Abstract
Magnetic source imaging was used to determine whether tonotopy in auditory cortex of individuals with tinnitus diverges from normative functional organization. Ten tinnitus subjects and 15 healthy controls were exposed to four sets of tones while magnetoencephalographic recordings were obtained from the two cortical hemispheres in sequence. A marked shift of the cortical representation of the tinnitus frequency into an area adjacent to the expected tonotopic location was observed. The Euclidean distance of the tinnitus frequency from the trajectory of the tonotopic map was 5.3 mm (SD = 3.1) compared with a distance of 2.5 mm (SD = 1.3) of a corresponding frequency in the healthy controls (t = 3.13, P < 0.01). In addition, a strong positive correlation was found between the subjective strength of the tinnitus and the amount of cortical reorganization (r = 0.82, P < 0.01). These results demonstrate that tinnitus is related to plastic alterations in auditory cortex. Similarities between these data and the previous demonstrations that phantom limb pain is highly correlated with cortical reorganization suggest that tinnitus may be an auditory phantom phenomenon.
Keywords: plasticity/magnetic source imaging
Subjective tinnitus is characterized by the perception of auditory signals experienced in the absence of any internal or external source of sound. This very disturbing phenomenon is experienced by 35% of the population at some point in their lives, and in about 1% of the population it seriously interferes with a person’s life (1–4). A central nervous system correlate of subjective tinnitus has long been sought, but has not been found (5–7). Based on previous findings from our group reporting a close association of phantom limb pain and reorganization of primary somatosensory cortex (8, 9), we hypothesized that tinnitus might be a phantom phenomenon (10) related to alterations of the tonotopic map in auditory cortex. To evaluate this possibility, magnetic source imaging was used to determine the tonotopic organization of the auditory cortex in groups of tinnitus subjects and healthy controls.
MATERIALS AND METHODS
Study Patients.
Ten right-handed tinnitus subjects with tonal tinnitus between 2,000 and 8,000 Hz and a maximal hearing loss of 25 dB below the normative hearing level and 15 right-handed controls with normal hearing and without tinnitus participated in the study. In the samples we have studied thus far, 80% of the patients complained of tonal as compared with noisiform tinnitus. This incidence is in accordance with that reported in the literature (11). The present data are thus relevant to the majority of tinnitus sufferers. The tinnitus subjects and controls were of comparable age [t = 1.81, not significant (n.s.)] and gender (χ2 = 0.03, n.s; see Table 1).
Table 1.
Demographic and clinical aspects of the tinnitus group
| Subject # | Age, yr | Gender | Affected ear | Tinnitus strength, MTI | Hearing loss at the tinnitus frequency, dB | Tinnitus frequency, Hz | Duration, yr |
|---|---|---|---|---|---|---|---|
| 2 | 31 | M | Left | 1.0 | 15 | 4,200 | 6 |
| 3 | 38 | M | Right | 5.3 | 10 | 4,000 | 2 |
| 5 | 32 | M | Both | 4.3 | 10 | 5,900 | 3 |
| 6 | 35 | M | Both | 4.0 | 14 | 6,000 | 1 |
| 7 | 18 | F | Right | 3.3 | 13 | 4,000 | 1 |
| 11 | 36 | F | Right | 2.0 | 6 | 4,200 | 6 |
| 13 | 31 | F | Both | 4.6 | 10 | 6,000 | 2 |
| 15 | 29 | M | Right | 3.6 | 25 | 3,000 | 7 |
| 17 | 32 | M | Both | 4.6 | 20 | 4,000 | 5 |
| 18 | 20 | M | Left | 4.3 | 15 | 3,700 | 1 |
| Mean | 32.4 | 3.7 | 4,500 | 3.4 |
Tinnitus strength was assessed by the tinnitus severity scale of the MTI, which is composed of three items referring to the severity of the sounds at the present time, during the previous week, and the amount of suffering related to the sounds on a seven-point scale ranging from 0 = none to 6 = extreme.
Auditory Testing.
Hearing loss and tinnitus frequency were assessed by an audiogram. Only subjects with simple tonal tinnitus were included. A standard audiogram procedure was used where sounds of various intensities and frequencies were delivered and the tinnitus sound was identified by matching it to the sounds that were presented. In an additional procedure, the subjects self-produced the tinnitus sound by moving a cursor on a video screen that varied frequency and intensity of a sound until it matched the tinnitus sound. The maximum deviation of the two procedures was 50 Hz and 4 db; thus the determination of the tinnitus sound was very reliable. Four tinnitus subjects had right ear, two subjects had left ear, and four subjects had bilateral tinnitus. Subjectively experienced tinnitus strength was assessed by the German version of the West Haven-Yale Multidimensional Pain Inventory (12, 13), which was modified so that sounds instead of pain were assessed (thus comprising the Multidimensional Tinnitus Inventory or MIT). Its convergent validity, assessed by correlating it with the commonly used visual analogue scale, yielded a high (for validity studies) correlation coefficient of 0.53. The stability and internal consistency of the MTI scale is also excellent: Cronbach’s alpha = 0.93; test-retest reliability r = 0.91. Informed consent was obtained after the nature and possible consequences of the study were explained.
Auditory Stimulation.
Four sets of 200 pure tone pulses of 500 msec duration and 70 dB above the individual hearing level were presented sequentially to each ear with an interstimulus interval of 2 sec. The sequence of sets of tones and order of initial side of stimulation were determined by a random process. In the healthy controls, standard carrier frequencies of 1,000, 2,000, 4,000, and 8,000 Hz were used. In the tinnitus group, the three standard frequencies that were most distant from the tinnitus frequency were used as standard stimuli. The fourth tone was chosen to match the tinnitus frequency (mean = 4,500 Hz, SD = 1,100) and the standard tone that was closest to this frequency (4,000 Hz in all cases) was deleted from the tone series.
Neuromagnetic Source Imaging.
During auditory stimulation, magnetoencephalographic recordings were carried out in a magnetically shielded room by using a 37-channel neuromagnetometer (Magnes Biomagnetic Technologies, San Diego, CA). The stimulus-related fields were recorded from an area under a circular sensor array 14.4 cm in diameter centered over the auditory cortex of the two hemispheres in an order counterbalanced between subjects. The magnetic signals were sampled at a rate of 296 Hz, digitally filtered by using 0.1 Hz high-pass and 20 Hz low-pass filters and selectively averaged for each stimulus frequency. The location of the peak of the N1m component of the stimulus-related field was identified within the range of 50–160 msec by using a single equivalent current dipole (ECD) model in a homogeneous spherical volume conductor. N1m has been shown to be tonotopically represented in primary auditory cortex, mainly Brodman’s area 41. Actual latencies varied between 77 and 138 msec across subjects with a mean latency of 97 msec; there were no significant differences between the tinnitus subjects and healthy controls. The dipole locations were computed from a selection of points within a 50-msec time segment around the maximum rms amplitude across the 37 channels. Points were selected if they met the following requirements: (i) rms indicating a signal-to-noise ratio >3, (ii) goodness of fit of the ECD model to the measured field >0.90, and (iii) a minimal confidence volume of the ECD location <100 mm3. The equivalent current dipole locations of the standard frequencies are a logarithmic function of the stimulus frequencies with a main orientation in the medial-lateral direction along Heschl’s gyrus (14–16). The confidence volumes of the patients and controls at both the tinnitus and a control frequency were not significantly different (maximum t = 1.17, maximum P = 0.25). The investigators were blind to the perceptual data when analyzing the magnetoencephalographic records.
RESULTS
Reorganization of the Tonotopic Map.
The N1m dipole locations corresponding to the four standard frequencies formed a trajectory in three-dimensional space representing the tonotopic map of the healthy controls. The entire tonotopic map in primary auditory cortex extends approximately 15 mm in the medial-lateral dimension. In the tinnitus group, the three locations other than the tinnitus frequency were used to reconstruct the tonotopic map of each patient. The Euclidean distance between the line connecting the three standard-frequency dipole locations and the dipole location corresponding to the tinnitus frequency then was calculated. Reorganization of auditory cortex related to tinnitus might lead to a shift of the tinnitus frequency representation in the upper or lower frequency range within the tonotopic map or to a shift into adjacent cortical areas. The deviation of the tinnitus frequency from the trajectory of the standard frequencies provided a measure of invasion of the tinnitus frequency into neighboring regions. This distance was 5.3 mm (SD = 3.1) for the contralateral hemisphere (mean of both contralateral hemispheres in bilateral tinnitus subjects) and 3.2 mm (SD = 1.8) for the ipsilateral hemisphere. For comparison purposes, 4,000 Hz was chosen as the corresponding “tinnitus frequency” of the healthy controls. The mean Euclidean distance between this frequency and the trajectory formed by the three remaining standard frequencies was 2.5 mm (SD = 1.3) for the two hemispheres of the healthy controls. Thus, this distance was more than twice as large in the contralateral hemisphere of the tinnitus subjects as in both hemispheres of the healthy controls (t = 3.13, P < 0.01), indicating that there was an expansion of the tonotopic map in the tinnitus subjects. The Euclidean distance measure in the ipsilateral hemisphere of the tinnitus subjects was not significantly different from this measure in both hemispheres of the controls (t = 1.05, n.s.) nor were the distances in the contralateral and ipsilateral hemispheres in the tinnitus subjects significantly different from each other (t = 0.96, n.s.). Typical examples of the tonotopic maps of a tinnitus subject and of a healthy control are shown in Fig. 1. There was a hearing loss at the tinnitus frequency of 14 dB in tinnitus subjects as compared with controls (see Table 1). However, the amount of hearing loss was not significantly correlated with either amount of cortical reorganization (r = −0.16) or subjective tinnitus strength (r = 0.09).
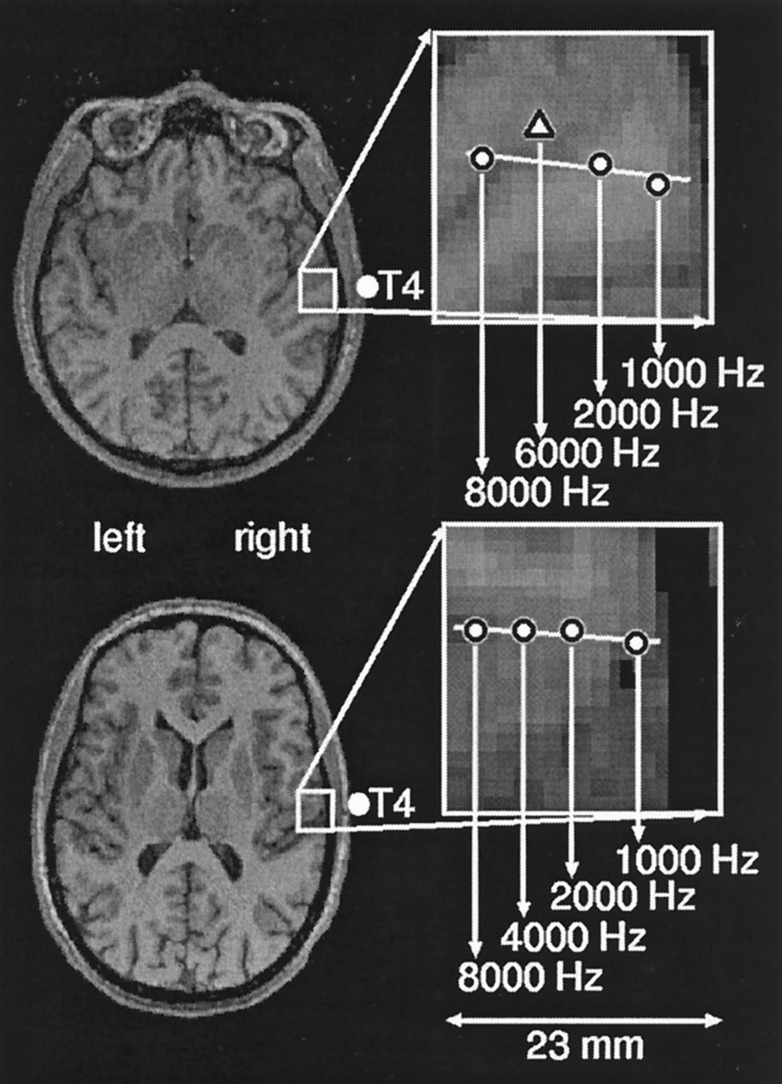
Figure 1.
A typical example of the tonotopic map is shown for a left ear tinnitus (Upper) and a control subject (Lower). Equivalent current dipoles elicited by auditory stimulation at the three standard and the tinnitus frequency in the tinnitus subject and the four standard frequencies in the healthy control are superimposed onto an axial slice of Brodman’s area 41 of the right hemisphere. The line in the upper portion of the figure shows the trajectory of the dipole locations of the three standard tones (circles). The triangle (Upper) represents the location of the tinnitus frequency (6,000 Hz in this case). Note that the trajectory of the dipole locations of the four standard frequencies in the healthy control subject (circles, Lower) is linear, whereas the dipole of the affected frequency in the tinnitus subject diverges from the linear trajectory established by the three standard frequencies. The location of T4 as well as the scale of measurement are marked.
Characterization of the Tonotopic Map.
The tonotopic map was further characterized by computing regression coefficients between the four log-transformed frequencies and their anatomical location in the medial-lateral dimension. In healthy subjects, the four frequencies typically are aligned in an ascending order from lateral to medial locations with regression coefficients above .90 (17). In the tinnitus subjects, the cortical map was distorted, showing a low mean regression coefficient for both the side contralateral (r = 0.29) and ipsilateral (r = 0.45) to the affected ear. Even if the highest correlation from either of the two hemispheres was chosen, it amounted only to r = 0.58 in the tinnitus subjects compared with 0.92 in the healthy controls (t = −2.80, P < 0.01), thus confirming the results from the Euclidean distance measure. When the tinnitus frequency was removed, the regression coefficient of the tinnitus subjects increased (mean r = 0.86) but was still lower than that of the healthy controls in this experiment (mean r = 0.98 calculated with three standard frequencies, t = −2.03, P < 0.05, one-sided) and those found in another study (17).
Relationship of Cortical Reorganization and Tinnitus Strength.
Spearman rank correlations were used to determine the relationship of tinnitus strength, as assessed with the MTI, and the deviation of the tinnitus frequency from the tonotopic map, as assessed with the Euclidean distance measure. This correlation was high for the contralateral hemisphere (r = 0.82, P < 0.01), although it was not significant (r = 0.15) for the ipsilateral hemisphere. Fig. 2 displays the relationship of tinnitus strength and amount of cortical reorganization. Tinnitus strength was uncorrelated with the latency of the tinnitus frequency (r = 0.20, n.s.), the dipole strength (r = 0.05, n.s.) and the regression coefficient characterizing the tonotopic map (r = 0.07, n.s.). The correlation between duration of tinnitus and amount of deviation from tonotopy at the tinnitus frequency also was not significant (r = −0.33).
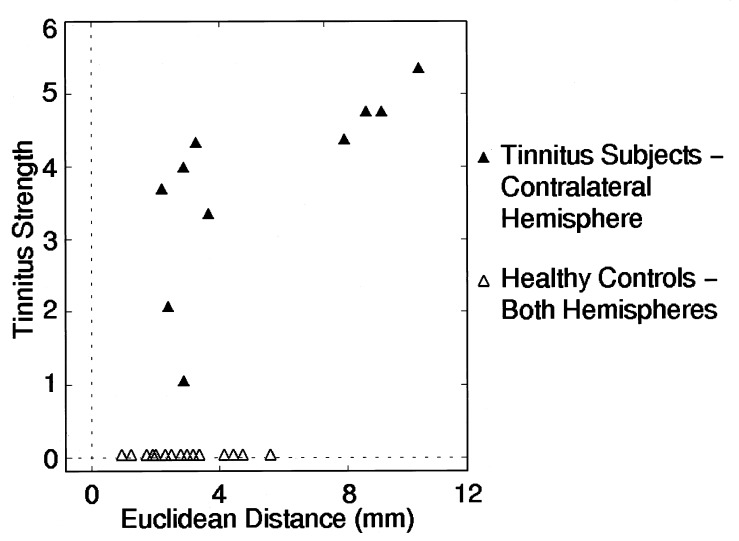
Figure 2.
Scatterplot of amount of subjective tinnitus strength and deviation of the tinnitus frequency from the tonotopic map in the contralateral hemisphere. A measure of deviation of the tinnitus frequency was obtained by determining the Euclidean distance between the trajectory of the standard tones and the location of the tinnitus frequency in tinnitus subjects or the corresponding comparison frequency in control subjects (see text). The tinnitus strength was assessed by the MTI. Greater subjective tinnitus strength was related to larger deviations from the trajectory of the standard frequencies. This figure suggests that there might not be a linear relationship between tinnitus and reorganization but rather a bimodal distribution of tinnitus sufferers with and without map distortions. The size of the sample studied is too small to clarify this point.
DISCUSSION
This study demonstrates that tinnitus is accompanied by a change of the tonotopic map in auditory cortex. Further, it is significant that there is a high positive association between subjective tinnitus strength and the amount of shift of the tinnitus frequency in auditory cortex. In previous magnetic source imaging research, our group showed that there is a very strong correlation between amount of reorganization of somatosensory cortex and amount of phantom limb pain in upper extremity amputees (9). There is a striking parallel between (i) the relationship of reorganization of the somatosensory cortex and phantom limb pain after upper extremity amputation and (ii) the relationship of the reorganization of auditory cortex and tinnitus. Moreover, both phenomena involve puzzling aversive perceptual experiences that are not fully accounted for by the status of structures in the periphery of the body. These parallels suggest that tinnitus is a type of auditory phantom phenomenon. This possibility had been the basis of previous speculations based on phenotypic analogizing to phantom limb pain (10), but the nature of the relationship could not be clearly identified before the association of phantom limb pain to cortical reorganization had been found (8).
Both increased input from peripheral stimulation (18–22) and loss of input caused by peripheral nerve (8, 9, 23–25) or dorsal root section (26) have been shown to produce reorganization of primary somatosensory cortex. Similar plastic reorganizational changes occur in auditory cortex with increases and decreases in input (27, 28). At this time, the mechanism and the causes of the displacement observed here are not known. Our data do not allow determination of whether it is loss of peripheral input or an increase of input along the auditory pathway that led to the changes that have been described here. However, they provide additional evidence that cortical reorganization has functional significance for the experience of an organism. It remains for future research to reveal whether tinnitus is maintained by cortical reorganization, whether cortical reorganization is a consequence of ongoing tinnitus, or whether both phenomena are triggered by a common source. Whatever the initial cause, however, the finding of a strong association between cortical reorganization and tinnitus opens the possibility that behavioral and pharmacological treatments aimed at altering cortical reorganization might be effective in relieving this thus far untreatable condition. Recanzone et al. (29) have shown that there is an increase in the representation of the frequencies involved in an auditory learning paradigm. These results could be used as a basis of a new therapeutic approach to tonal tinnitus by having patients attend to and discriminate some features of acoustic stimuli that are close to the tinnitus frequency to drive cortical reorganization of the nontinnitus frequencies into the tinnitus representation, thereby reducing it. Masking sounds have been used extensively in the treatment of tinnitus without reliable success (7). The new approach would differ by creating a situation where the stimuli were behaviorally relevant and were adjacent to and not in the tinnitus range.
Acknowledgments
This paper is based on W.M.’s doctoral dissertation. The majority of the data were collected while T.E. and W.M. were at the Institute of Experimental Audiology, University of Münster, Germany. This research was supported by grants from the Deutsche Forschungsgemeinschaft, the German American Academic Council (Transcoop Program), and Grant B95-975R from the Rehabilitation Research and Development Service, U.S. Department of Veterans Affairs.
ABBREVIATIONS
- n.s.
not significant
- MTI
Multidimensional Tinnitus Inventory
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.McFadden D. Tinnitus: Facts, Theories, and Treatments. Washington, DC: Natl. Acad. Press; 1982. [PubMed] [Google Scholar]
- 2.Coles R R. J Laryng Otol Suppl. 1984;9:7–15. doi: 10.1017/s1755146300090041. [DOI] [PubMed] [Google Scholar]
- 3.Coles R R. J Laryng Otol Suppl. 1984;9:195–202. doi: 10.1017/s1755146300090041. [DOI] [PubMed] [Google Scholar]
- 4.Axelsson A, Ringdahl A. In: Proceedings of the Third International Tinnitus Seminar. Feldmann H, editor. Karlsruhe, Germany: Hausch; 1987. pp. 154–158. [Google Scholar]
- 5.Hoke M, Feldmann H, Pantev C, Lütkenhöner B, Lehnertz K. Hear Res. 1989;37:281–286. doi: 10.1016/0378-5955(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 6.Tonndorf J. In: Tinnitus: Diagnosis/Treatment. Schulman A, Aran J M, Feldmann H, Tonndorf J, Vernon J A, editors. Philadelphia: Lea & Febiger; 1987. pp. 41–49. [Google Scholar]
- 7.Feldmann H, Lenarz T, von Wedel H. Tinnitus. Stuttgart, Germany: Thieme; 1992. [Google Scholar]
- 8.Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W, Taub E. NeuroReport. 1994;5:2593–2597. doi: 10.1097/00001756-199412000-00047. [DOI] [PubMed] [Google Scholar]
- 9.Flor H, Birbaumer N, Braun C, Elbert T, Ross B, Hoke M. Nature (London) 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 10.Jastreboff P W. Neurosci Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- 11.Harrop-Griffiths J, Katon W, Dobie R, Sakai C, Russo J. J Pysochosom Res. 1987;31:613–621. doi: 10.1016/0022-3999(87)90040-7. [DOI] [PubMed] [Google Scholar]
- 12.Flor H, Rudy T E, Birbaumer N, Streit B, Schugens M M. Der Schmerz. 1990;4:82–87. doi: 10.1007/BF02527839. [DOI] [PubMed] [Google Scholar]
- 13.Kerns R D, Turk D C, Rudy T E. Pain. 1985;23:345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 14.Romani G L, Williamson S J, Kaufman L. Science. 1982;216:1339–1340. doi: 10.1126/science.7079770. [DOI] [PubMed] [Google Scholar]
- 15.Pantev C. Electroenceph Clin Neurophysiol. 1988;25:54–61. [Google Scholar]
- 16.Yamamoto T, Williamson S J, Kaufman L, Nicholson C, Llinas R. Proc Natl Acad Sci USA. 1988;85:8732–8736. doi: 10.1073/pnas.85.22.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantev C, Bertrand O, Eulitz C, Verkindt C, Hampson S, Schuierer G, Elbert T. Electroenceph Clin Neurophysiol. 1995;94:26–40. doi: 10.1016/0013-4694(94)00209-4. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins W M, Merzenich M M, Ochs M T, Allard T, Guic-Robles E. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 19.Recanzone G H, Jenkins W M, Hradek G T, Merzenich M M. J Neurophysiol. 1992;67:1015–1030. doi: 10.1152/jn.1992.67.5.1015. [DOI] [PubMed] [Google Scholar]
- 20.Recanzone G H, Merzenich M M, Jenkins W M. J Neurophysiol. 1992;67:1057–1070. doi: 10.1152/jn.1992.67.5.1057. [DOI] [PubMed] [Google Scholar]
- 21.Recanzone G H, Merzenich M M, Jenkins W M, Grajski K A, Dinse H R. J Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 22.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 23.Yang T T, Gallen C, Schwartz B, Bloom F E, Ramachandran V S, Cobb S. Nature (London) 1994;368:592–593. doi: 10.1038/368592b0. [DOI] [PubMed] [Google Scholar]
- 24.Merzenich M M, Nelson R J, Stryker M P, Cynader M S, Shoppmann A, Zook J. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- 25.Florence S L, Kaas J H. J Neurosci. 1995;15:8083–8095. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pons T P, Garraghty P E, Ommaya A K, Kay J H, Taub E, Mishkin M M. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 27.Rajan R, Irvine D R, Wise L Z, Heil P. J Comp Neurol. 1993;338:17–49. doi: 10.1002/cne.903380104. [DOI] [PubMed] [Google Scholar]
- 28.Robertson D, Irvine D R. J Comp Neurol. 1989;89:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- 29.Recanzone G H, Schreiner C E, Merzenich M M. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]