X-ray diffraction data were collected to 1.9 Å from crystals of HLA-G. Cobalt ions were found to be essential for the production of diffracting crystals.
Keywords: class I major histocompatibility complexes, HLA-G
Abstract
HLA-G is a nonclassical class I major histocompatibility complex (MHC) molecule that is primarily expressed at the foetal–maternal interface. Although the role of HLA-G has not been fully elucidated, current evidence suggests it protects the foetus from the maternal immune response. In this report, HLA-G (44 kDa) is characterized by expression in Escherichia coli. The inclusion bodies were refolded in complex with a peptide derived from histone H2A (RIIPRHLQL), purified and subsequently crystallized. Correct refolding was determined using two conformation-dependent antibodies. Cobalt ions were shown to be an essential ingredient for obtaining diffraction-quality crystals. The crystals, which diffracted to 1.9 Å resolution, belonged to space group P3221, with unit-cell parameters a = b = 77.15, c = 151.72 Å.
1. Introduction
HLA-G is a product of the class I major histocompatibility complex (MHC-I). Class I MHC molecules comprise a membrane-spanning heavy chain noncovalently associated with β2-microglobulin (β2m). Peptides are bound in the antigen-binding cleft formed by the α1 and α2 domains, whereas the α3 domain can bind co-receptors (Gao et al., 2000 ▶). A distinctive feature of HLA-G is its expression on the foetal trophoblast cells that invade the maternal endometrium during placenta formation (LeMaoult et al., 2003 ▶; Kovats et al., 1990 ▶; Ishitani et al., 2003 ▶). In this location, HLA-G may play a role in maternal tolerance of the semiallogenic foetus by mediating protection from NK cells, CTL, macrophages and mononuclear cells (LeMaoult et al., 2003 ▶; Mandelboim et al., 1996 ▶; Munz et al., 1997 ▶; Le Bouteiller & Blaschitz, 1999 ▶; Hofmeister & Weiss, 2003 ▶). Evidence suggests that HLA-G interacts with the inhibitory receptors LIR-1 (leukocyte Ig-like receptor 1 or ILT2) and LIR-2 (leukocyte Ig-like receptor 2 or ILT4) that can be expressed on these cells (Colonna et al., 1998 ▶; Allan et al., 2002 ▶). Although LIR-1 and LIR-2 interact with the α3 domain of several class I MHC molecules (LeMaoult et al., 2003 ▶; Rajagopalan & Long, 1999 ▶), both bind to HLA-G with higher affinity than to class Ia MHC molecules (Shiroishi et al., 2003 ▶). This suggests that differences in the α3 domain are important for LIR-1/2 affinity (Chapman et al., 1999 ▶).
Class Ia MHC molecules are highly polymorphic, with many of the polymorphisms clustered around the antigen-binding cleft (Margulies & McCluskey, 2003 ▶; Bjorkman & Parham, 1990 ▶). Even a single amino-acid difference can influence both peptide loading (Zernich et al., 2004 ▶) and the diversity of the peptide-binding repertoire (Macdonald et al., 2003 ▶). In contrast, the class Ib genes HLA-G, HLA-E and HLA-F display limited polymorphism (Robinson et al., 2003 ▶; Shawar et al., 1994 ▶). The peptide-binding repertoire of HLA-G is therefore restricted, with a defined peptide-binding motif (Ishitani et al., 2003 ▶; Diehl et al., 1996 ▶; Lee et al., 1995 ▶). Indeed, in the placenta a single peptide accounts for up to 15% of recovered ligand (Ishitani et al., 2003 ▶).
Alternative spicing of HLA-G mRNA produces up to seven different variants (Ishitani & Geraghty, 1992 ▶; Moreau et al., 1998 ▶), although the function of these is unclear (Bainbridge et al., 2001 ▶). Full-length HLA-G1 is expressed as a cell-surface glycoprotein which may form a disulfide-bonded homodimer that is cross-linked by the cysteine at position 42 of the heavy chain (Boyson et al., 2002 ▶; LeMaoult et al., 2003 ▶). This may have implications for receptor binding as dimerization may increase the affinity of HLA-G for LIR-1/2 (Clements et al., 2005 ▶).
In this report, we describe the expression in Escherichia coli and refolding of both the native and the Cys42Ser mutant of HLA-G1 in complex with a natural endogenous peptide from histone H2A (RIIPRHLQL). We have crystallized both complexes and found the addition of cobalt to be essential for the production of diffracting crystals. A 1.9 Å data set was collected for the Cys42Ser mutant, which subsequently enabled the structure to be determined (Clements et al., 2005 ▶)
2. Materials and methods
2.1. Cloning of the HLA-G*0101 gene
The gene encoding HLA-G*0101 was cloned from the human choriocarcinoma cell line JEG-3. RNA was reverse transcribed with oligo-dT and PCR-amplified using the oligonucleotides CGCCATATGGGATCCCACTCCATGAGGTATTTCAGCG and CCCAAGCTTTTAGGAAGACTGCTTCCATCTCAGCA. The PCR-amplified DNA fragment was then TA-cloned into p-GEM T Easy (Promega, Madison, WI, USA) and verified by sequencing. Another form of HLA-G*0101 was also made in which the codon for cysteine at position 42 was changed to encode serine by QuikChange site-directed mutagenesis (Stratagene, La Jolla, CA, USA). The HLA-G genes were then cloned as NdeI–HindIII fragments into the pET30 expression vector (Novagen, San Diego, CA, USA). The expression vectors were thus predicted to encode residues 1–278 of the three extracellular domains of the splice isoform HLA-G1.
2.2. Expression and purification of HLA-G
The two forms of HLA-G1 were expressed separately in BL21 E. coli and inclusion-body protein was prepared, refolded and purified as described by Clements et al. (2002 ▶). Briefly, the inclusion bodies were resuspended in 20 mM Tris pH 8.0, 8 M urea, 0.5 mM EDTA, 1 mM DTT before freezing at 203 K. Inclusion-body aliquots were added separately, with 30 mg of RIIPRHLQL peptide, to 1 l of refolding buffer containing 100 mM Tris pH 8.5, 0.4 M arginine, 0.5 mM oxidized glutathione, 5 mM reduced glutathione, 2 mM EDTA, 0.2 mM PMSF and 1 µg ml−1 pepstatin A at 277 K. Two additional aliquots of HLA-G heavy chain (hc) were added to the refolding buffer after 12 and 24 h. 24 h after the last addition of hc, the refold mix was dialysed against 15 l of 5 mM Tris pH 8.0 for 24 h. Dialysed protein was captured on a column containing DE52 resin and eluted with 10 mM Tris–HCl, 150 mM NaCl. Eluted HLA-G was concentrated and loaded onto a gel-filtration column. Fractions containing HLA-G were pooled and purifed further on a MonoQ column. Peak fractions were pooled and concentrated to 18 mg ml−1 in 10 mM Tris pH 8.0, 150 mM NaCl.
2.3. Conformation-dependant ELISA
Two mAbs reactive against HLA-G were used in an enzyme-linked immunosorbent assay (ELISA). The ELISA assay was performed in triplicate. 100 µl BM-63 (Sigma, catalogue No. M7398; ascites, 1:50 dilution into PBS), a human β2-microglobulin-reactive IgG1 mAb, was added to 96-well ELISA plates (U96 Maxisorp, Nunc) at 277 K for 16 h. Plates were then blocked with 200 µl PBS/1% BSA at 310 K for 1 h. Positive-control HLA-B8/FLR, HLA-G or negative-control LC13 TCR proteins were then added at 0.01, 0.1, 1 or 10 µg ml−1 for 1 h. The biotinylated IgG2a mAb W6/32 (Sigma catalogue No. H1650) reactive against human HLA class I heavy chain was then added at 10 µg ml−1, after which streptavidin-conjugated horse radish peroxidase (Chemicon Australia, catalogue No. 8A2D2) was added. O-Phenylenediamine substrate (Sigma, catalogue No. P8287) was added, the reaction was terminated with HCl and ELISA plates were read at 492 nm on a Labsystems Multiscan ELISA plate reader.
2.4. Crystallization and data collection
Crystallization experiments were performed at 277 K using the hanging-drop vapour-diffusion technique. The crystals were flash-cooled prior to data collection using 10%(v/v) glycerol as the cryoprotectant. A 1.9 Å data set was collected at the BioCars beamline using a Quantum 4 CCD detector. The data were processed and scaled using the HKL package.
3. Results and discussion
3.1. Expression, refolding and functional verification of HLA-G
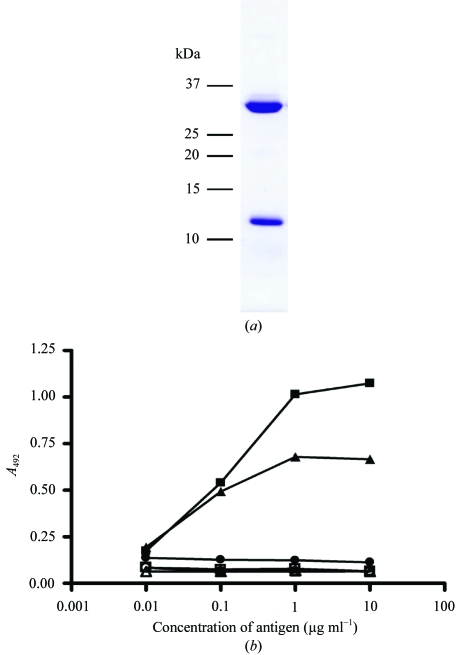
The HLA-G heavy chain and β2-microglobulin were expressed separately in E. coli as denatured inclusion-body protein and then refolded in the presence of an excess of the histone H2A peptide RIIPRHLQL. Following capture of the refolded HLA-G–β2m–RIIPRHLQL complex on a DE52 anion-exchange column, purification by gel filtration and a further MonoQ anion-exchange step were performed (Fig. 1 ▶ a). Correct refolding of HLA-G was confirmed by ELISA using two separate conformation-dependent antibodies (Fig. 1 ▶ b).
Figure 1.
HLA-G refolding and functional testing. (a) SDS–PAGE analysis of refolded HLA-G–RIIPRHLQL complex following anion-exchange and size-exclusion chromatography. (b) ELISA of refolded HLA-G. Two antibodies specific for human MHC molecules were used in a sandwich ELISA with HLA-G (filled squares) and HLA-B8 (filled triangles) as positive controls and LC13 (filled circles) as a negative control. Open symbols indicate the control experiment without primary antibody. Data represents the mean of three experiments.
3.2. Crystallization
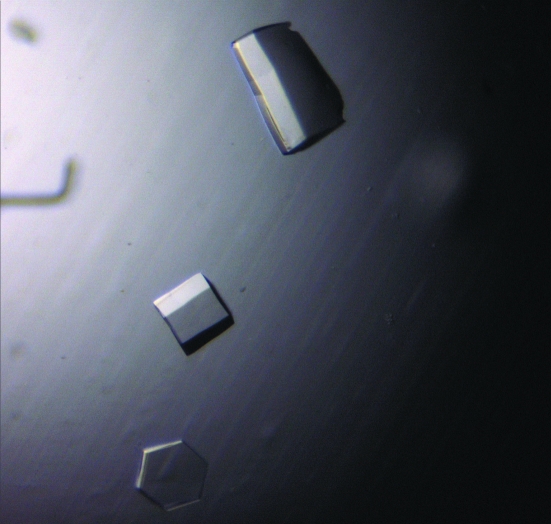
Needle-like crystals were initially obtained at 277 K in condition No. 34 of the PEG/Ion screen (Hampton Research, Riverside, CA, USA). Subsequent screening produced thicker crystals (Fig. 2 ▶) in 20%(w/v) PEG 3350, 0.1 M HEPES pH 7.0, 0.2 M potassium formate; however, these diffracted poorly. The morphology of the crystals was improved through use of Additive Screens 1, 2 and 3 (Hampton Research, Riverside, CA, USA). Initially, only a small improvement in crystal quality was seen at pH 7.0. However, subsequent screening at pH 6.8 produced diffracting crystals (0.2 × 0.2 × 0.2 mm) of the Cys42Ser mutant using condition No. 4 of Additive Screen 1, cobalt chloride (Fig. 3 ▶). The crystals were grown in 5–7 d by mixing equal volumes of 18 mg ml−1 HLA-G with reservoir buffer [18–21%(w/v) PEG 3350, 0.1 M HEPES pH 6.8, 0.2 M potassium formate and 10 mM CoCl2]. The crystals belong to space group P3221, with unit-cell parameters a = b = 77.15, c = 151.72 Å. The crystals were flash-frozen prior to data collection using 10% glycerol as the cryoprotectant. Data-collection statistics are given in Table 1 ▶. The data were processed and scaled using the HKL package. The HLA-G Cys42Ser mutant crystallized as a monomer in the asymmetric unit. Crystals of native HLA-G were produced under identical conditions, suggesting that the native protein was also monomeric.
Figure 2.
Preliminary crystals of HLA-G grown in 20% PEG 3350, 0.1 M HEPES pH 7.0, 0.2 M potassium formate at 277 K.
Figure 3.
Diffracting crystals of HLA-G grown in 18–21% PEG 3350, 0.1 M HEPES pH 6.8, 0.2 M potassium formate and 10 mM CoCl2 at 277 K.
Table 1. Data-collection statistics.
Values in parentheses are for the highest resolution bin (approximate interval 0.1 Å).
| Temperature (K) | 100 |
| X-ray source | BioCars, APS |
| Detector | Quantum 4 CCD |
| Space group | P3221 |
| Unit-cell parameters (Å) | a = b = 77.15, c = 151.72 |
| Resolution (Å) | 1.9 |
| Total No. of observations | 130133 |
| No. of unique observations | 40820 |
| Multiplicity | 3.19 |
| Data completeness (%) | 96.9 (83.6) |
| Data > 2σ(I) (%) | 83.7 (57.8) |
| 〈I/σ(I)〉 | 28.94 (3.07) |
| Rmerge† (%) | 4.0 (28) |
R
merge = 100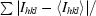
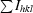 .
.
Analysis of the structure (Clements et al., 2005 ▶) demonstrated the importance of the addition of cobalt ions: a single cobalt ion was observed to mediate crystal contacts. Interestingly, heavy atoms have been found to assist the crystallization of other MHCs, including HLA-B8, which required cadmium for crystallization (Kjer-Nielsen et al., 2002 ▶). In this structure, cadmium ions mediated interactions between the HLA-B8 heavy chain and β2-microglobulin.
In this report, we demonstrate that we have expressed and refolded native and the Cys42Ser mutant of HLA-G with a peptide derived from histone H2A (RIIPRHLQL). Both forms of HLA-G were crystallized. The addition of cobalt to the crystallization condition produced crystals of the Cys42Ser mutant that diffracted to 1.9 Å. This permitted us to investigate the structural basis for the limited peptide repertoire of HLA-G and to correlate structural differences in the α3 domain with differences in co-receptor affinity.
References
- Allan, D. S., Lepin, E. J., Braud, V. M., O’Callaghan, C. A. & McMichael, A. J. (2002). J. Immunol. Methods, 268, 43–50. [DOI] [PubMed] [Google Scholar]
- Bainbridge, D., Ellis, S., Le Bouteiller, P. & Sargent, I. (2001). Trends Immunol.22, 548–552. [DOI] [PubMed] [Google Scholar]
- Bjorkman, P. J. & Parham, P. (1990). Annu. Rev. Biochem.59, 253–288. [DOI] [PubMed] [Google Scholar]
- Boyson, J. E., Erskine, R., Whitman, M. C., Chiu, M., Lau, J. M., Koopman, L. A., Valter, M. M., Angelisova, P., Horejsi, V. & Strominger, J. L. (2002). Proc. Natl Acad. Sci. USA, 99, 16180–16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, T. L., Heikeman, A. P. & Bjorkman, P. J. (1999). Immunity, 11, 603–613. [DOI] [PubMed] [Google Scholar]
- Clements, C. S., Kjer-Nielsen, L., Kostenko, L., Hoare, H. L., Dunstone, M. A., Moses, E., Freed, K., Brooks, A. G., Rossjohn, J. & McCluskey, J. (2005). Proc. Natl Acad. Sci. USA, 102, 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements, C. S., Kjer-Nielsen, L., MacDonald, W. A., Brooks, A. G., Purcell, A. W., McCluskey, J. & Rossjohn, J. (2002). Acta Cryst. D58, 2131–2134. [DOI] [PubMed] [Google Scholar]
- Colonna, M., Samaridis, J., Cella, M., Angman, L., Allen, R. L., O’Callaghan, C. A., Dunbar, R., Ogg, G. S., Cerundolo, V. & Rolink, A. (1998). J. Immunol.160, 3096–3100. [PubMed] [Google Scholar]
- Diehl, M., Munz, C., Keilholz, W., Stevanovic, S., Holmes, N., Loke, Y. W. & Rammensee, H.-G. (1996). Curr. Biol.6, 305–314. [DOI] [PubMed] [Google Scholar]
- Gao, G. F., Willcox, B. E., Wyer, J. R., Boulter, J. M., O’Callaghan, C. A., Maenaka, K., Stuart, D. I., Jones, E. Y., Van Der Merwe, P. A., Bell, J. I. & Jakobsen, B. K. (2000). J. Biol. Chem.275, 15232–15238. [DOI] [PubMed] [Google Scholar]
- Hofmeister, V. & Weiss, E. (2003). Semin. Cancer Biol.13, 317–323. [DOI] [PubMed] [Google Scholar]
- Ishitani, A. & Geraghty, D. (1992). Proc. Natl Acad. Sci. USA, 89, 3947–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani, A., Sageshima, N., Lee, N., Dorofeeva, N., Hatake, K., Marquardt, H. & Geraghty, D. E. (2003). J. Immunol.171, 1376–1384. [DOI] [PubMed] [Google Scholar]
- Kjer-Nielsen, L., Clements, C. S., Brooks, A. G., Purcell, A. W., Fontes, M. R., McCluskey, J. & Rossjohn, J. (2002). J. Immunol.169, 5153–5160. [DOI] [PubMed] [Google Scholar]
- Kovats, S., Main, E., Librach, C., Stubblebine, M., Fisher, S. & DeMars, R. (1990). Science, 248, 220–223. [DOI] [PubMed] [Google Scholar]
- Le Bouteiller, P. & Blaschitz, A. (1999). Immunol. Rev.167, 233–244. [DOI] [PubMed] [Google Scholar]
- Lee, N., Malacko, A., Ishitani, A., Chen, M., Bajorath, J., Marquardt, H. & Geraghty, D. E. (1995). Immunity, 3, 591–600. [DOI] [PubMed] [Google Scholar]
- LeMaoult, J., Le Discorde, M., Rouas-Freiss, N., Moreau, P., Menier, C., McCluskey, J. & Carosella, E. D. (2003). Tissue Antigens, 62, 273–284. [DOI] [PubMed] [Google Scholar]
- Macdonald, W. A., Purcell, A. W., Mifsud, N. A., Ely, L. K., Williams, D. S., Chang, L., Gorman, J. J., Clements, C. S., Kjer-Nielsen, L., Koelle, D. M., Burrows, S. R., Tait, B. D., Holdsworth, R., Brooks, A. G., Lovrecz, G. O., Lu, L., Rossjohn, J. & McCluskey, J. (2003). J. Exp. Med.198, 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelboim, O., Davis, D. M., Reyburn, H. T., Valés-Gómez, M., Sheu, E. G., Pazmany, L. & Strominger, J. L. (1996). Science, 274, 2097–2100. [DOI] [PubMed] [Google Scholar]
- Margulies, D. H. & McCluskey, J. (2003). In Fundamental Immunology, edited by W. Paul. Philadelphia: Lippincott Williams & Wilkins.
- Moreau, P., Paul, P., Rouas-Freiss, N., Kirszenbaum, M., Dausset, J. & Carosella, E. D. (1998). Am. J. Reprod. Immunol.40, 136–144. [DOI] [PubMed] [Google Scholar]
- Munz, C., Holmes, N., King, A., Loke, Y. W., Colonna, M., Schild, H. & Rammensee, H.-G. (1997). J. Exp. Med.185, 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan, S. & Long, E. O. (1999). J. Exp. Med.189, 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J., Waller, M. J., Parham, P., de Groot, N., Bontrop, R., Kennedy, L. J., Stoehr, P. & Marsh, S. G. E. (2003). Nucleic Acids Res.31, 311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawar, S. M., Vyas, J. M., Rodgers, J. R. & Rich, R. R. (1994). Annu. Rev. Immunol.12, 839–880. [DOI] [PubMed] [Google Scholar]
- Shiroishi, M., Tsumoto, K., Amano, K., Shirakihara, Y., Colonna, M., Braud, V. M., Allan, D. S. J., Makadzange, A., Rowland-Jones, S., Willcox, B., Jones, E. Y., van der Merwe, P. A., Kumagai, I. & Maenaka, K. (2003). Proc. Natl Acad. Sci. USA, 100, 8856–8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernich, D., Purcell, A. W., Macdonald, W. A., Kjer-Nielsen, L., Ely, L. K., Laham, N., Crockford, T., Mifsud, N. A., Bharadwaj, M., Chang, L., Tait, B. D., Holdsworth, R., Brooks, A. G., Bottomley, S. P., Beddoe, T., Peh, C. A., Rossjohn, J. & McCluskey, J. (2004). J. Exp. Med.200, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]