Cytosolic class II α-mannosidase from T. maritima (TM1851), a family 38 glycoside hydrolase, was crystallized. A diffraction data set was collected to 2.9 Å resolution.
Keywords: class II α-mannosidase, glycoside hydrolase family 38, Thermotoga maritima, TM1851
Abstract
Class II α-mannosidase cleaves off α-1,2-, α-1,3- and α-1,6-mannose residues. In this paper, the crystallization and preliminary X-ray analysis of cytosolic class II α-mannosidase from Thermotoga maritima (TM1851), a family 38 glycoside hydrolase, is described. The crystal of recombinant TM1851 belongs to the C-centred monoclinic space group C2, with unit-cell parameters a = 244.7, b = 87.4, c = 166.6 Å, β = 124.7°. X-ray diffraction data were collected to a resolution of 2.9 Å.
1. Introduction
Class II α-mannosidases exhibit exo-type activity toward α-1,2-, α-1,3- and α-1,6-mannose linkages (Herscovics, 1999 ▶) and are classified into glycoside hydrolase family 38, based on sequence similarity (Carbohydrate-Active Enymes Server; http://afmb.cnrs-mrs.fr/CAZY; Coutinho & Henrissat, 1999 ▶). In eukaryotic cells, several types of class II α-mannosidases are expressed, e.g. Golgi α-mannosidase II, ER/cytosolic α-mannosidase, lysosomal α-mannosidase etc. The first enzyme plays a role in N-glycan biosynthesis and the latter two play roles in glycoprotein catabolism. To date, the crystal structures of two class II α-mannosidases, Drosophila melanogaster Golgi α-mannosidase II (DGMII; van den Elsen et al., 2001 ▶) and bovine lysosomal α-mannosidase (BLAM; Heikinheimo et al., 2003 ▶), have been determined. The zinc ion at the catalytic site of DGMII contributes to substrate binding by coordinating hydroxyl O atoms O2 and O3 of substrate analogues. The zinc ion also appears to play a role in the catalysis by stabilizing intermediate states of the sugar ring.
We have previously reported the cloning of a putative cytosolic α-mannosidase gene from Thermotoga maritima and characterization of the recombinant enzyme (TM1851) expressed in Escherichia coli (Nakajima et al., 2003 ▶). Cobalt and cadmium ions are the most favourable divalent ions for the activity of TM1851 and the enzyme showed no activity in their absence, indicating that an apo form was obtained. Such metal dependency is unique to TM1851; the zinc ion in DGMII, for example, is tightly bound and cannot be removed with EDTA (Rabouille et al., 1999 ▶). TM1851 exhibits about 20% amino-acid sequence identity to ER/cytosolic α-mannosidase from Rattus norvegicus. On the other hand, it exhibits very low identities to DGMII and BLAM; in particular, the C-terminal regions of these enzymes cannot be aligned at all. Therefore, TM1851 is an ER/cytosolic type α-mannosidase. In this paper, we describe the crystallization and preliminary X-ray analysis of TM1851.
2. Experimental procedures and results
2.1. Expression and purification
Recombinant TM1851 was expressed in E. coli as described previously (Nakajima et al., 2003 ▶). A crude extract was heated to 343 K for 30 min to remove E. coli proteins. After centrifugation (15 000g for 30 min), the supernatant was filtered through a 0.22 µm filter and loaded onto a Q-Sepharose Fast Flow (Amersham Biosciences, Piscataway, NJ, USA) column equilibrated with 20 mM Tris–HCl pH 7.5. The sample was eluted with a linear gradient of 0–1.0 M NaCl. The active fractions were combined and dialyzed against 20 mM sodium phosphate pH 6.8. The sample was loaded onto a hydroxylapatite (Bio-Rad Laboratories, Hercules, CA, USA) column and eluted with linear gradient of sodium phosphate (20–400 mM). The concentrated protein was then loaded onto a Superdex 200 HiLoad 16/60 column (Amersham Biosciences) equilibrated with 5 mM sodium phosphate pH 6.8 (buffer A) containing 150 mM NaCl and eluted with the same buffer. The amount of purified protein obtained from 6 l of culture was about 93 mg. The specific enzymatic activity of the purified sample was 4.2 µmol min−1 mg−1 and the sample migrated as a single band on native PAGE.
2.2. Crystallization
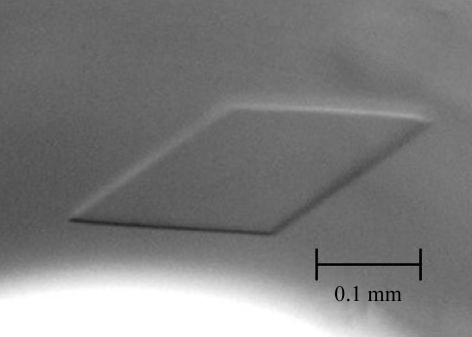
The crystallization conditions were initially screened by means of the sitting-drop vapour-diffusion method at 298 K using the sparse-matrix kits Crystal Screens 1 and 2, Index Screen (Hampton Research, Aliso Viejo, CA, USA), Wizard 1 and 2 and Cryo 1 and 2 (Emerald BioSystems, Bainbridge Island, WA, USA) and Structure Screens 1 and 2 (Molecular Dimensions Inc., FL, USA). 1 µl of a 10 mg ml−1 solution of TM1851 in buffer A was mixed with an equal volume of reservoir solution. Crystals of TM1851 were grown from drops of various compositions, including No. 8 from the Wizard 2 kit [10%(w/v) polyethylene glycol 8000, 100 mM sodium/potassium phosphate pH 6.2, 0.2 M NaCl]. Finally, the optimal crystallization condition was obtained with a solution containing 4%(w/v) polyethylene glycol 6000, 50 mM sodium phosphate pH 6.0, 0.5 M NaCl and 5.3 mg ml−1 protein by the oil-batch method. After incubation for about 1 d at 298 K, crystals of approximately 0.3 × 0.2 × 0.05 mm in size (Fig. 1 ▶) were formed.
Figure 1.
Crystal of TM1851.
2.3. Data collection
Diffraction data were collected using a charge-coupled device (CCD) camera (ADSC Quantum 210) at the NW12A station of the Photon Factory AR, High Energy Accelerator Research Organization (KEK), Tsukuba, Japan (λ = 1.000 Å). Prior to data collection, the crystals were briefly immersed in a cryoprotectant solution comprising 25%(v/v) polyethylene glycol 400 in the mother liquor and then flash-cooled in a stream of nitrogen gas maintained at 100 K. Diffraction images were indexed, integrated and scaled using the HKL2000 program suite (Otwinowski & Minor, 1997 ▶).
The crystals of TM1851 belong to the C-centred monoclinic space group C2 and diffract to a resolution of 2.9 Å. The data-collection statistics are presented in Table 1 ▶. Assuming the presence of two monomers of TM1851 per asymmetric unit, the calculated V M value (Kantardjieff & Rupp, 2003 ▶) and solvent content were 3.3 Å3 Da−1 and 62.9%, respectively. The native Patterson map did not show a strong peak, indicating that the molecules in the asymmetric unit are not related by pseudo-translation. A plot of self-rotation functions calculated in polar coordinates with the rotation angle (κ) set to 180° displayed two strong peaks separated by 90°. Both of these peaks are perpendicular to the peak of the crystallographic twofold rotation axis (unit cell b axis) and parallel to the ac plane. Plots of rotation functions calculated with κ = 72, 90 or 120° showed only diffuse features. These results indicate that there are two molecules in the asymmetric unit related by a non-crystallographic twofold axis perpendicular to the crystallographic twofold axis. All attempts at molecular replacement using search models based on the DGMII and BLAM structures, including the whole molecule and truncated fragments of various lengths, failed. Further attempts involving multiple isomorphous replacement or multiwavelength anomalous diffraction methods are required to solve the three-dimensional structure of TM1851.
Table 1. Data-collection statistics.
Values in parentheses are for the highest resolution shell.
| Space group | C2 |
| Unit-cell parameters | |
| a (Å) | 244.7 |
| b (Å) | 87.4 |
| c (Å) | 166.6 |
| β (°) | 124.7 |
| Resolution range (Å) | 50.00-2.90 (3.00–2.90) |
| Measured reflections (I > 3σ) | 113074 (9043) |
| Unique reflections | 60563 (5011) |
| Completeness (%) | 94.3 (78.8) |
| Redundancy | 1.8 (1.8) |
| Mean I/σ(I) | 9.6 (1.8) |
| Rmerge† (%) | 8.9 (29.1) |
| Space group | C2 |
R
merge = 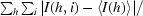
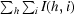 , where I(h, i) is the intensity of the ith measurement of reflection h and 〈I(h)〉 is the mean value of I(h, i) for all i measurements.
, where I(h, i) is the intensity of the ith measurement of reflection h and 〈I(h)〉 is the mean value of I(h, i) for all i measurements.
Acknowledgments
We wish to thank the staff of the Photon Factory, KEK for data collection. This work was supported by the National Project on Protein Structural and Functional Analysis and the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN).
References
- Coutinho, P. M. & Henrissat, B. (1999). Recent Advances in Carbohydrate Bioengineering, edited by H. J. Gilbert, G. J. Davies, B. Henrissat & B. Svensson, pp. 3–12. Cambridge: The Royal Society of Chemistry.
- Elsen, J. M. van den, Kuntz, D. A. & Rose, D. R. (2001). EMBO J.20, 3008–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikinheimo, P., Helland, R., Leiros, H. K., Leiros, I., Karlsen, S., Evjen, G., Ravelli, R., Schoehn, G., Ruigrok, R., Tollersrud, O. K., McSweeney, S. & Hough, E. (2003). J. Mol. Biol.327, 631–644. [DOI] [PubMed] [Google Scholar]
- Herscovics, A. (1999). Biochim. Biophys. Acta, 1473, 96–107. [DOI] [PubMed] [Google Scholar]
- Kantardjieff, K. A. & Rupp, B. (2003). Protein Sci.12, 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, M., Imamura, H., Shoun, H. & Wakagi, T. (2003). Arch. Biochem. Biophys.415, 87–93. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Rabouille, C., Kuntz, D. A., Lockyer, A., Watson, R., Signorelli, T., Rose, D. R., van den Heuvel, M. & Roberts, D. B. (1999). J. Cell Sci.112, 3319–3330. [DOI] [PubMed] [Google Scholar]