Crystals of dengue serotype 2 and West Nile virus NS2B–NS3 protease complexes have been obtained and the crystals of both diffract to useful resolution. Sample homogeneity was essential for obtaining X-ray-quality crystals of the dengue protease. Controlled proteolysis produced a crystallizable fragment of the apo West Nile virus NS2B–NS3 and crystals were also obtained in the presence of a peptidic inhibitor.
Keywords: viral protease, dengue virus, West Nile virus, proteolysis, mutation
Abstract
Both dengue and West Nile virus infections are an increasing risk to humans, not only in tropical and subtropical areas, but also in North America and parts of Europe. These viral infections are generally transmitted by mosquitoes, but may also be tick-borne. Infection usually results in mild flu-like symptoms, but can also cause encephalitis and fatalities. Approximately 2799 severe West Nile virus cases were reported this year in the United States, resulting in 102 fatalities. With this alarming increase in the number of West Nile virus infections in western countries and the fact that dengue virus already affects millions of people per year in tropical and subtropical climates, there is a real need for effective medicines. A possible therapeutic target to combat these viruses is the protease, which is essential for virus replication. In order to provide structural information to help to guide a lead identification and optimization program, crystallizations of the NS2B–NS3 protease complexes from both dengue and West Nile viruses have been initiated. Crystals that diffract to high resolution, suitable for three-dimensional structure determinations, have been obtained.
1. Introduction
Dengue and West Nile viruses both belong to the flavivirus family, which are widespread human pathogens that can cause haemorrhagic fevers. While dengue infections are more prevalent in tropical and subtropical areas, placing some 2.5 billion people (or 40% of the world population) at risk, West Nile virus has continued to spread throughout North America over the past ten years, causing fever, meningitis, encephalitis and fatalities. The virus-transmitting mosquitoes Aedes aegypti and A. albopictus are found in all continents except Europe, but this may change in coming years as a result of a global warming trend. It is estimated that about 5% of the haemorrhagic cases (or 50 000 persons) are fatal (Gubler & Clark, 1995 ▶) and at present there is no vaccine available to protect against either dengue or West Nile virus infections. A therapeutic agent against these targets would potentially save the lives of hundreds of thousands of people throughout the world, which is why there is growing interest from the pharmaceutical industry in developing treatments to combat these diseases. The viral protease of flaviviruses is necessary for the processing of its polyprotein and subsequently for virus replication. The NS2B cofactor is essential for the catalytic activity of NS3 protease and the NS2B–NS3 protease complex is therefore an attractive target for therapeutic intervention in flavivirus infections. A three-dimensional structure for a physiologically relevant NS2B–NS3 complex from either dengue or West Nile virus would provide important insights into the role of the cofactor and the active-site architecture. The three-dimensional structure of a dengue protease lacking the NS2B cofactor has been solved (Murthy et al., 1999 ▶) in the presence and absence of a Bowman–Birk inhibitor (PDB codes 1df9 and 1bef, respectively). However, no successful crystallization experiment has been reported of a cofactor–enzyme complex for either dengue or West Nile proteases. In this work, we describe the production of crystals of both dengue serotype 2 (DEN2) and West Nile virus (WNV) NS2B–NS3 protease complexes that diffract to high resolution and should facilitate the rapid resolution of their three-dimensional structures. For the WNV NS2B–NS3 protein, crystals of a different form were also obtained in the presence of a peptidic inhibitor.
2. Experimental
2.1. Cloning, expression and purification of DEN2 NS2B–NS3
The DEN2 pET-15b-NS2B-NS3 expression construct (PlasNova No. NPL006926) was assembled as the 47-amino-acid hydrophilic core sequence of NS2B (cNS2B; amino acids 1394–1440) linked via a nine-amino-acid linker (G4-S-G4) to the N-terminal 185 amino acids of NS3 (cNS3; amino acids 1476–1660) in vector pET15b (Novagen, Madison, USA). The plasmid pGEM-T-(E-NS3) encoding the NS2B–NS3 cDNA sequence from dengue virus serotype 2 (strain TSV01, GenBank accession No. AY037116; obtained from S. G Vasudevan, Novartis Institutes of Tropical Diseases, Singapore) was used as a template for PCR of cNS2B and cNS3. To obtain the cNS2B sequence, PCR was carried out using the primers DEN2-cNS2B-FOR, 5′- TATACTCGAGGCTGATTTGGAACTGGAGAG-3′, and DEN2-cNS2B-REV, 5′-CCCGCCTCCCACCACTACCTCCGCCCCCCAGTGTTTGTTCTTCTTCTTCA-3′. To obtain the NS3 sequence, PCR was carried out using the primers DEN2-NS3-FOR, 5′-GGGGGCGGAGGTAGTGGTGGAGGCGGGGCCGGAGTATTGTGGGATGT-3′, and DEN2-NS3-REV, 5′-TAATGGATCCTTACTTTCGAAAGATGTCATCTTCA-3′. The NS2B–NS3 chimeric sequence was generated in an overlap PCR reaction with these two PCR products, digested with XhoI/BamHI and cloned into the same sites in pET15b.
Cultures of Escherichia coli BL21 (DE3) transformed with the DEN pET-15b-NS2B-NS3 expression plasmid were grown in 2 l LB medium containing 10 µg ml−1 ampicillin at 310 K until the OD600 reached 0.8. After cooling the cultures to 293 K, expression of the recombinant protein was induced by the addition of 0.5 mM isopropyl β-d-thiogalactopyranose (IPTG) and the culture was incubated for 16 h. The cells were harvested by centrifugation and resuspended in 50 ml lysis buffer [50 mM NaCl, 20 mM Tris pH 8.5, 5%(v/v) glycerol] and stored at 253 K.
For purification, the cells were thawed, lysed by sonification and then centrifuged at 50 000g for 30 min at 277 K. The supernatant was then passed through 0.22 µm syringe filters and injected onto a Chelating Sepharose Fast Flow column (all column materials were purchased from Amersham GE Healthcare, Freiburg, Germany), washed with four column volumes of 20 mM imidazole and eluted with 125 mM imidazole. The N-terminal His6 tag was cleaved by incubating overnight with 500 units of thrombin per 100 mg of protein while dialyzing against a dialysis buffer (50 mM Tris pH 8.5) at 277 K. The cleaved His6 tag and uncleaved protein were removed by applying the protein to the same chelating column and the flowthrough was loaded onto a Mono Q HR10/10 anion-exchange column pre-equilibrated with the dialysis buffer. The protein, which was eluted by application of a linear 0–300 mM NaCl gradient to this column, was concentrated in an Amicon Ultra centrifugal filter device with a 10 kDa filter and then loaded onto a HiLoad 26/60 Superdex 75 column equilibrated in the above lysis buffer. The protein was concentrated to 60 mg ml−1 for crystallization trials using centrifugal filter units (Millipore, Volketswil, Switzerland) with a molecular-weight cutoff of 10 kDa, flash-frozen as 35 µl aliquots in 50 mM NaCl, 20 mM Tris pH 8.5, 5%(v/v) glycerol and stored at 193 K.
2.2. Cloning, expression and purification of WNV NS2B–NS3
The WNV pET-15b-NS2B-NS3 expression construct (PlasNova No. NPL007345) was assembled as the 47-amino-acid hydrophilic core sequence of NS2B (cNS2B; amino acids 1424–1470) linked via a nine-amino-acid linker (G4-S-G4) to the N-terminal 187 amino acids of NS3 (cNS3; amino acids 1506–1692) in vector pET15b (Novagen, Madison, USA). The viral RNA of an infectious West Nile virus strain (originally isolated by G. Wengler, GenBank accession No. M12294) was extracted from the supernatant from infected cells and used as a template for PCR of cNS2B and cNS3. Reverse transcription was performed using primer WNV-REV, 5′-GGACATCACACTCAATAGTGCAATC-3′. To obtain the cNS2B sequence, PCR was carried out using the primers WNV-NdeF, 5′-GACACTACATATGACAGACATGTGGATTGAGAG-3′, and WNV-LinR, 5′-CCCGCCTCCACCACTACCTCCGCCCCCTTTCCATGGTGCCCCGGGGTCATTCAT-3′. To obtain the cNS3 sequence, PCR was carried out using the primers WNV LinF, 5′-GGGGGCGGAGGTAGTGGTGGAGGCGGGGGAGGTGTTCTTTGGGACACA-3′, and WNV NdeR, 5′-GGATACCCATATGTTATTTCTTCCTCAACATTTCAGGTTCG-3′. The cNS2B–NS3 chimeric sequence was generated in an overlap PCR reaction with these two PCR products, digested with NdeI and cloned into the same site in pET15b.
Cultures of E. coli BL21 (DE3) transformed with the WNV pET-15b-NS2B-NS3 expression plasmid were grown in 2 l LB medium containing 100 µg ml−1 ampicillin at 310 K until the OD600 reached 0.8. After cooling the cultures to 293 K, expression of the recombinant protein was induced by the addition of 0.5 mM IPTG and the culture was incubated for 16 h. The cells were harvested by centrifugation and resuspended in 50 ml lysis buffer [50 mM Tris pH 8.5, 5%(v/v) glycerol] and stored at 253 K.
For purification, the cells were thawed, lysed by sonification and then centrifuged at 50 000g for 30 min at 277 K. The supernatant was then passed through 0.22 µm syringe filters and injected onto a Chelating Sepharose Fast Flow column, washed with four column volumes of lysis buffer and eluted with 125 mM imidazole. The N-terminal His6 tag was cleaved by incubating overnight with thrombin (Amersham; 500 units per 100 mg of protein) while dialyzing against the lysis buffer at room temperature. The cleaved His6 tag and uncleaved protein were removed by applying the protein to the same chelating column and the flowthrough was loaded onto a 50 ml Source 15Q anion-exchange column pre-equilibrated with the lysis buffer. The protein, which was eluted by application of a linear 0–300 mM NaCl gradient to this column, was concentrated in an Amicon Ultra centrifugal filter device with a 10 kDa filter and then loaded onto a HiLoad 16/60 Superdex 75 column equilibrated in the lysis buffer.
The protein was concentrated to between 60 and 80 mg ml−1 for crystallization trials using centrifugal filter units with a molecular-weight cutoff of 10 kDa, flash-frozen as 35 µl aliquots in 20 mM Tris pH 8.5, 5%(v/v) glycerol and stored at 193 K.
3. Analysis
3.1. Dynamic light scattering
Both proteins were analysed at concentrations of between 6 and 10 mg ml−1 using dynamic light scattering to determine their suitability for crystallization as described by Ferre-D’Amare & Burley (1994 ▶) and Zulauf & D’Arcy (1992 ▶) using a DynaPro instrument (ProteinSolutions, Charlottesville, USA). The proteins were filtered through 0.22 µm centrifugal units prior to analysis and all measurements were made at 296 K.
3.2. Mass spectrometry
The purified proteins were analyzed by HPLC/ESI–MS. Separation of proteins was performed on a HP1100 HPLC system (Hewlett Packard, Palo Alto, USA) employing a 1 × 150 mm LC column packed with POROS R1/H (Dr Maisch High Performance LC-GmbH, Ammerbuch, Germany). Samples were injected onto the column using a CTC PAL autosampler (CTC, Zwingen, Switzerland) fitted with a Valco model C6UW HPLC valve (Valco, Houston, USA) and a 10 µl injection loop. HPLC was controlled by MassLynx software (Micromass, Manchester, England). UV detection was performed at 214 nm. Eluent A was water containing 0.05% TFA. Eluent B was a 1:9 mixture of water and acetonitrile containing 0.045% TFA. A gradient from 20% B to 90% B was run in 20 min. The flow rate was typically 60 µl min−1. The total flow from the LC system was introduced into the UV detection cell prior to introduction into the ESI source. The HPLC system and the signal from the UV detector were controlled using MassLynx software and the data were processed using the same software. Mass spectrometry was carried out using a Q-TOF quadrupole time-of-flight hybrid tandem mass spectrometer (Micromass, Manchester, England) equipped with a Micromass Z-type electrospray ionization source. The acquisition mass range was typically m/z 500–2000. Data were recorded and processed with MassLynx software. Calibration of the 500–2000 m/z scale was achieved by using the multiple-charged ion peaks of horse heart myoglobin (MW 16 951.5 Da).
3.3. Isolation of crystals for SDS–PAGE and mass-spectrometric analysis
Crystals were isolated using a standard cryoloop and washed three times in 5 µl drops containing the corresponding reservoir solutions. After the final wash, the excess reservoir was removed with a micropipette and 5 µl water was added to dissolve the crystals.
4. Crystallization
4.1. Crystallization of DEN2 NS2B–NS3 complex
Vapour-diffusion crystallization experiments were performed using an Oryx crystallization robot (Douglas Instruments, East Garston, England). In a typical experiment, 0.3 µl screening solution was added to 0.3 µl protein solution on 96-well Intelliplates (Robbins Instruments, Sunnyvale, USA); reservoir wells contained 90 µl screening solution.
The screening solutions used for the experiments were Index and SaltRx from Hampton Research (Aliso Viejo, USA) and the PEGs Suite (Nextal Biotechnologies, Montreal, Canada).
All crystallization trials were performed at 296 K. For data collection, the crystals were transferred to the respective reservoir solutions without additional cryoprotection. The crystals were mounted onto cryoloops and flash-cooled to 100 K in a stream of gaseous nitrogen produced by an Oxford Cryostream 700 series (Oxford Cryosystems, Oxford, England). Crystals were tested for diffraction on a MAR Research 345 image plate at 100 K using Cu Kα radiation generated by a Nonius FR 591 rotating-anode generator equipped with an Osmic mirror system.
The most promising conditions were optimized using the hanging-drop vapour-diffusion method (McPherson, 1982 ▶). 500 µl of solution corresponding to the initial hit reservoir was pipetted into the desired well of the Linbro plate, 2 µl of protein was dispensed onto a 22 mm siliconized square glass cover slide and either 1 or 2 µl of the reservoir solution was added without mixing. The slides were then placed over the pre-greased well and sealed by applying pressure.
4.2. Crystallization of WNV NS2B–NS3 complex
Concentration and screening for initial crystallization conditions were performed as described for DEN2 NS2B–NS3. Trypsin digestions were carried out by mixing 35 µl WNV NS2B–NS3 protease solution at 80 mg ml−1 with 1 µl trypsin at 1 mg ml−1 and the mixture was incubated for 16 h at 296 K. For controlled auto-digestion, the protein was incubated at 310 K for 18, 42 and 90 h.
5. Results and discussion
5.1. Impact of purification on the crystallization of DEN2 NS2B–NS3
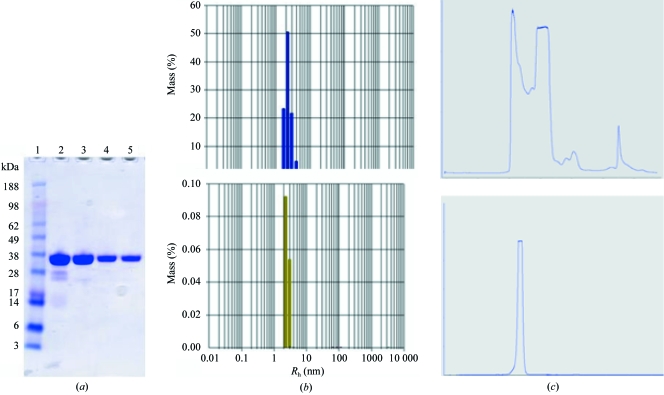
Den2 NS2B–NS3 was originally purified using nickel-chelate chromatography followed by thrombin digestion, a second nickel column to remove free tag and undigested viral protease and finally a size-exclusion chromatography (SEC) column. For the optimized purification, dialysis steps were carried out at 277 K instead of 296 K and a wash buffer containing 20 mM imidazole was substituted after the first nickel-chelate column to remove nonspecifically bound proteins from the column. A Mono-Q ion-exchange column was introduced after the second nickel-chelate column and finally the SEC column was run in the presence of buffer augmented with 50 mM NaCl. The final products from the two purification schemes were loaded onto an SDS gel and the contaminants in the original preparation were clearly visible (Fig. 1 ▶). This is confirmed by SEC profiles, which show a single peak for the optimized purification, whereas the original preparation has multiple peaks. Examination of the two preparations by dynamic light scattering also indicated a much narrower size distribution for the optimized purification. A summary of these comparisons is shown in Fig. 1 ▶.
Figure 1.
Comparison of initial and optimized purification with DEN2 NS2B–NS3. (a) SDS–PAGE of initial purification (lanes 2 and 4) and final purification (lanes 3 and 5). (b) Size-distribution profiles from DLS. (c) SEC profiles.
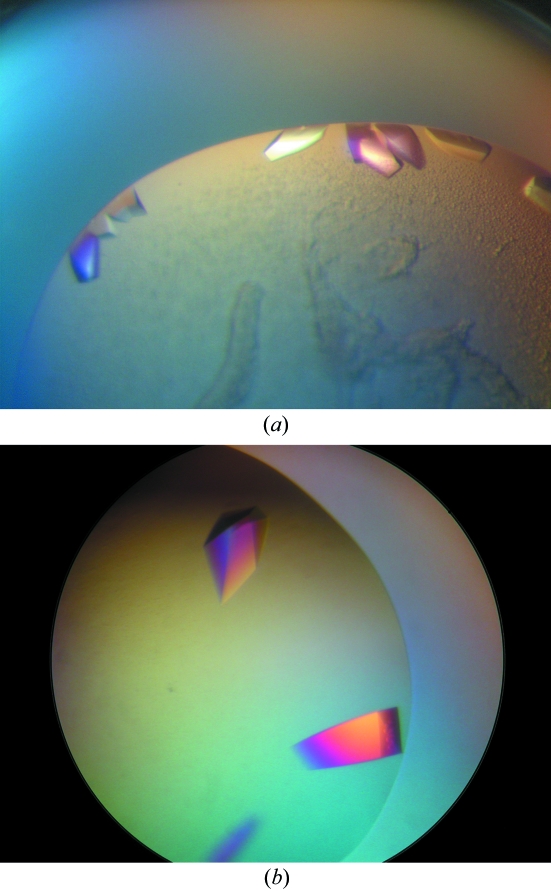
No crystals were obtained from the less pure sample and the optimally purified protein only produced crystals in two very similar conditions out of more than 300 tested. This suggests that the protein is not readily crystallisable and a high degree of purity is required to obtain any initial hits. Large single crystals were observed after 5 d in the Nextal PEG screen in condition Nos. 1 (0.1 M acetic acid pH 4.6, 40% PEG 200) and 7 (0.1 M MES pH 6.5, 40% PEG 200). The crystals are shown in Fig. 2 ▶.
Figure 2.
Protein crystals of DEN2 NS2B–NS3 complex grown in (a) 0.1 M acetic acid pH 4.6, 40% PEG 200, (b) 0.1 M MES pH 6.5, 40% PEG 200.
Drops of protein from the original purification were set up under identical conditions as for the optimized preparation and were streak-seeded with crushed crystals from the screen hits. The fact that no crystals were again obtained underlines the fact that with this particular protein a high degree of homogeneity is essential. The crystals obtained in the initial screens were large enough for X-ray analysis without further optimization and diffract to 1.5 Å on an in-house X-ray source; the crystallographic data is summarized in Table 2.
5.2. Crystallization of WNV NS2B–NS3
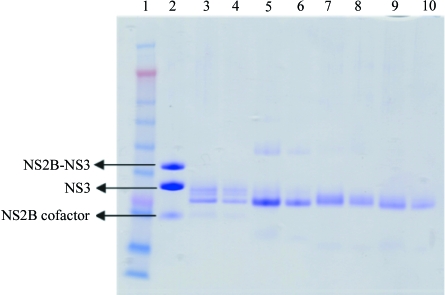
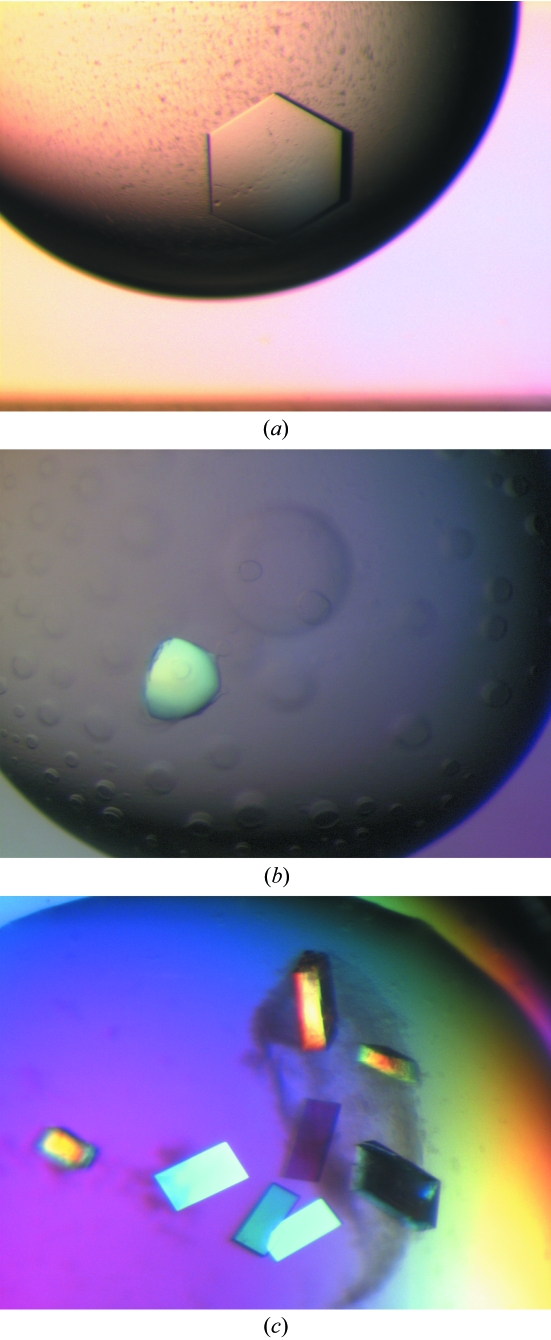
The protein appeared to be suitable for crystallization as judged by light-scattering experiments, which showed a unimodal size distribution. Interestingly, SDS–PAGE gels of the starting material indicated that the NS2B–NS3 protease is nicked and mass-spectrometric analysis showed that a fragments of 20 740 and 5855 Da were present. Further examination of this data indicates that the nick occurs in the linker between the NS2B and NS3. Initial screens with this protein only produced crystals after a period of approximately 19 d in Index condition 43 (25% PEG 3350, 100 mM Bis-Tris pH 6.5). It subsequently proved very difficult to reproduce these crystallization conditions and we suspected that the protein was susceptible to proteolytic digestion during the crystallization trials at room temperature. A truncated form may have been generated which crystallized more readily than the original protein. This was confirmed when crystals were isolated and analyzed using mass spectrometry (Table 1 ▶). This analysis showed a major fragment (91% of the mass) with a molecular weight of 18 560 Da, compared with 20 740 Da for the full-length protein. As the cleavage occurs between residues Lys76 and Gly77, it seems likely that we are observing autoproteolysis, as this site corresponds to a consensus recognition site for flaviviruses and autoproteolysis for WNV has been reported by Shiryaev et al. (2006 ▶). When the fresh protein preparation was complexed with a peptidic inhibitor Bz-Nle-Lys-Thr-Arg-H, crystals were observed in two conditions in the Index Screen that were different from the non-inhibited protein (1.0 M sodium/potassium phosphate pH 5.6 and 0.1 M HEPES pH 7.5, 2.0 M ammonium sulfate). It also proved difficult to reproduce these crystals and subsequent analysis on SDS–PAGE gels of the inhibited crystals again showed that proteolytic cleavage had occurred (Fig. 3 ▶). Although these crystals had a strange rounded morphology (Fig. 4 ▶), they diffracted well and data could be collected to 2.3 Å.
Table 1. Mass spectrometry of WNV NS2B–NS3 samples.
| Sample | Molecular weight of major fragments (Da) |
|---|---|
| Full length | 20740.5, 5854.6 |
| Crystals of spontaneous digest | 18252.5–18787.5 |
| Solution of trypsin digest | 18439.0–18859.5 |
| Solution of 90 h autodigest | 20740.5, 18566.5 (6%) |
Figure 3.
SDS–PAGE analysis of crystals of WNV NS2B–NS3. Lane 1, molecular-weight markers; lane 2, freshly prepared WNV NS2B–NS3; lanes 3 and 4, crystals of nontreated protein; lanes 5 and 6, crystals of trypsin-digested protein; lanes 7 and 8, crystals of 18 h digested protein; lanes 9 and 10, crystals of 90 h digested protein.
Figure 4.
(a) Crystals of trypsin-digested WNV NS2B–NS3, (b) crystals of WNV NS2B–NS3 complexed with Bz-Nle-Lys-Thr-Arg-H, (c) crystals of WNV NS2B–NS3 after 90 h incubation at 310 K.
With the aim of establishing a more reproducible crystallization system by controlling proteolysis, the protein was incubated with trypsin and crystallization screens were set up after 16 h of incubation at 296 K. SDS–PAGE gels of the trypsin-treated sample showed the cleavage product to be fragments with apparent molecular weights of around 18 000 Da and this was confirmed by mass-spectrometric analysis, which indicated masses of between 18 439 and 18 859 (Table 1 ▶). The trypsin-digested protein produced crystals from a precipitant solution consisting of 0.2 M ammonium sulfate, 0.1 M Tris pH 8.5, 25%(w/v) PEG 3350 (Fig. 4 ▶) which diffracted to 2.1 Å and appear to belong to space group P321. Once again these crystals could not be reproduced easily and we attempted to reproduce the spontaneous autodigestion which occurred in the original crystallization to determine whether this product could be crystallized more reproducibly. The protein at 80 mg ml−1 was incubated at 310 K for periods of 18, 42 and 90 h before initiating crystallization screening. Crystals were observed after about two weeks with the protein that had been incubated for 90 h and after three weeks with the 18 and 42 h digests. The conditions were similar to those originally identified for the spontaneous proteolysis [0.1 M HEPES pH 7.5, 25%(w/v) PEG 3350] and crystals could be grown in a reproducible manner (Fig. 4 ▶). It could be shown that the 90 h autodigested sample had generated only a small percentage (6%) of a fragment corresponding to a molecular weight of 18 566 Da as observed by mass-spectrometric analysis. A similar analysis of the crystals produced from this preparation proved difficult as the polyethylene glycol interfered with measurements; however, SDS–PAGE clearly showed that they contained only proteolytically digested protein of similar molecular weight to those observed in the original crystallization, trypsin-digested protein and inhibited protein. A summary of the mass-spectrometric analysis is shown in Table 1 ▶ and SDS–PAGE gels in Fig. 3 ▶.
The crystals of the autodigested protein diffracted to at least 2.3 Å resolution and belong to space group P21. A summary of the crystallographic data of the different West Nile virus NS2B–NS3 crystals is presented in Table 2 ▶.
Table 2. Summary of crystallographic analysis of WNV NS2B–NS3 and DEN2 NS2B–NS3 crystals.
Values in parentheses are for the highest resolution shell.
| WNV NS2B–NS3 | ||||
|---|---|---|---|---|
| Complexed with Bz-Nle-Lys-Thr-Arg-H | 90 h digested | Trypsin digested | DEN2 NS2B–NS3 | |
| Space group | P65 | P21 | P321 | C2221 |
| Unit-cell parameters (Å, °) | a = b = 99.4, c = 50.0, α = β = 90, γ = 120 | a = 30.4, b = 82.7, c = 31.9, α = γ = 90, β = 98.9 | a = b = 41.5, c = 178.3, α = β = 90, γ = 120 | a = 61.3, b = 61.1, c = 114.0, α = β = γ = 90 |
| No. of molecules in ASU | 1 | 1 | 1 | 1 |
| Solvent content (%) | 60 | 38 | 47 | 44 |
| Wavelength (Å) | 1.00882 | 1.00123 | 1.54178 | 1.54178 |
| Resolution range (Å) | 20.0–2.3 (2.40–2.30) | 28.2–1.96 (2.00–1.96) | 20–2.23 (2.30–2.23) | 43.0–1.50 (1.56–1.50) |
| Unique reflections | 12682 | 11151 | 9308 | 34417 |
| Completeness (%) | 99.9 | 99.3 | 99.4 | 99.2 (98.7) |
| Redundancy | 4.1 | 3.5 | 9.9 | 3.0 |
| Rmerge (%) | 7.8 (30.2) | 3.6 (7.0) | 7.8 (45.1) | 7.9 (24.9) |
| I/σ(I) | 14.1 (5.2) | 30.8 (12.4) | 18.8 (6.1) | 11.7 (5.0) |
6. Conclusions
6.1. DEN2 NS2B–NS3
Many proteins crystallize readily and under multiple conditions even after relatively crude purifications, whereas others may only produce crystals in a very narrow ‘window’. It may prove difficult to identify initial hits in these cases if the protein is not of sufficient purity. This was the case with the DEN2 NS2B–NS3 complex: although the protein was well behaved and soluble to extremely high concentrations, very pure sample preparation was a prerequisite for identifying crystallization conditions and obtaining X-ray-quality crystals. It has recently been suggested by Doye et al. (2004 ▶) that many proteins may go through a negative evolutionary design process and have evolved to avoid native-state aggregation in vivo, which results amongst other things in making them less likely to crystallize. This theory has been partially substantiated by experimental work using crystal engineering to modify or introduce crystal contacts into proteins that were recalcitrant to crystallization (McElroy et al., 1992 ▶; Dasgupta et al., 1997 ▶). Dasgupta and coworkers observed that lysine residues are amongst those most seldom found in crystals contacts, whereas arginines are amongst the most common. To try to improve the chances of obtaining crystals, we also produced two mutants during the course of this study, replacing surface lysines (Lys61/Lys63 and Lys73/Lys74) with arginine residues. When crystallization conditions were finally identified, it was clear that the mutations had no influence on the way the protein crystallized (data not shown). The crystals of DEN2 NS2B–NS3 are of very high quality and should facilitate the rapid determination of the three-dimensional structure.
6.2. WNV NS2B–NS3
An autoproteolytic digestion of the WNV NS2B–NS3 protease has facilitated the production of X-ray-quality crystals of this potentially important target. A fragment of the protein produced by autodigestion provided a system in which the protein could be reproducibly crystallized. The crystals grown in the presence of a peptide inhibitor belong to a different space group, suggesting that a conformational change may have taken place upon binding. The X-ray structure determination is in progress, which should provide important information on the inhibitor-binding mode and provide a basis for the design of lower molecular-weight inhibitors.
Acknowledgments
The authors wish to thank Dr Ulrich Hommel and Dr Subash Vasudevan for their support and encouragement throughout the project, Aruna Sampath for cloning WNV CF40glyNS3pro and Fong Chii Shyang for cloning DEN2 CF40glyNS3pro.
References
- Dasgupta, S., Iyer, G. H., Bryant, S. H., Lawrence, C. E. & Bell, J. A. (1997). Proteins, 28, 494–514. [DOI] [PubMed] [Google Scholar]
- Doye, J. P. K., Louis, A. A. & Vendruscolo, M. (2004). Phys. Biol.1, P9–P13. [DOI] [PubMed] [Google Scholar]
- Ferre-D’Amare, A. R. & Burley, S. K. (1994). Structure, 25, 357–359. [DOI] [PubMed]
- Gubler, D. J. & Clark, G. G. (1995). Emerg. Infect. Dis.1, 55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy, H. E., Sisson, G. W., Schoettlin, W. E., Aust, R. M. & Villafranca, J. E. (1992). J. Cryst. Growth, 122, 265–272.
- McPherson, A. (1982). Preparation and Analysis of Protein Crystals. New York: John Wiley & Sons.
- Murthy, H. M., Clum, S. & Padmanabhan, R. (1999). J. Mol. Biol.274, 5573-5580. [DOI] [PubMed]
- Shiryaev, S. A., Ratnikov, B. I., Chekanov, A. V., Sikora, S., Rozanov, D. V., Godzik, A., Wang, J., Smith, J. W., Huang, Z., Lindberg, I., Samuel, M. A., Diamond, M. S. & Strongin, A. Y. (2006). Biochem. J.393, 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulauf, M. & D’Arcy, A. (1992). J. Cryst. Growth, 122, 102–106.