Abstract
In this issue, Short et al. report the discovery of a protein named Golgin-45 that is located on the surface of the middle (or medial) cisternae of the Golgi complex. Depletion of this protein disrupts the Golgi complex and leads to the return of a resident, lumenal, medial Golgi enzyme to the endoplasmic reticulum. These findings suggest that Golgin-45 serves as a linchpin for the maintenance of Golgi complex structure, and offer hints as to the mechanisms by which the polarized Golgi complex is constructed.
The Golgi apparatus is a central way station for proteins to be secreted from cells or to be delivered to the cell surface or to lysosomes (Farquhar and Palade, 1998; Warren and Malhotra, 1998). It comprises a set of membrane-bound compartments in which each houses different sets of glycosyltransferases and other proteins. These compartments take the form of flattened cisternae and associate with each other in the form of a stack (Fig. 1) . Proteins enter the stack at one face, called the cis-Golgi and eventually exit the stack at the other face, the so-called trans-Golgi network. Proteins passing through the Golgi complex are modified by the sequential action of enzymes present in each of the individual cisternae. Protein sorting occurs at the exit site, the trans-Golgi network.
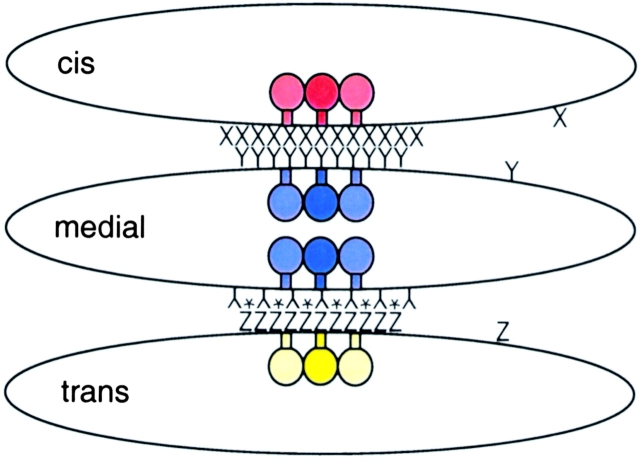
Figure 1.
The Golgi is comprised of minimally three distinct cisternae: cis, medial, and trans. Each cisternae houses different sets of resident Golgi enzymes that may be associated with one another within a given cisterna. One model for Golgi assembly would be that each cisterna has its own matrix: X, Y, and Z. If the medial matrix, Y, could bind both the cis matrix, X and the trans matrix, Z, but X and Z could not interact, a polarized structure could be generated. This model has a major flaw: a medial Golgi might bind two cis- or two trans-cisternae instead of one cis- and one trans-cisterna. Thus, the model shown includes medial Golgi matrix Y*, which can only bind the trans-Golgi. Y and Y* may represent different proteins or the same proteins, differently modified.
A fundamental question in cell biology is how the Golgi complex is formed. The organelle is a polar structure that disassembles during cell division and reforms after distribution of Golgi fragments to daughter cells (Lowe et al., 1998). The Golgi also disassembles in cells treated with brefeldin A (Klausner et al., 1992). In these cells, most resident Golgi enzymes are returned to the ER. It was recently discovered that a specific class of Golgi proteins fails to return to the ER in brefeldin-treated cells. These Golgi “matrix” proteins may actually define the structure and identity of the Golgi complex (Seemann et al., 2000). A paper in this issue by Francis Barr and coworkers (Short et al., 2001) supports this notion and provides an exciting new clue to the mechanism by which the polarity of the Golgi complex may be established.
Building a Golgi complex requires that membrane-bound cisternae be filled with distinct glycosyltransferases. Moreover, the stack of cisternae must be generated with the correct orientation and layering: the cis-Golgi must be separated from the trans-Golgi by an intervening medial Golgi compartment. The enzymes act within the interior (or lumen) of the Golgi; stacking must involve protein–protein interactions on the outer surface of the Golgi.
Initial clues to enzyme packaging come from a classic experiment that revealed direct interaction of two distinct medial Golgi enzymes: GlcNAc transferase I and mannosidase II (Nilsson et al., 1994). These workers attached an ER retention signal onto these enzymes, individually, and found that ER retention of one medial enzyme led to ER accumulation of the other untagged endogenous enzyme, and vice versa. In contrast, ER retention of the trans-Golgi enzyme galactosyltransferase had no effect on the distribution of the medial Golgi enzymes. Thus, at least some enzymes may occur as assemblies within a given cisterna, which simplifies their packaging.
A detergent-insoluble Golgi matrix was identified that bound specifically to GlcNAc transferase I and mannosidase II (Slusarewicz et al., 1994) and was later found to contain the protein GM130 (Nakamura et al., 1995). GRASP65, a cis-Golgi surface protein required for stacking of Golgi cisternae in vitro (Barr et al., 1997), binds to GM130. GM130 also binds to the vesicle docking protein, p115 (Nakamura et al., 1997), as well as to Rab1, a GTPase needed for ER-to-Golgi transport (Moyer et al., 2001; Weide et al., 2001). p115 also interacts with Rab1 (Allan et al., 2000).
Preliminarily, then, a cis-Golgi matrix, comprised minimally of GRASP65 and GM130, exists as an independent unit that can be recognized by the vesicle docking and tethering machinery constituents Rab1 and p115. These proteins may represent the blueprints for building the first cisterna: incoming vesicles could recognize the compartment and deliver nascent secretory cargoes.
GRASP55 is a medial Golgi matrix protein that is also needed for Golgi stack formation in vitro (Shorter et al., 1999). In this issue, Short et al. report that GRASP55 interacts with a novel protein, Golgin-45. Golgin-45 behaves like a Golgi matrix protein in that it does not return to the ER upon brefeldin A treatment. In addition, depletion of Golgin-45 from cells using RNA interference triggers the redistribution of the medial Golgi enzyme, GlcNAc transferase I, to the ER and disruption of cis- and medial Golgi morphology. Under these conditions, a normally secreted protein (VSV-G protein) accumulates in the ER.
This paper represents the discovery of a key Golgi structural constituent that organizes a medial Golgi-specific matrix and also, apparently, the ability of that matrix to bind and retain medial Golgi enzymes. The findings support the notion that the Golgi matrix defines the Golgi complex (Seemann et al., 2000) since depletion of Golgin-45 causes the Golgi to fall apart and resident enzymes to return to the ER.
Also reported is the ability of Golgin-45 to bind to the Rab2 GTPase, but not to Rab1 (Short et al., 2001). This finding recapitulates the theme in which the components that define a Golgi cisterna are also recognized by the vesicle trafficking machinery: at the cis-Golgi, GM130 and p115 can bind to Rab1-GTP; at the medial Golgi, Golgin-45 binds Rab2-GTP.
Like Rab1, Rab2 is believed to function in ER-to-Golgi transport (Tisdale et al., 1992; Tisdale and Balch, 1996). The fact that Golgin-45 is a Rab2-specific effector implies that Rab2 plays a role quite distinct from Rab1. If Rab2 participates in transport through the Golgi, one could imagine that Rab1 brings vesicles to the cis-Golgi, whereas Rab2 helps dock vesicles at the medial Golgi. Alternatively, Rab2 may regulate some structural aspect of Golgi stack assembly. It will be of interest to localize Rab2 in cells lacking Golgin-45, and to interfere with Golgin-45's ability to bind Rab2, to learn more about Rab2's precise function.
GRASP55 and Golgin-45 thus appear to define the medial Golgi as the medial Golgi matrix. An appealing possibility is that a gradient of interactions between cisterna-specific Golgi matrix proteins could itself provide the basis by which a polarized Golgi complex is formed. For example, one could imagine a scenario in which GM130 interacts preferentially with GRASP65 on the cis-Golgi and less strongly with GRASP55 on the medial Golgi. Since the Golgi matrix proteins seem to occur as large oligomeric complexes, perhaps the secondary interactions between the medial and cis-matrices set up the stacking of cis and medial cisternae. Rab GTPases may in some way control these (or similar) interactions.
The finding that Golgin-45 depletion disrupted the cis- and medial cisternae further suggests that the medial Golgi may control the overall structure of the Golgi stack. Indeed, distinct cis–medial and medial–trans interactions would be sufficient to build a correctly polarized organelle (Fig. 1). An alternative possibility is that a single matrix holds together all of the Golgi compartments. In this case, the Golgi remnant generated upon depletion of Golgin-45 would contain cis- and trans-compartments, still attached to one another. Although this latter prediction has not yet been tested, it seems likely that a trans-Golgi–specific matrix awaits identification. Future experiments with appropriately tagged cisterna-specific markers should also permit visualization of stack formation in live cells in real time.
We have likely only seen the tip of the iceberg, in terms of the complete list of constituents that comprise the Golgi matrices and how they interface with components of the trafficking machinery. How the Golgi matrix links specifically to the cisternae-specific glycosyltransferases, and how transported cargoes and vesicle coats are segregated from the matrix and resident enzymes while traversing the Golgi complex, remain important and fascinating questions that await resolution.
References
- Allan, B.B., B.D. Moyer, and W.E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 289:444–448. [DOI] [PubMed] [Google Scholar]
- Barr, F.A., M. Puype, J. Vandekerckhove, and G. Warren. 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 91:253–262. [DOI] [PubMed] [Google Scholar]
- Farquhar, M.G., and G.E. Palade. 1998. The Golgi-apparatus—100 years of progress and controversy. Trends Cell Biol. 8:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner, R.D., J.G. Donaldson, and J. Lippincott-Schwartz. 1992. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116:071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, M., N. Nakamura, and G. Warren. 1998. Golgi division and membrane traffic. Trends Cell Biol. 8:40–44. [DOI] [PubMed] [Google Scholar]
- Moyer, B.D., B.B. Allan, and W.E. Balch. 2001. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis—Golgi tethering. Traffic. 2:268–276. [DOI] [PubMed] [Google Scholar]
- Nakamura, N., C. Rabouille, R. Watson, T. Nilsson, N. Hui, P. Slusarewicz, T.E. Kreis, and G. Warren. 1995. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131:1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, N., M. Lowe, T.P. Levine, C. Rabouille, and G. Warren. 1997. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 89:445–455. [DOI] [PubMed] [Google Scholar]
- Nilsson, T., M.H. Hoe, P. Slusarewicz, C. Rabouille, R. Watson, F. Hunte, G. Watzele, E.G. Berger, and G. Warren. 1994. Kin recognition between medial Golgi enzymes in HeLa cells. EMBO J. 13:562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann, J., E. Jokitalo, M. Pypaert, and G. Warren. 2000. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature. 407:1022–1026. [DOI] [PubMed] [Google Scholar]
- Short, B., C. Preisinger, R. Körner, R. Kopajtich, O. Byron, and F.A. Barr. 2001. A GRASP55-Rab2 effector complex linking Golgi structure to membrane traffic. J. Cell Biol. 155:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter, J., R. Watson, M.E. Giannakou, M. Clarke, G. Warren, and F.A. Barr. 1999. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 18:4949–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarewicz, P., T. Nilsson, N. Hui, R. Watson, and G. Warren. 1994. Isolation of a matrix that binds medial Golgi enzymes. J. Cell Biol. 124:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale, E.J., and W.E. Balch. 1996. Rab2 is essential for the maturation of pre-Golgi intermediates. J. Biol. Chem. 271:29372–29379. [DOI] [PubMed] [Google Scholar]
- Tisdale, E.J., J.R. Bourne, R. Khosravi-Far, C.J. Der, and W.E. Balch. 1992. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 119:749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, G., and V. Malhotra. 1998. The organisation of the Golgi apparatus. Curr. Opin. Cell Biol. 10:493–498. [DOI] [PubMed] [Google Scholar]
- Weide, T., M. Bayer, M. Koster, J.P. Siebrasse, R. Peters, and A. Barnekow. 2001. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2:336–331. [DOI] [PMC free article] [PubMed] [Google Scholar]