Abstract
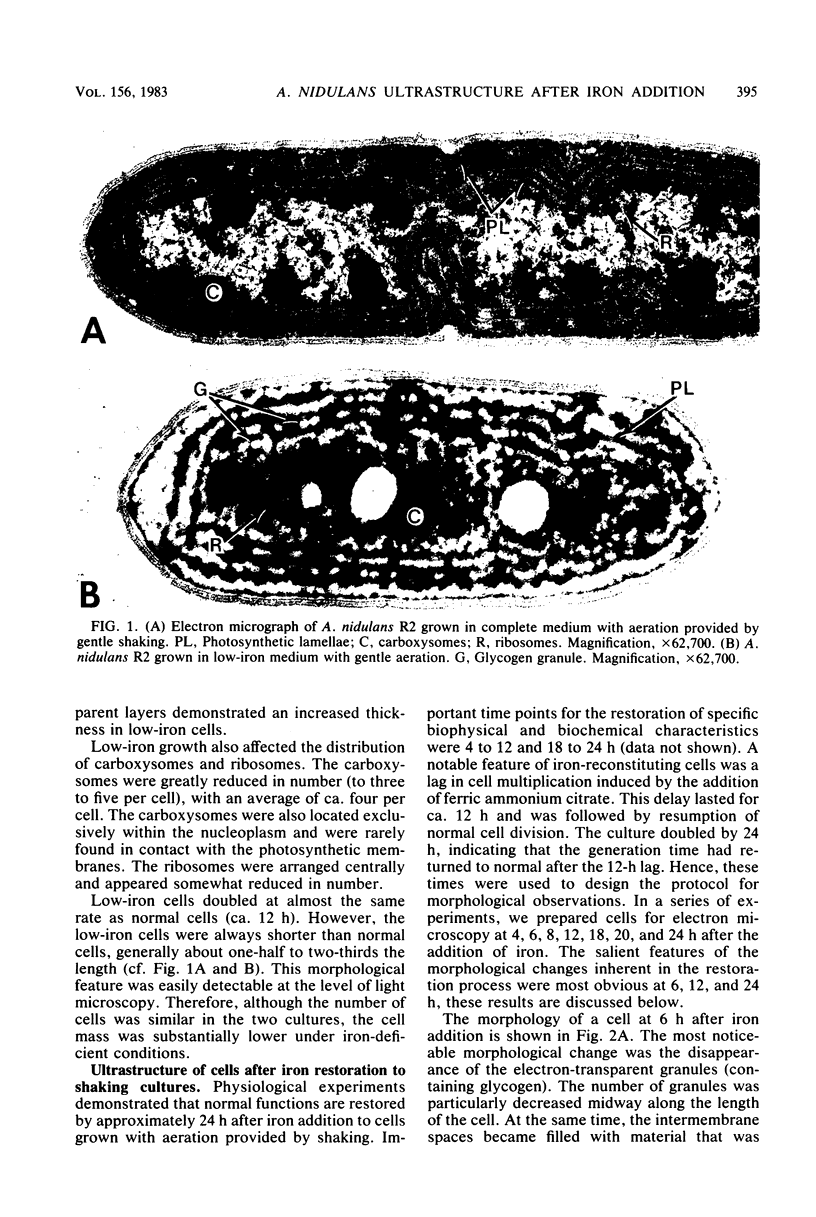
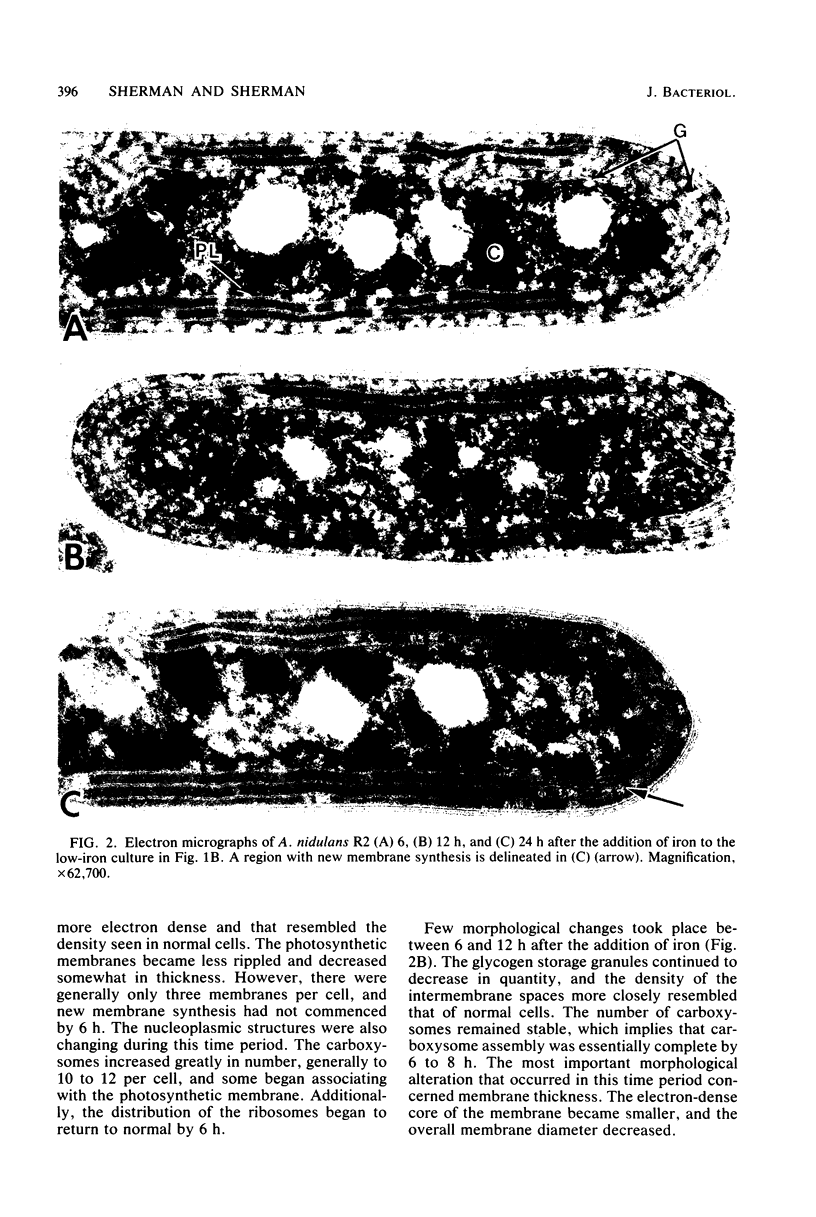
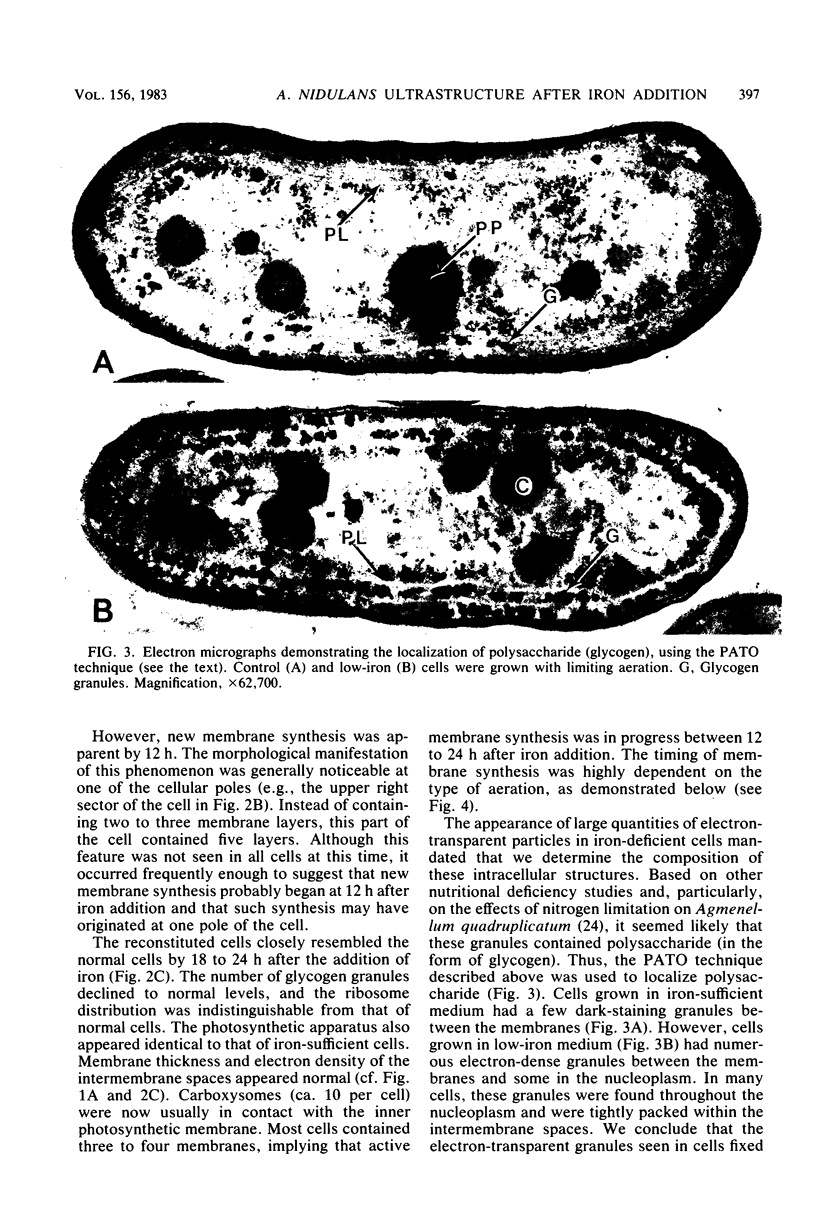
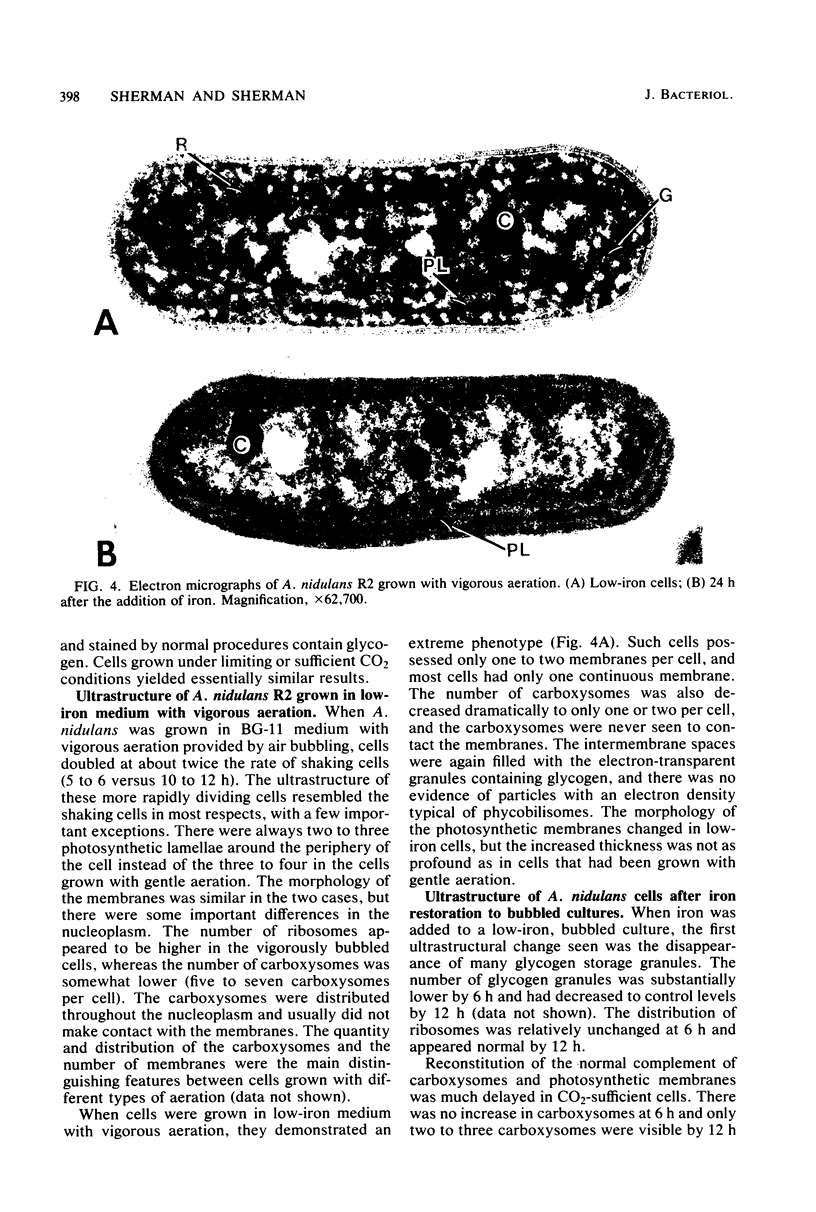
The effects of iron deficiency and iron reconstitution on the ultrastructure of the unicellular cyanobacterium Anacystis nidulans R2 were studied by electron microscopy. Low-iron cells, grown with different amounts of aeration, were analyzed at 6, 12, and 24 h after the addition of iron. Low-iron cells had a decrease in the quantities of membranes, phycobilisomes, and carboxysomes and a large increase in glycogen storage granules. In cells aerated with gentle shaking, the addition of iron caused the number of carboxysomes to increase rapidly within 6 h. This was paralleled by a decrease in the quantity of glycogen storage granules. Carboxysomes were associated with the nucleoplasmic face of the inner photosynthetic membrane in normal, but not low-iron, cells; they once more contacted the membrane by 6 h after iron addition. Phycobilisome assembly was apparent by 6 h, and the number of phycobilisomes increased throughout reconstitution. Membrane restoration was accomplished in two stages: (i) components were added to preexisting membranes until about 12 h, and (ii) new membranes were synthesized beginning at 12 to 18 h. Low-iron cells grown by bubbling with air had only one to two concentric layers of membrane per cell. The addition of iron led to a pattern of reconstitution that was similar to that described above with two important exceptions. Under these conditions, the number of carboxysomes remained low and the carboxysomes rarely contacted the photosynthetic membranes. New membranes were not synthesized until the culture had reached the late-logarithmic growth phase and after all other morphological features had returned to normal.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M. Photosynthetic membrane system in Anacystis nidulans. J Bacteriol. 1968 Sep;96(3):836–841. doi: 10.1128/jb.96.3.836-841.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Allen M. M. Ultrastructure of the cell wall and cell division of unicellular blue-green algae. J Bacteriol. 1968 Sep;96(3):842–852. doi: 10.1128/jb.96.3.842-852.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANKER J. S., SEAMAN A. R., WEISS L. P., UENO H., BERGMAN R. A., SELIGMAN A. M. OSMIOPHILIC REAGENTS: NEW CYTOCHEMICAL PRINCIPLE FOR LIGHT AND ELECTRON MICROSCOPY. Science. 1964 Nov 20;146(3647):1039–1043. doi: 10.1126/science.146.3647.1039. [DOI] [PubMed] [Google Scholar]
- Lehmann M., Wöber G. Accumulation, mobilization and turn-over of glycogen in the blue-green bacterium Anacystis nidulans. Arch Microbiol. 1976 Dec 1;111(1-2):93–97. doi: 10.1007/BF00446554. [DOI] [PubMed] [Google Scholar]
- Miller L. S., Holt S. C. Effect of carbon dioxide on pigment and membrane content in Synechococcus lividus. Arch Microbiol. 1977 Nov 18;115(2):185–198. doi: 10.1007/BF00406374. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Connelly M., Sherman D. M. Infection of Synechococcus cedrorum by the cyanophage AS-1M. I. Ultrastructure of infection and phage assembly. Virology. 1976 May;71(1):1–16. doi: 10.1016/0042-6822(76)90089-1. [DOI] [PubMed] [Google Scholar]
- Shively J. M., Ball F., Brown D. H., Saunders R. E. Functional organelles in prokaryotes: polyhedral inclusions (carboxysomes) of Thiobacillus neapolitanus. Science. 1973 Nov 9;182(4112):584–586. doi: 10.1126/science.182.4112.584. [DOI] [PubMed] [Google Scholar]
- Stevens S. E., Paone D. A. Accumulation of Cyanophycin Granules as a Result of Phosphate Limitation in Agmenellum quadruplicatum. Plant Physiol. 1981 Apr;67(4):716–719. doi: 10.1104/pp.67.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J. Cytoplasmic reserve polysaccharide of Selenomonas ruminantium. Appl Environ Microbiol. 1980 Mar;39(3):630–634. doi: 10.1128/aem.39.3.630-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood N. B., Haselkorn R. Control of phycobiliprotein proteolysis and heterocyst differentiation in Anabaena. J Bacteriol. 1980 Mar;141(3):1375–1385. doi: 10.1128/jb.141.3.1375-1385.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos L., Fay P. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. I. The effect of nitrogen starvation. Arch Mikrobiol. 1974 Mar 28;96(4):271–279. [PubMed] [Google Scholar]