The tRNase domain of colicin D, which is specific to tRNAArgs, has been crystallized. A diffraction data set has been collected to a resolution of 1.05 Å.
Keywords: arginine tRNAs, atomic resolution, colicin D, tRNases
Abstract
The tRNase domain of colicin D, which cleaves only tRNAArgs at the 3′ side of their anticodon loops, has been expressed in Escherichia coli with its inhibitor protein and purified to a form free from the inhibitor using a low-pH buffer. Crystals were obtained by the hanging-drop vapour-diffusion method at 278 K from a buffer containing 100 mM Tris–HCl pH 8.5, 22% PEG MME 2000 and 1 mM nickel(II) chloride. Diffraction data to 1.05 Å resolution were collected at BL41XU, SPring-8. The crystals belong to space group P212121, with unit-cell parameters a = 34.7, b = 65.5, c = 96.5 Å.
1. Introduction
Colicin D (697 residues) is a Col plasmid-encoded antibacterial protein which only cleaves the four isoaccepting tRNAs for Arg of sensitive Escherichia coli cells; the anticodon sequences of these tRNAs are ICG, CCG, U*CU (where U* is 5-methylaminomethyluridine) and CCU (Tomita et al., 2000 ▶). The cleavage site is between positions 38 and 39 at the 3′ junction of the anticodon loop, where the residue is A at 38 and G or C at 39 in the four isoacceptors. These sequences at positions 38 and 39 are shared by some other tRNAs, so that colicin D should recognize some higher order structural features of tRNAArgs with or without recognizing bases at (or close to) the cleavage site.
The N-terminal domain of colicin D is required for receptor binding and translocation into sensitive cells and the remaining C-terminal domain includes the active domain (CRD; C-terminal RNase domain; Roos et al., 1989 ▶; de Zamaroczy & Buckingham, 2002 ▶). ImmD, which is the inhibitor protein of colicin D and is produced from the colD operon on plasmid ColD, binds specifically to the CRD and neutralizes its tRNase activity. We have determined that the CRD consists of 97 amino acids by sequence comparison with other bacteriocins which share homology with the N-terminal domain of colicin D. We also demonstrated that the CRD shows the same specific tRNase activity to tRNAArgs as does the whole colicin D molecule. It is therefore of interest to clarify how this small domain recognizes the higher order structure of a tRNA and cleaves it.
Specific recognition of tRNA molecules by proteins has been studied intensively for aminoacyl-tRNA synthetases (aaRSs); only a few nucleotides on tRNAArg molecules have proved to be important for specific recognitions (Chakraburtty, 1975 ▶; Atilgan et al., 1986 ▶; McClain et al., 1990 ▶). Since there is no significant homology between the CRD of colicin D and ArgRS and because ArgRS is substantially larger than the colicin D CRD (576 residues for the E. coli ArgRS), it is not clear whether the CRD of colicin D has a similar molecular-recognition mechanism to ArgRS. It is therefore difficult to infer the substrate-recognition mechanism of the CRD by analogy with ArgRS.
We have been studying the specific substrate-recognition mechanism of the CRD. We have previously reported the crystal structure of the CRD–ImmD complex at 2.3 Å (Yajima et al., 2004 ▶) and found that several Lys/Arg residues line up along a curve on the protein surface, suggesting an interaction with a tRNA backbone. Graille et al. (2004 ▶) also determined the CRD–ImmD complex structure at 2.0 Å. We report here a crystal of CRD which is free from ImmD and diffracts to an atomic resolution of 1.05 Å. Since only the CRD is believed to be translocated into the sensitive cells, its structure would directly reflect the active form that binds to and cleaves the target tRNAs.
2. Materials and methods
2.1. Expression and purification
The CRD–ImmD complex protein was expressed by a plasmid which has operons consisting of ORFs coding for the CRD595 (amino-acid positions 595–697) and ImmD (94 residues) tagged with six histidines at the C-terminus (Yajima et al., 2004 ▶).
1 l of Luria broth supplemented with ampicillin (100 mg l−1) was inoculated with 10 ml of an overnight culture of E. coli strain RR1 harbouring the plasmid at 310 K. Overexpression of the CRD–ImmD complex was induced by adding 0.4 µg ml−1 Mytomycin C (Seikagaku Corporation) at an OD600 of about 0.8. The cells were cultivated for 3 h and harvested by centrifugation at 6000g for 15 min at 277 K (Beckman J-25). The pellet obtained from 1 l of expression culture was resuspended in 40 ml buffer A (20 mM potassium phosphate buffer pH 7.0) and sonicated. To remove nucleic acid from the lysate, 400 µl 10% polyethyleneimine was added and the mixture was stirred slowly, followed by centrifugation at 15 000g for 20 min. Cell debris was also removed.
The supernatant was loaded onto an Ni2+-charged Hi-trap column (Amersham Biosciences) and washed with buffer A supplemented with 0.5 M KCl and 45 mM imidazole. The protein was eluted with buffer A supplemented with 0.5 M KCl and 120 mM imidazole. The fractions containing the protein were dialyzed with buffer B (pH 2.5) consisting of 100 mM NaH2PO4, 50 mM citric acid and 500 mM NaCl. The protein solution was then applied onto a Mono S column. At such a low pH, only ImmD was eluted by a gradient of 0.5–1.0 M KCl. The CRD remaining on the column was then eluted with a gradient of 0–1.0 M KCl after replacing the column buffer with potassium phosphate pH 7.4. The fractions containing the CRD were dialyzed with 20 mM Tris–HCl pH 8.5 for 8 h and concentrated to 16–24 mg ml−1 using a Vivaspin 5000 MWCO (Vivascience) prior to crystallization experiments.
2.2. Crystallization and preliminary X-ray diffraction
The initial crystallization conditions were screened using Hampton Research Crystal Screens I and II (Jancarik & Kim, 1991 ▶); the final condition was 100 mM Tris–HCl pH 8.5, 22% PEG MME 2000 and 1 mM nickel(II) chloride as the reservoir buffer. All crystallization experiments were performed using the hanging-drop vapour-diffusion method in a 24-well VDX plate at 278 K. Needle-shaped crystals were obtained with Crystal Screen 2 solution 45 [10 mM nickel(II) chloride, 100 mM Tris–HCl pH 8.5, 20%(w/v) PEG MME 2000]. After the Additive Screen (Hampton Research, USA) had been applied to optimize conditions, crystals suitable for diffraction experiments were grown by mixing 3 µl protein solution with 0.6 µl 2 M sodium thiocyanate, 2.4 µl 100 mM Tris–HCl pH 8.5, 1 mM nickel(II) chloride and 25% PEG MME 2000. The crystal reached maximum dimensions after 10 d, with dimensions of 0.3 × 0.7 × 0.1 mm.
Before the crystals were flash-frozen in the nitrogen stream at 100 K, they were soaked for 1 min in drops, which were made by a 1:1 mixture of 20 mM Tris–HCl pH 8.5, 20% glycerol with the reservoir buffer containing 100 mM Tris–HCl pH 8.5, 22% PEG MME 2000, 1 mM nickel(II) chloride containing 20% glycerol, and equilibrated with the reservoir buffer overnight. Diffraction data were collected on beamline BL41XU at SPring-8 and were processed using the HKL2000 program suite (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
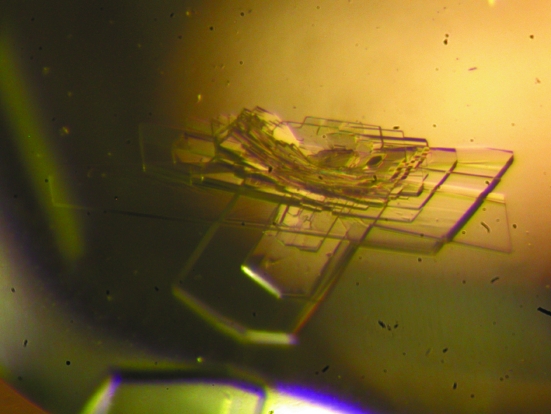
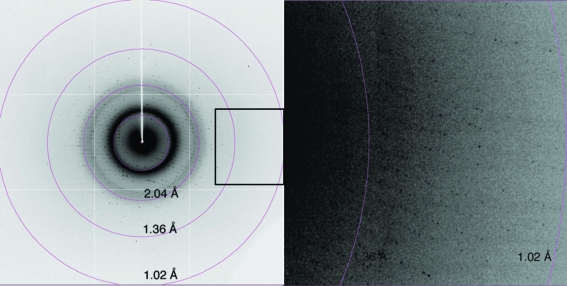
The CRD was expressed as a complex with ImmD in E. coli to prevent suicide of the host cells. After purification of the complex protein, ImmD was detached from the CRD on a Mono S column using buffer of pH 2.5, from which the CRD was recovered in a yield of 30 mg from 1 l culture. Upon optimizing the crystallization conditions, the crystals grew in a plate-like shape with typical dimensions of 0.3 × 0.7 × 0.1 mm in a heavily stacked condition, as shown in Fig. 1 ▶. Without the addition of sodium thiocyanate, the crystals only grew in needle shapes which were unsuitable for further experiments. The plate-shaped crystal diffracted to a resolution of 1.05 Å using X-rays of wavelength 0.7900 Å and the ADSC Quantum 315 detector (Fig. 2 ▶). Data-collection and processing statistics are given in Table 1 ▶.
Figure 1.
Crystals of the CRD obtained from 100 mM Tris–HCl pH 8.5, 25% PEG MME 2000, 1 mM nickel(II) chloride with sodium thiocyanate, having a maximum size of 0.7 mm in the longest dimension.
Figure 2.
Typical diffraction image of the CRD crystal collected on beamline BL41XU at SPring-8 using an ADSC Quantum 315 detector. The box in the left image indicates the position of the magnified image shown on the right.
Table 1. Data-collection and processing statistics.
Values in parentheses correspond to the outer resolution shell.
| Wavelength (Å) | 0.7900 |
| Space group | P212121 |
| Unit-cell parameters | |
| a (Å) | 34.7 |
| b (Å) | 65.5 |
| c (Å) | 96.5 |
| Matthews coefficient (Å3 Da−1) | 2.29 |
| Molecules per ASU | 2 |
| Solvent content (%) | 45.8 |
| Resolution range (Å) | 50–1.05 (1.09–1.05) |
| Total observations | 741756 |
| Unique reflections | 103580 |
| Average I/σ(I) | 33.2 (4.6) |
| Rmerge | 0.084 (0.370) |
| Completeness (%) | 100.0 (100.0) |
A molecular-replacement solution using the CRD structure from the complex (PDB code 1tfo; Yajima et al., 2004 ▶) as a search model has been obtained using the MOLREP program (Vagin & Teplyakov, 1997 ▶). From the solution, the crystal contains two molecules in an asymmetric unit, giving the solvent content as 45.8%. We also attempted to use direct methods to determine the structure using the programs SnB (Weeks & Miller, 1999 ▶) or ACORN (Yao, 2002 ▶); preliminary attempts using these approaches were unsuccessful, probably because of the number of residues (206 residues, ∼2000 non-H atoms) in the two molecules in an asymmetric unit.
Since we have previously studied the complex structure at only 2.3 Å resolution, we expect more precise structural information from the present data set. Model building and refinement of this structure are under way.
Acknowledgments
We are grateful for access to and user support at the synchrotron facilities of SPring-8.
References
- Atilgan, T., Nicholas, H. B. & McClain, W. H. (1986). Nucleic Acids Res.14, 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraburtty, K. (1975). Nucleic Acids Res.2, 1793–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graille, M., Mora, L., Buckingham, R. H., van Tilbeurgh, H. & de Zamaroczy, M. (2004). EMBO J.23, 1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411. [Google Scholar]
- McClain, W. H., Foss, K., Jenkins, R. A. & Schneider, J. (1990). Proc. Natl Acad. Sci. USA, 87, 9260–9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Roos, U., Harkness, R. E. & Braun, V. (1989). Mol. Microbiol.3, 891–902. [DOI] [PubMed] [Google Scholar]
- Tomita, K., Ogawa, T., Uozumi, T., Watanabe, K. & Masaki, H. (2000). Proc. Natl Acad. Sci. USA, 97, 8278–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin, A. & Teplyakov, A. (1997). J. Appl. Cryst.30, 1022–1025. [Google Scholar]
- Weeks, C. M. & Miller, R. (1999). J. Appl. Cryst.32, 120–124. [Google Scholar]
- Yajima, S., Nakanishi, K., Takahashi, K., Ogawa, T., Hidaka, M., Kezuka, Y., Nonaka, T., Ohsawa, K. & Masaki, H. (2004). Biochem. Biophys. Res. Commun.322, 966–973. [DOI] [PubMed] [Google Scholar]
- Yao, J.-X. (2002). Acta Cryst. D58, 1941–1947. [DOI] [PubMed] [Google Scholar]
- Zamaroczy, M. de & Buckingham, R. H. (2002). Biochimie, 84, 423–432. [DOI] [PubMed] [Google Scholar]