recreating the reassembly of the Golgi apparatus after mitosis (Rabouille et al., 1995; Barr et al., 1997; Nakamura et al., 1997; Shorter and Warren, 1999; Shorter et al., 1999; Lesa et al., 2000). How the stacking of cis-Golgi cisternae is promoted by the p115-GM130-GRASP65 complex and giantin is unclear; we favor a model whereby cisternae arise by vesicle docking and fusion, but at some point cisternal fusion is prevented, allowing Golgi stack formation (Barr et al., 1997; Shorter and Warren, 1999). This process may be regulated by the Golgi-associated rab GTPases, since rab1 associates with p115 and GM130 (Allan et al., 2000; Moyer et al., 2001; Weide et al., 2001), and nonhydrolyzable GTP analogs promote the stacking of Golgi cisternae in vitro, although having no effect on membrane fusion (Rabouille et al., 1995).
Studies to date have almost entirely addressed Golgi matrix proteins present on the cis-Golgi, and it is unclear what mechanism would operate at other levels of the Golgi stack. Previously, we identified GRASP55 on the medial-Golgi as a factor important for the formation of Golgi stacks in vitro (Shorter et al., 1999). Therefore, we set out to identify and characterize proteins present in complexes with both GRASP55 and Golgi rab GTPases and to address the function of these complexes in vivo.
Results and discussion
We adopted a two-pronged approach to identify GRASP55-interacting proteins: comparing proteins identified using the yeast two-hybrid system and biochemical isolation of GRASP55 complexes from Golgi membranes. First, a cDNA library was screened with GRASP55 as the bait, and several clones were identified corresponding to the Golgi matrix protein GM130 and a ubiquitously expressed 45-kD protein described previously as a potential transcription factor (Tong et al., 1998). For reasons outlined below, we refer to this protein as golgin-45 (Fig. 1 A). The presence of GM130 was expected, since it is known to interact with GRASP55 in the yeast two-hybrid system (Shorter et al., 1999), and therefore we focused on the characterization of golgin-45. The only obvious sequence feature of golgin-45 was a predicted coiled-coil region (Fig. 1 A) reminiscent of GM130 and the golgin family of coiled-coil proteins (Fritzler et al., 1993; Nakamura et al., 1995). Golgin-45 binds to GRASP55 via a COOH-terminal sequence, since deletion of its last seven amino acids abolishes the interaction (Fig. 1 A), similar to the binding of GM130 to GRASP65. GRASP65 also showed an interaction with golgin-45 in the two-hybrid system (Fig. 1 A). These results were confirmed by immunoprecipitation of in vitro–translated GRASP proteins, golgin-45, and GM130 (Fig. 1 B). Golgin-45 bound more GRASP55 than GRASP65 in vitro, whereas the converse was true for GM130 (Fig. 1 B). The second part of our approach was to purify endogenous GRASP55 complexes from detergent extracts of Golgi membranes (Fig. 1 C). Analysis of the interacting proteins by mass spectrometry revealed that the major protein of 55 kD was GRASP55, and a doublet of 45–50 kD was the rat homologue of golgin-45 (Fig. 1 C). These results confirm the two-hybrid data and demonstrate that endogenous GRASP55 and golgin-45 are present in the same complex. We next asked if golgin-45 is a GRASP55-specific binding protein in vivo. For this purpose, antibodies were raised against golgin-45; these recognize a protein of the expected size in Golgi membranes competed by the addition of the recombinant antigen (Fig. 1 D). Western blot analysis of GRASP55, GRASP65, golgin-45, and GM130 immunoprecipitates showed that golgin-45 is only found in a complex with GRASP55, whereas GRASP65 is only found together with GM130 and not golgin-45 (Fig. 1 E). Some GM130 was found in GRASP55 immunoprecipitates, but since the amount of GRASP55 was below the level of detection in the reciprocal immunoprecipitation with GM130 we conclude this is not the major GRASP55 complex in Golgi membranes. These data suggest that GRASP55 and GRASP65 form specific complexes in vivo with golgin-45 and GM130, respectively.
Figure 1.
Golgin-45 is a novel GRASP55 interacting protein. (A) Amino acids 12–404 of golgin-45 were identified using the yeast two- hybrid system by screening GRASP55 against a human testis cDNA library. Golgin-45 and a COOH-terminal deletion mutant were tested for interaction with GRASP55 and GRASP65 in the yeast two-hybrid system compared with GM130 and empty vector as positive and negative controls, respectively. (B) Coimmunoprecipitations of in vitro–translated GRASP55 and GRASP65 with golgin-45 and GM130. (C) GRASP55 complexes were affinity purified from detergent extracts of Golgi membranes. Specifically, interacting proteins were excised (arrowheads) and tryptic digests of the proteins contained therein analyzed by mass spectrometry. (D) Golgi membranes (10 μg) were Western blotted with antibodies to golgin-45 in the presence (+) or absence (−) of 10 μg/ml of recombinant golgin-45. Control blots are also shown for the antibodies to GRASP65, GRASP55, and GM130. (E) Immunoprecipitations (IPs) were performed from 20 μg Golgi membranes with the following antibodies: sheep anti-GRASP55, mouse anti-GRASP65, sheep anti-GM130, and rabbit anti–golgin-45. Immunoprecipitates were blotted with the following antibodies: GRASP55 and GRASP65 IPs with rabbit anti-GM130 and rabbit anti–golgin-45, GM130 IPs with rabbit anti-GRASP55 and mouse anti-GRASP65, and golgin-45 IPs with sheep anti-GRASP55 and mouse anti-GRASP65. Asterisks indicate a nonspecific cross-reactivity of the rabbit anti-GRASP55 antibody to the sheep antibody used for the IP.
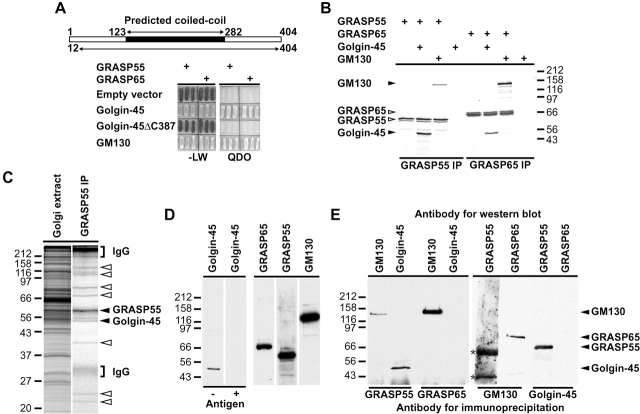
Several Golgi-associated coiled-coil proteins have been shown to interact with the activated or GTP forms of the rab family of GTPases involved in membrane traffic. In particular, both p115 and GM130 bind to the GTP form of the small GTPase rab1 (Allan et al., 2000; Moyer et al., 2001; Weide et al., 2001). Since golgin-45 shares several properties with GM130, we tested if it interacted with Golgi-localized rab GTPases. Golgin-45 from Golgi membranes bound quantitatively and specifically to the GTP form of rab2 and showed negligible binding to other inactive or activated rabs (Fig. 2 A). GRASP55 was also specifically eluted from rab2-GTP beads consistent with its interaction with golgin-45. Intriguingly both GM130 and p115 bound to both activated rab1 and rab2 but not the inactive forms of these rabs or any form of rab6 (Fig. 2 A). The yeast two-hybrid system was then used to screen for interactions between golgin-45, GM130, or p115 and the Golgi rabs 1, 2, and 6 with endosomal rab5 as a negative control. Golgin-45 interacted strongly with the rab2 activated mutant but only weakly with wild-type rab2 (Fig. 2 B). None of the other rab proteins tested showed an interaction with golgin-45. The ability to detect the known interactions between an activated form of rab1 and p115 or GM130 (Fig. 2 B) demonstrates the validity of this approach for studying specific rab-effector protein interactions. GM130 was also able to interact strongly with the activated mutant of rab2 and much less with the wild-type protein (Fig. 2 B), confirming the results of the rab2 binding assays from Golgi membranes. These findings are consistent with direct interaction between the active form of rab2 and golgin-45, rab1 and p115, rab 1 or rab2, and GM130. The presence of p115 in rab2-GTP binding assays is probably due to its binding to GM130, since it interacts with rab1 but not with rab2 in the two-hybrid system. The rab2-binding site in golgin-45 lies in the predicted coiled-coil domain, demonstrating the functional significance of the weak homology to other golgins in this region (Fig. 2 C).
Figure 2.
Golgin-45 is a specific binding partner for activated rab2, whereas GM130 binds both rab1 and 2. (A) Rab1, 2, and 6 beads loaded with either GDP or GTPγS were incubated with Golgi extract and the specifically eluted proteins analyzed by Western blotting. Note that golgin-45 is depleted from the supernatant of rab2-GTP beads. (B) Full-length golgin-45 and p115 were tested for interaction with wild-type rab2 and the following rab proteins carrying activating point mutations rab1Q70L, rab2Q65L, rab5Q79L, and rab6Q72L. (C) Full-length and truncation mutants of golgin-45 were tested for interaction with rab2Q65L and GRASP55 in the yeast two-hybrid system. Interaction was scored by assessing growth on QDO plates, from none (−) to strong (+++).
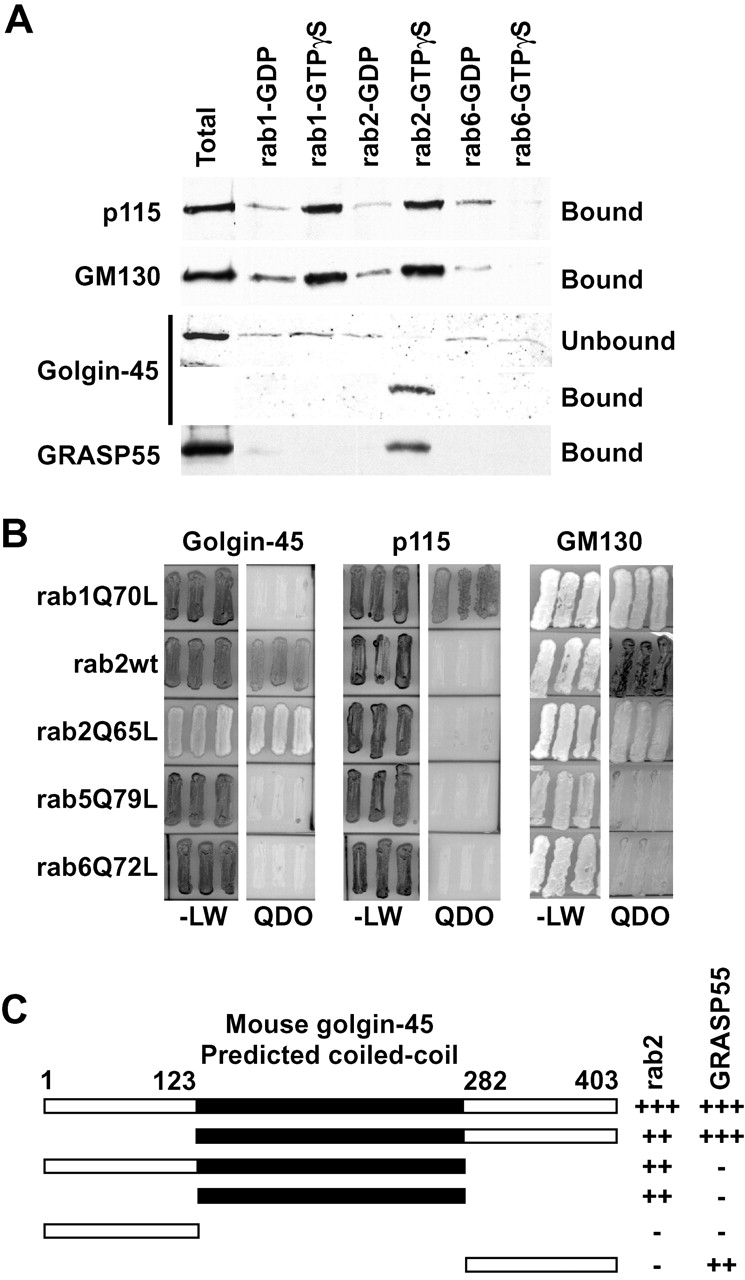
Golgin-45 was localized to the Golgi apparatus using immunofluorescence microscopy (Fig. 3 A). Comparison of GRASP55 and golgin-45 staining revealed extensive colocalization consistent with these proteins existing in a complex on Golgi membranes (Fig. 3 A). Treatment of cells with the drug brefeldin A (BFA)* causes Golgi enzymes to redistribute back to the ER, whereas Golgi matrix proteins remain in Golgi remnants (Seemann et al., 2000a). As expected for a Golgi matrix protein, BFA treatment caused golgin-45 staining to become vesiculated and dispersed throughout the cell to Golgi remnants rather than redistribution to the ER (Fig. 3 B). Similarly, the microtubule depolymerizing drug nocodazole, known to disrupt the Golgi apparatus into smaller “mini-stacks” (Rogalski et al., 1984), caused golgin-45 staining to become dispersed (Fig. 3 B). Overexpression of full-length golgin-45 resulted in a disruption of the Golgi similar to the structures observed with nocodazole treatment (Fig. 3 C). Therefore, Golgin-45 displays the behavior of a Golgi matrix protein rather than a Golgi enzyme or vesicle coat protein and may be important for Golgi structure.
Figure 3.
Golgin-45 behaves like a Golgi matrix protein upon BFA treatment. (A) Normal rat kidney (NRK) cells were fixed and then stained with antibodies to golgin-45 and GRASP55. In the merged images, DNA is blue, golgin-45 is indicated in red, GRASP55 in green, with yellow indicating areas of golgin-45 and GRASP55 overlap. (B) NRK cells were treated with 5 μg/ml BFA or 200 ng/ml nocodazole for 30 and 60 min, respectively, before fixation and antibody staining. (C) NRK cells were transfected with GFP–golgin-45 for 12 h and then fixed and stained with antibodies to GRASP55. Bar, 10 μm.
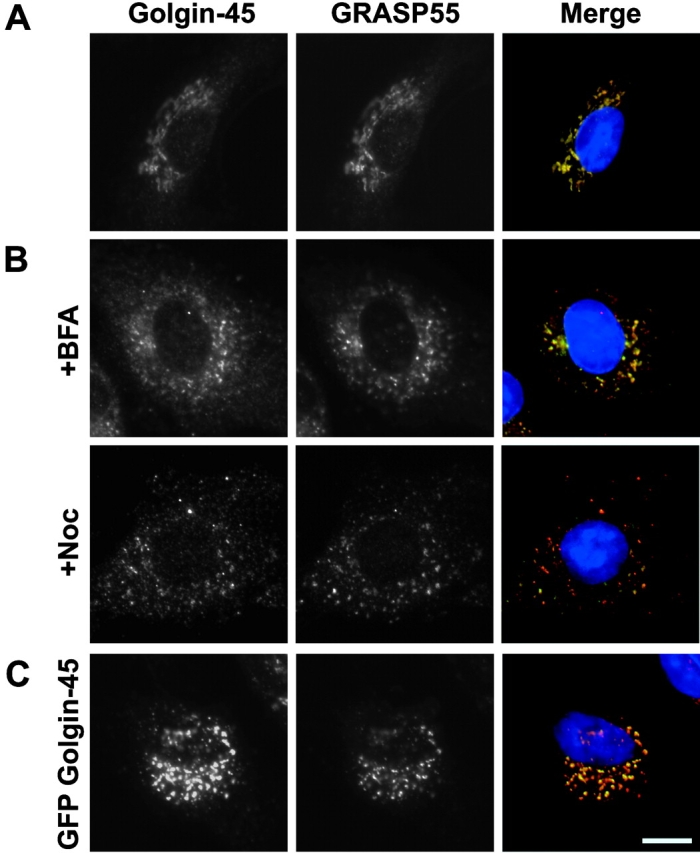
To investigate the requirement for golgin-45 in maintaining normal Golgi structure, we used RNA interference with 21 base RNA duplexes (Elbashir et al., 2001). Duplexes targeting golgin-45 but not a control protein lamin-A (unpublished data) were able to deplete golgin-45 to an undetectable level. This resulted in the redistribution of the Golgi enzyme n-acetylglucosaminyltransferase-I to give a nuclear envelope plus reticular ER-like staining (Fig. 4 A), whereas GRASP55 (Fig. 4 B) and GM130 (Fig. 4 C) were found in small punctate structures with some diffuse cytosolic and reticular staining. Therefore, depletion of golgin-45 disrupts the Golgi apparatus as defined by components of the cis- and medial-cisternae and a resident medial-Golgi enzyme.
Figure 4.
Depletion of golgin-45 disrupts the Golgi apparatus. (A) HeLa cells were treated with duplex RNA to target golgin-45 for 36 h and then transfected for a further 18 h with a plasmid encoding GFP-tagged n-acetylglucosaminyltransferase-I before fixation and staining with antibodies to golgin-45. (B and C) HeLa cells were treated with RNA duplexes to target golgin-45 for 48 h before fixation and staining with antibodies to golgin-45, GRASP55, and GM130. Bars, 10 μm.
We then examined whether or not golgin-45 is required for secretory protein transport in light of its rab2-binding properties. A biochemical assay relying on protein glycosylation was not used due to the redistribution of Golgi enzymes to the ER in golgin-45–depleted cells (Fig. 4 A), something that would complicate the analysis. Therefore, we made use of a GFP-tagged temperature-sensitive allele of the vesicular stomatitis virus G protein (VSV-G ts045), a well-characterized secretory marker used previously to demonstrate the role of GM130 and other factors in protein transport with microscopy-based assays (Pepperkok et al., 1993; Scales et al., 1997; Seemann et al., 2000b). At the nonpermissive temperature of 39.5°C, VSV-G accumulated in the endoplasmic reticulum of both control (Fig. 5 A) and golgin-45–depleted cells (Fig. 5 B). After chase periods of 30 or 60 min at 31.5°C, VSV-G was transported efficiently via the Golgi apparatus to the cell surface in control (Fig. 5 A) but not in golgin-45–depleted cells (Fig. 5 B). Quantitation of this effect revealed an almost complete block in transport of VSV-G after golgin-45 depletion (Fig. 5 C). Live cell imaging confirmed these observations and revealed that VSV-G ts045 is trapped in the ER when the Golgi apparatus is disrupted by golgin-45 depletion (see videos 1 and 2 available at http://www.jcb.org/cgi/content/full/jcb.200108079/DC1). Therefore, Golgin-45 is not only required for normal Golgi structure but also for protein transport from the ER through the Golgi apparatus to the cell surface.
Figure 5.
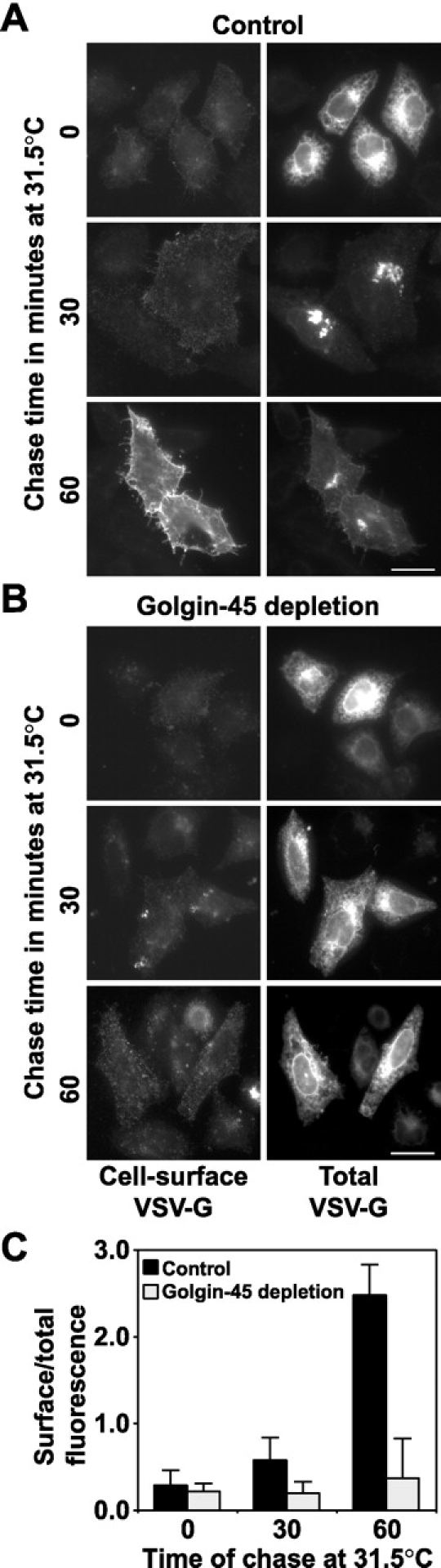
Depletion of golgin-45 disrupts transport of VSV-G protein from the ER to the cell surface. Control (A) or golgin-45 (B) RNAi cells were transfected with a plasmid encoding GFP-tagged VSV-G ts045 protein. VSV-G ts045was arrested in the ER at 39.5°C and then chased out at 31.5°C for 30 and 60 min. Images are shown of cells fixed after 0, 30, and 60 min of chase for both total and cell surface–associated VSV-G. (C) The extent of VSV-G transport after 0, 30, and 60 min chase at 31.5°C was measured in control and golgin-45–depleted cells. This ratio does not approach unity due to the different dyes used to measure surface and total fluorescence. The data shown is representative of three experiments with n = 20 for all data points in each experiment. Live cell videos showing VSV-G ts045 transport in control and golgin-45 depleted cells are available at http://www.jcb.org/cgi/content/full/jcb.200108079/DC1. Bars, 20 μm.
To maintain normal secretion and Golgi function, there must be a balance of membrane traffic to and from the Golgi apparatus; otherwise it would either be consumed or grow uncontrollably (Warren and Malhotra, 1998). Golgi matrix proteins together with rab GTPases are positioned perfectly to control both Golgi structure and membrane traffic, thereby balancing the surface area available for membrane fusion with that sequestered in the stacked cisternal core of the Golgi apparatus. Our observations that the GRASP55- and GRASP65-golgin complexes interact with multiple rab GTPases help to explain why nonhydrolyzable GTP analogs promote the stacking of Golgi cisternae in vitro (Rabouille et al., 1995) and support the hypothesis that stacking is a form of membrane tethering (Barr et al., 1997; Shorter and Warren, 1999; Waters and Pfeffer, 1999). The cis to trans polarity of the Golgi stack may therefore be established by the sequential action of different GRASP-golgin vesicle docking complexes together with the rab GTPases. These results also question the idea that vesicle transport does not occur between Golgi cisternae and that cisternal maturation is the only anterograde transport mechanism (Bonfanti et al., 1998). Rab2 has been implicated previously in anterograde vesicular transport (Tisdale et al., 1992), and we show that the rab2 effector protein golgin-45 is in a complex with the medial-Golgi protein GRASP55, indicating that vesicle transport to this Golgi subcompartment is likely to occur.
Materials and methods
Purification of GRASP55 complexes
GRASP55 affinity matrix was made by covalently coupling 1 mg purified antibody to 1 ml of protein G–sepharose (Amersham-Pharmacia Biotech) using dimethylpimelidate (Harlow and Lane, 1998). Purified Golgi membranes (Hui et al., 1998) were extracted for 15 min on ice at a concentration of 1 mg/ml in HNT buffer (50 mM Hepes-KOH, pH 7.2, 200 mM NaCl, and 0.5% Triton X-100) containing protease inhibitors (Mini EDTA-free; Roche Diagnostics). Insoluble material was removed by centrifugation at 20,000 g for 20 min at 4°C. GRASP55 complexes were isolated by incubating 4 mg Golgi membrane extract with 100 μl of the GRASP55 affinity matrix for 2 h at 4°C. After four washes with 500 μl HNT, bound proteins were eluted in 100 μl of 3% SDS then precipitated with 12% (wt/vol) trichloracetic acid.
Mass spectrometry
Proteins were extracted from Coomassie blue–stained gel slices and digested with sequencing-grade porcine trypsin (Promega) for analysis by peptide mass fingerprinting using a MALDI-TOF instrument (Reflex III; Bruker) and probability-based database searching (Perkins et al., 1999).
Rab-effector binding assays
Recombinant rab proteins (0.5 mg) bound to 50 μl glutathione-sepharose (Amersham Pharmacia Biotech) were loaded with either GDP or GTPγS (Christoforidis and Zerial, 2000). These beads were incubated in the presence of 10 μM GDP or GTPγS and 200 μg Golgi extract in HNTM (HNT containing 5 mM MgCl2) in a total volume of 400 μl for 1 h on ice. Beads were washed three times with 500 μl HNTM containing 10 μM GDP or GTPγS, and then bound proteins were eluted with HNT containing 20 mM EDTA, precipitated with 12% (wt/vol) trichloracetic acid, and analyzed by Western blotting.
Molecular biology and two-hybrid screening
Standard molecular biology techniques were used for all constructs; primer sequences are available upon request. The rat GRASP55 cDNA was inserted into the EcoRI and SalI sites of the two-hybrid bait vector pGBT9, and this plasmid transformed into the reporter strain PJ69-4A according to the CLONTECH Laboratories yeast protocol handbook. A human testis cDNA library was transformed into this bait strain and plated on synthetic media lacking leucine, tryptophan, histidine, and adenine with 2% glucose as the carbon source (QDO). Library plasmids were rescued and retransformed into PJ69-4A together with either pGBT9 or the GRASP55 bait plasmid on synthetic medium lacking leucine and tryptophan (−LW) and then restreaked onto QDO. Those showing strong growth on QDO after 2 d at 30°C were taken as positive clones and the inserts sequenced. Light colored streaks indicate strong interaction, whereas dark colored streaks indicate weak or no interaction. Golgin-45 (sequence data available from GenBank/EMBL/DDBJ under accession no. AK006544) was amplified from mouse liver and rab proteins from human cDNA for cloning into vectors for bacterial and mammalian expression. Mutagenesis was performed using the Quickchange protocol (Stratagene), and constructs were confirmed by DNA sequencing (Medigenomix).
Cell culture and RNA interference
NRK and HeLa cells were cultured at 37°C and 5% CO2 in DME containing 10% calf serum (Life Technologies). RNA interference was performed on HeLa cells transfected using oligofectamine (Life Technologies) with duplex RNA (Dharmacon Research, Inc.) for 24 h; coverslips were then placed in fresh growth medium for a further 48 h and processed for fluorescence microscopy (Elbashir et al., 2001). Golgin-45 was targeted with the sequence AATCCGAGGAGCAGGAGATGGAA, and the lamin-A control was described previously (Elbashir et al., 2001). Images were collected using a ZEISS Axioskop-2 with 63× Plan Apochromat oil immersion objective with a 1.4 NA, a 1,300 by 1,030 pixel cooled CCD camera (Princeton Instruments), and Metaview software (Universal Imaging Corp.).
VSV-G protein transport assays
VSV-G ts045 protein transport assays were performed using an adaptation of a published protocol (Seemann et al., 2000b). HeLa cells plated on glass coverslips were treated with golgin-45 or lamin-A–specific RNA duplexes for 36 h at 37°C and then transfected with a plasmid encoding green fluorescent protein (GFP)–tagged VSV-G protein for 2 h at 37°C then 12 h at 39.5°C (Toomre et al., 1999). The cells were then incubated at 4°C to promote VSV-G protein folding (Scales et al., 1997), and afterwards the growth medium was replaced with prewarmed medium at 31.5°C. After the required chase period, cells were fixed with 3% paraformaldehyde in PBS. Cell surface VSV-G was detected with a monoclonal antibody to the VSV-G lumenal domain and a donkey anti–mouse secondary coupled to CY3 (Jackson ImmunoResearch Laboratories) and total VSV-G by GFP fluorescence. The ratio of surface to total measured fluorescence was used to calculate the extent of VSV-G protein transport (Seemann et al., 2000b).
Online supplemental material
Videos 1 and 2 (available at http://www.jcb.org/cgi/content/full/jcb.200108079/DC1) are supplemental materials for Fig. 5. Cells were prepared for live cell imaging of VSV-G transport essentially identically to the fixed cell procedure except that cells were plated in Labtek coverglass chamber slides (Nunc), and the chase was performed in CO2-independent medium with 10% calf serum. A ZEISS Axiovert-2 with Plan Neofluar 63× oil immersion objective (1.3 NA), a heated sample stage, and a Till Photonics imaging system were used to collect and process data. Prior to imaging, the media was overlaid with mineral oil (Sigma-Aldrich) to prevent evaporation. GFP fluorescence was imaged every 30 s with 100 ms exposure time using excitation at 470 nm from a monochromator. Five focal planes spaced by 100 μm were captured to enable visualization of the ER, Golgi, and cell surface.
Supplemental Material
Acknowledgments
We thank E. Nigg and his laboratory, T. Tuschl for the RNA interference protocol, S. Ponnambalam, U. Grüneberg, and S. Urbé for reading the article, G. Warren for useful discussion, and I. Mellman, P. Keller, and M. Lowe for reagents.
This work was supported by grants from the Max-Planck-Gesellschaft and the Wellcome Trust. B. Short is the recipient of a Wellcome Trust Ph.D. award jointly held with F.A. Barr and O. Byron.
The online version of this article contains supplemental material.
Footnotes
Abbreviations used in this paper: BFA, brefeldin A; ER, endoplasmic reticulum; GFP, green fluorescent protein; IP, immunoprecipitation; VSV-G, vesicular stomatitis virus G protein.
References
- Allan, B.B., B.D. Moyer, and W.E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 289:444–448. [DOI] [PubMed] [Google Scholar]
- Barr, F.A., M. Puype, J. Vandekerckhove, and G. Warren. 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 91:253–262. [DOI] [PubMed] [Google Scholar]
- Bonfanti, L., A.A. Mironov, Jr., J.A. Martinez-Menarguez, O. Martella, A. Fusella, M. Baldassarre, R. Buccione, H.J. Geuze, A.A. Mironov, and A. Luini. 1998. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 95:993–1003. [DOI] [PubMed] [Google Scholar]
- Christoforidis, S., and M. Zerial. 2000. Purification and identification of novel rab effectors using affinity chromatography. Methods Enzymol. 20:403–410. [DOI] [PubMed] [Google Scholar]
- Elbashir, S.M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 411:494–498. [DOI] [PubMed] [Google Scholar]
- Farquhar, M.G., and G.E. Palade. 1998. The Golgi apparatus—100 years of progress and controversy. Trends Cell Biol. 8:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzler, M.J., J.C. Hamel, R.L. Ochs, and E.K. Chan. 1993. Molecular characterization of two human autoantigens: unique cDNAs encoding 95- and 160-kD proteins of a putative family in the Golgi complex. J. Exp. Med. 178:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1998. Using Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. 495 pp.
- Hui, N., N. Nakamura, P. Slusarewicz, and G. Warren. 1998. Purification of rat liver Golgi stacks. Cell Biology: A Laboratory Handbook. 2nd ed. Vol. 2. J. Celis, editor. Academic Press, Inc., Orlando, FL. 46–55.
- Lesa, G.M., J. Seemann, J. Shorter, J. Vandekerckhove, and G. Warren. 2000. The amino-terminal domain of the Golgi protein giantin interacts directly with the vesicle-tethering protein p115. J. Biol. Chem. 275:2831–2836. [DOI] [PubMed] [Google Scholar]
- Linstedt, A.D., and H.-P. Hauri. 1993. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell. 7:679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer, B.D., B.B. Allan, and W.E. Balch. 2001. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic. 2:268–276. [DOI] [PubMed] [Google Scholar]
- Nakamura, N., C. Rabouille, R. Watson, T. Nilsson, N. Hui, P. Slusarewicz, T.E. Kreis, and G. Warren. 1995. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131:1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, N., M. Lowe, T.P. Levine, C. Rabouille, and G. Warren. 1997. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 89:445–455. [DOI] [PubMed] [Google Scholar]
- Pepperkok, R., J. Scheel, H. Horstmann, H.-P. Hauri, G. Griffiths, and T.E. Kreis. 1993. Beta-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 74:71–82. [DOI] [PubMed] [Google Scholar]
- Perkins, D.N., D.J. Pappin, D.M. Creasy, and J.S. Cottrell. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 20:3551–3567. [DOI] [PubMed] [Google Scholar]
- Rabouille, C., T. Misteli, R. Watson, and G. Warren. 1995. Reassembly of Golgi stacks from mitotic Golgi fragments in a cell-free system. J. Cell Biol. 129:605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski, A.A., J.E. Bergmann, and S.J. Singer. 1984. Effect of microtubule assembly status on the intracellular processing and surface expression of an integral protein of the plasma membrane. J. Cell Biol. 99:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales, S.J., R. Pepperkok, and T.E. Kreis. 1997. Visualisation of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 90:1137–1148. [DOI] [PubMed] [Google Scholar]
- Seemann, J., E. Jokitalo, M. Pypaert, and G. Warren. 2000. a. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature. 407:1022–1026. [DOI] [PubMed] [Google Scholar]
- Seemann, J., E.J. Jokitalo, and G. Warren. 2000. b. The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol. Biol. Cell. 11:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter, J., and G. Warren. 1999. A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system. J. Cell Biol. 146:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter, J., R. Watson, M.E. Giannakou, M. Clarke, G. Warren, and F.A. Barr. 1999. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 18:4949–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarewicz, P., T. Nilsson, N. Hui, R. Watson, and G. Warren. 1994. Isolation of a matrix that binds medial Golgi enzymes. J. Cell Biol. 124:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale, E.J., J.R. Bourne, R. Khosravi-Far, C.J. Der, and W.E. Balch. 1992. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 119:749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, J.H., X. Fant, E. Duprez, G. Benoit, C.C. Uphoff, H.G. Drexler, J.C. Pla, E. Lofvenberg, and M. Lanotte. 1998. Expression patterns of the JEM-1 gene in normal and tumor cells: ubiquity contrasting with a faint, but retinoid-induced, mRNA expression in promyelocytic NB4 cells. Leukemia. 12:1733–1740. [DOI] [PubMed] [Google Scholar]
- Toomre, D., P. Keller, J. White, J.C. Olivo, and K. Simons. 1999. Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J. Cell Sci. 112:21–33. [DOI] [PubMed] [Google Scholar]
- Warren, G., and V. Malhotra. 1998. The organisation of the Golgi apparatus. Curr. Opin. Cell Biol. 10:493–498. [DOI] [PubMed] [Google Scholar]
- Waters, M.G., and S.R. Pfeffer. 1999. Membrane tethering in intracellular transport. Curr. Opin. Cell Biol. 11:453–459. [DOI] [PubMed] [Google Scholar]
- Weide, T., M. Bayer, M. Koster, J.P. Siebrasse, R. Peters, and A. Barnekow. 2001. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.