Abstract
Objective
We assessed birth patterns of patients with idiopathic inflammatory myopathies (IIM), hypothesizing that seasonal early environmental exposures influence later development of autoimmune disease.
Methods
Juvenile-onset patients (n = 307) and healthy controls (n = 3,942) were born from 1970−1999, and adult-onset patients (n = 668) and controls (n = 6,991) from 1903−1982. Birth dates were analyzed as circular data. Seasonal clustering was assessed by the Rayleigh test, and differences between groups by a rank-based Uniform-Scores Test.
Results
The overall birth distributions of juvenile IIM patients and controls did not significantly differ nor did those of adult IIM patients vs. controls. Subgroups of juvenile IIM showed seasonal birth distributions: Hispanic juvenile IIM patients had a seasonal birth pattern (mean October 16), differing (p = 0.002) from those of Hispanic controls (uniform birth distribution) and from non-Hispanic juvenile patients (mean May 2, p < 0.001). Juvenile dermatomyositis patients with p155 autoantibody differed in birth distribution (p = 0.003) from p155 antibody-negative juvenile dermatomyositis patients. Juvenile IIM patients with the human leukocyte antigen (HLA) risk factor allele DRB1*0301 differed in birth distribution (p = 0.021) from those without the allele. Similar results were observed for juvenile and adult IIM patients with the linked allele DQA1*0501. No significant seasonal birth patterns were found for other subgroups.
Conclusion
Birth distributions appear to have stronger seasonality in juvenile than adult IIM subgroups, suggesting greater influence of perinatal exposures on childhood-onset illness. Seasonal early-life exposures may influence the onset of some autoimmune diseases later in life.
Keywords: Myositis, Environmental Factors, Autoimmune Diseases
Recent studies have directed attention to the potential role that environmental exposures during early development may play in a variety of health outcomes later in life, including the development of cardiovascular disease, Type 1 and Type 2 diabetes, cancer, asthma, osteoporosis, and neurological illness (1). The potential role of such exposures in the development of autoimmune disease is beginning to be explored (2).
If an environmental exposure experienced during a particular interval during prenatal or early postnatal life contributes to the risk of a disease later in life and varies seasonally, then a corresponding seasonal pattern in birth dates of patients with the resulting disease should be observed. Identifying such seasonal birth patterns could be a first step in identifying environmental exposures that might contribute to the disease process during development.
There is some evidence of seasonal patterns of birth in patients with immune mediated diseases such as childhood asthma (3) and potentially immune mediated diseases such as narcolepsy (4) and schizophrenia (5;6). Several studies have examined whether there are early life influences on the later development of autoimmune disease. Studies of Type 1 diabetes, multiple sclerosis, and inflammatory bowel disease have shown inconsistent evidence of seasonal birth patterns (7-11). A number of these studies, however, may have been limited by examining birth season in heterogeneous patient populations (12), or by use of insensitive statistical approaches to examine seasonality, with the treatment of birth date as a linear, rather than a circular, variable (13;14). The relevance of these studies of organ-specific autoimmune disease to systemic autoimmunity is also unknown.
The idiopathic inflammatory myopathies (IIM), or myositis syndromes, are a heterogeneous group of rare, systemic autoimmune diseases characterized by chronic muscle weakness due to inflammation of unknown cause. The IIM can be classified into clinical subgroups based on the presence of certain clinical and histopathologic features and serologic subgroups based on the presence of myositis-specific or myositis-associated autoantibodies (15). There are several genetic risk factors for the IIM, particularly in the major histocompatibility (MHC) region (16), but no genetic risk factor is sufficient to cause disease, indicating a potential role for environmental exposures.
The present study examines whether the birth dates of patients with the IIM show seasonal patterns, and thus a potential role for seasonally varying early environmental factors on disease development later in life. Because of the heterogeneity of the IIM, the study also examines whether there is birth seasonality in demographic, clinical, serologic or immunogenetic subgroups of juvenile- and adult-onset IIM.
Methods
Subjects
Previously established databases of juvenile-onset and adult-onset IIM, with patients enrolled under informed consent, were used in this study. The source of the juvenile-onset IIM patients was a database that had been previously established for National Institutes of Health (NIH) or Food and Drug Administration (FDA) laboratory, immunogenetic, and autoantibody studies as part of the ongoing Childhood Myositis Heterogeneity Study (17). Juvenile IIM patients were included in the current study if they met the following criteria: met probable or definite Bohan and Peter criteria for IIM with disease onset before age 18 (18), had birth date reported, were born between 1970 and 1999, and resided in the contiguous U.S., with a resulting sample size of 307 juvenile IIM patients.
The source of the adult-onset IIM patients was a database that had been previously established for National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) ongoing therapeutic trials or natural history studies of myositis (19-22) or FDA immunogenetic and autoantibody studies. Geographic information was not available for these patients, but they came to the NIH Clinical Research Center in Bethesda, Maryland to participate in the study. Adult IIM patients were included in the current study if they met the following criteria: met probable or definite Bohan and Peter criteria for adult IIM (18), had birth date reported, and were born between 1903 and 1982, with a resulting sample size of 668 adult IIM patients.
Serum samples of a subset of patients were tested for myositis-specific and myositis-associated autoantibodies by immunoprecipitation methods (23). Anti-p155 autoantibodies were assessed as previously described (17). A subset of IIM patients was genotyped for MHC class II alleles (16).
Because birth season has been shown to vary in the general population (24), a control group was used for comparison. The source of controls was a NIH Healthy Volunteer Office database. Self-reported medical history information was available for 223 of the potential controls and seventy-six of these individuals reported to have autoimmune or immune-mediated conditions were excluded. Controls were included in the study if they had self-reported information on birth date and resided in the contiguous U.S. Juvenile controls were born between 1970 and 1999, with a sample size of 3,942, and adult controls were born between 1903 and 1982, with a sample size of 6,991. Institutional review board approval was granted for this study, and included use of the control database. Race/ethnicity was self-reported for patients and controls.
Statistical Methods
Directional (circular) statistical methods (14) were used to allow for the cyclic nature of time (December 31 being adjacent to January 1), with birth dates represented as angles on the unit circle (14). These angles indicate the direction of unit vectors such that the mean direction, or the direction of the resultant, is an angle that corresponds to the mean birth date. Mean birth dates and estimates of 95% confidence intervals were calculated on the circle, using Nicholas Cox's SSC package “Circular” in STATA 8 (StataCorp, College Station, Texas, USA), and then converted back to the nearest birth date. If birth dates are uniformly distributed throughout the year (around the circle), then the 95% confidence interval will be so wide that the mean birth date is not useful.
In addition to a direction corresponding to the mean birth date, the resultant has a length associated with the strength of seasonality (Figure 1). The vector sum, with the mean resultant length, R, is equal to ((mean cosine)2 + (mean sine)2)1/2. As described by Mardia & Jupp, if birth dates are tightly clustered in a season, the corresponding unit vectors will tend to point in the same direction and the mean resultant (R) will consequently be long, or close to one (14). In contrast, if birth dates are completely uniform around a circle, then the mean resultant length will be zero. Thus, the mean resultant length provides a way to test a distribution of birth dates for seasonality (14).
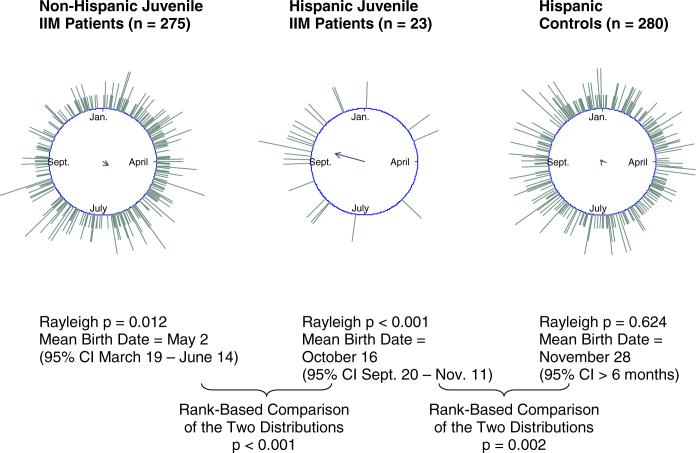
Figure 1. Hispanic juvenile idiopathic inflammatory myopathy (IIM) patients have a seasonal birth distribution that differs from non-Hispanic juvenile IIM patients and Hispanic controls.
When birth dates are represented on a unit circle, the direction of the resultant is the angle that corresponds to the mean birth date. The mean resultant length, along with the sample size, is used in the Rayleigh test to assess the strength of seasonality against the null hypothesis of uniformity (14). The long resultant for the birth distribution for Hispanic juvenile IIM patients indicates strong seasonality, while the short resultant for the birth distribution for Hispanic controls indicates a birth distribution that is not significantly different from uniformity.
Birth distributions of individual samples were tested for seasonality (i.e. violations of uniformity) using the Rayleigh Test, in which the test statistic 2nR2 is compared to a chi-square distribution with two degrees of freedom (14). The null hypothesis of a uniform distribution of births throughout the year is rejected when the test statistic is sufficiently large.
In order to test whether the birth distributions of two samples differed significantly from each other, a non-parametric rank-based test, the “Uniform-Scores Test”, was used (14). The null hypothesis is that the pattern of births throughout the year (distribution around the circle) is the same in the two populations. Ranked observations from the two samples are treated as a combined sample of distinct equidistant points on a circle. The distribution of points originating from one of the samples is then tested for uniformity (14). The analyses were conducted using Nicholas Cox's SSC package “Circular” in STATA 8 (StataCorp, College Station, Texas, USA).
Analyses were conducted to assess the need for stratifying by birth decade or by geography. Birth distributions were analyzed by decade for patients. In order to assess the potential impact of latitude within the U.S., the contiguous U.S. was divided at approximately 40 degrees north latitude into 24 northern states and 25 southern states including Washington, D.C. Birth distributions were compared between northern and southern states for juvenile IIM patients, juvenile controls, and adult controls. In addition, because the majority of controls were from Maryland, Virginia, or Washington, D.C., birth distributions for individuals from this region were compared to those from the rest of the country. These analyses could not be conducted for adult IIM patients because their geographic information was not available.
The birth distributions of juvenile- and adult-onset IIM patients were compared to those of controls, and the birth distributions of demographic, clinical, serologic, and immunogenetic subgroups of juvenile- and adult-onset IIM were compared to each other. As this was an exploratory study, p-values of less than 0.05 were considered significant, and adjustment was not made for multiple comparisons.
Results
The overall birth distributions for all IIM patients did not show significant seasonality and did not differ from the birth distributions of controls. The birth distributions of juvenile-onset IIM patients and controls were similar, with mean birth dates in early May (Table 1). Neither the birth distribution of adult IIM patients nor that of adult controls displayed a significant seasonal pattern, and these two distributions did not differ from each other (Table 2).
Table 1.
Subgroups of Juvenile Idiopathic Inflammatory Myopathy (IIM) Patients and Controls That Did Not Differ in Birth Distribution.
| Group | Sample Size | Mean Birth Date | 95% Confidence Interval | Rayleigh p-value for Uniformity | Rank-Based p-value for Difference |
|---|---|---|---|---|---|
| Juvenile IIM | 307 | May 3 | * | p = 0.177 | p = 0.545 |
| Juvenile Controls | 3,942 | May 10 | March 17 — July 3 | p = 0.048 | |
| Non-Caucasian Juvenile IIM | 85 | June 4 | * | p = 0.777 |  |
| Caucasian Juvenile IIM | 206 | May 8 | * | p = 0.175 | |
| Caucasian Juvenile Controls | 2,372 | May 15 | March 30 — June 30 | p = 0.021 | |
| Non-African-American Juvenile IIM | 249 | May 10 | * | p = 0.635 | 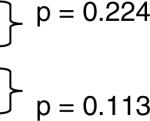 |
| African American Juvenile IIM | 51 | May 4 | March 3 — July 5 | p = 0.077 | |
| African American Juvenile Controls | 835 | July 23 | * | p = 0.302 | |
| Juvenile Dermatomyositis | 270 | April 25 | * | p = 0.318 | p = 0.672 |
| Juvenile Polymyositis | 37 | May 26 | * | p = 0.369 | |
| Myositis-Specific Autoantibody Positive (Anti-Jo1, anti-alanyl tRNA synthetase, anti-Mi2, or anti-signal recognition particle) Juvenile IIM | 22 | May 18 | * | p = 0.509 | p = 0.692 |
| Myositis-Specific Autoantibody Negative Juvenile IIM | 171 | April 9 | * | p = 0.589 | |
| Female Juvenile IIM | 221 | May 20 | * | p = 0.236 | p = 0.645 |
| Male Juvenile IIM | 86 | March 23 | * | p = 0.486 | |
95% confidence interval is wider than six months.
Table 2.
Comparison of Birth Distributions between Subgroups of Adult Idiopathic Inflammatory Myopathy (IIM) Patients and Adult Controls or Subgroups.
| Group | Sample Size | Mean Birth Date | 95% Confidence Interval | Rayleigh p-value for Uniformity | Rank-Based p-value for Difference |
|---|---|---|---|---|---|
| Adult IIM | 668 | September 6 | * | p = 0.301 | p = 0.375 |
| Adult Controls | 6,991 | June 25 | * | p = 0.205 | |
| Non-Caucasian Adult IIM | 196 | October 6 | * | p = 0.160 | 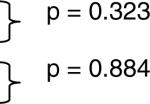 |
| Caucasian Adult IIM | 472 | July 29 | * | p = 0.615 | |
| Caucasian Adult Controls | 4,199 | June 29 | * | p = 0.160 | |
| Non-African American Adult IIM | 537 | August 30 | * | p = 0.582 | 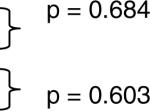 |
| African American Adult IIM | 131 | September 15 | * | p = 0.362 | |
| African American Adult Controls | 1,690 | August 15 | * | p = 0.197 | |
| Anti-synthetase Autoantibody Positive (anti-Jo1, anti-alanyl, glycyl, threonyl, or isoleucyl tRNA synthetase) | 88 | November 24 | * | p = 0.709 | p = 0.396 |
| Myositis-Specific Autoantibody Negative | 195 | August 8 | * | p = 0.165 | |
| Adult Polymyositis | 351 | September 2 | June 15 — November 21 | p = 0.131 | 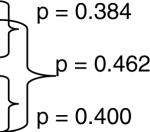 |
| Adult Dermatomyositis | 237 | May 27 | * | p = 0.828 | |
| Adult Inclusion Body Myositis | 70 | November 27 | * | p = 0.499 | |
| HLA DRB1*0301 Positive Adult IIM | 125 | September 17 | * | p = 0.754 | p = 0.966 |
| HLA DRB1*0301 Negative Adult IIM | 150 | September 18 | * | p = 0.870 | |
| HLA DQA1*0501 Positive Adult IIM | 153 | September 19 | July 9 — November 29 | p = 0.118 | p = 0.050 |
| HLA DQA1*0501 Negative Adult IIM | 133 | March 13 | * | p = 0.447 | |
| Female Adult IIM | 439 | August 27 | * | p = 0.474 | p = 0.931 |
| Male Adult IIM | 229 | September 23 | * | p = 0.592 | |
95% confidence interval is wider than six months
When patients' birth patterns were assessed by decade, no significant seasonal patterns were found in any decade (results not shown). No significant differences were observed between birth patterns of juvenile IIM patients (rank-based p = 0.459), juvenile controls (rank-based p = 0.485), or adult controls (rank-based p = 0.178) from southern vs. northern states. No significant differences were observed between the birth distributions of juvenile IIM patients (rank-based p = 0.263), juvenile controls (rank-based p = 0.951), or adult controls (rank-based p = 0.599) from Maryland, Virginia, or Washington D.C. compared to those from all other states. Based on these findings, all further analyses were conducted over the entire study time period and geographic region in order to increase statistical power.
Certain subgroups of myositis patients had birth distributions that demonstrated seasonal patterns. Hispanic juvenile IIM patients had a non-uniform birth distribution with a mean birth date of October 16 (Rayleigh p < 0.001). This distribution differed from that of juvenile IIM patients who are not Hispanic (rank-based p < 0.001) and from that of Hispanic controls (rank-based p = 0.002) (Figure 1). Caucasian and African American juvenile IIM patients did not have seasonal birth distributions and did not differ in birth distribution from other juvenile IIM patients or controls (Table 1). No significant differences in birth distribution were found between the major clinical subgroups of juvenile IIM: dermatomyositis (DM) and polymyositis, or between male and female juvenile IIM patients (Table 1).
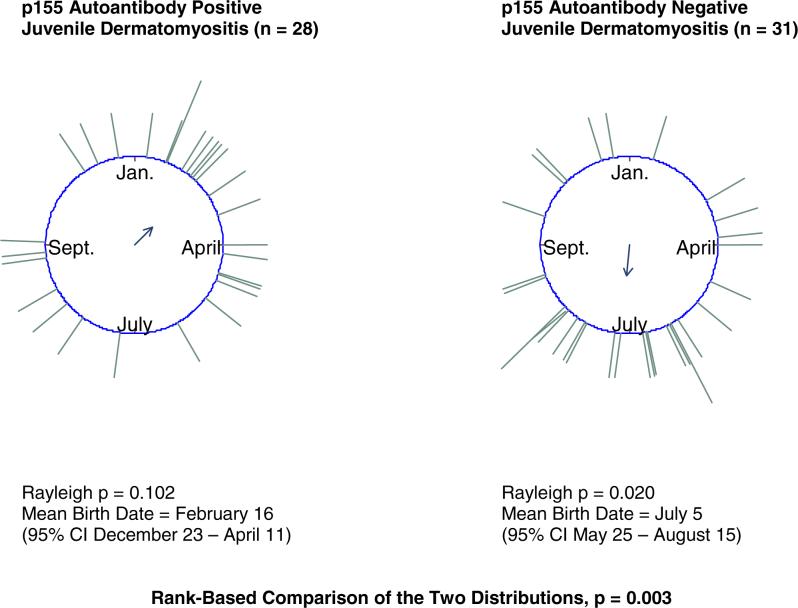
The birth distribution of juvenile DM patients with the p155 autoantibody, a newly described myositis-associated autoantibody strongly associated with DM (17), differed (rank-based p = 0.003) from that of juvenile DM patients negative for the autoantibody (Figure 2). The birth distribution for juvenile DM patients with the p155 autoantibody appeared skewed towards late winter, but the pattern was not statistically significantly seasonal (Rayleigh p = 0.102). The birth distribution for juvenile DM patients who tested negative for p155 had a seasonal pattern with a mean birth date of July 5 (Rayleigh p = 0.020). There was no significant difference in birth distribution between juvenile IIM patients with and without myositis-specific autoantibodies (Table 1).
Figure 2.
p155 autoantibody positive juvenile dermatomyositis patients differ in birth distribution from p155 autoantibody negative juvenile dermatomyositis patients.
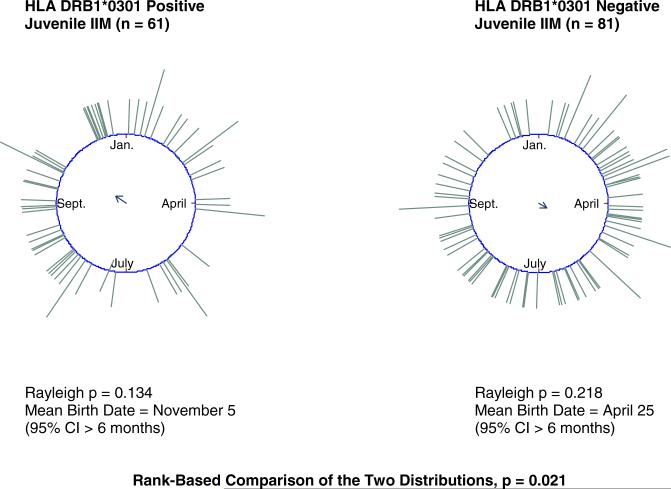
The birth distribution of juvenile IIM patients positive for the human leukocyte antigen (HLA) risk factor allele DRB1*0301 differed (rank-based p = 0.021) from that of juvenile IIM patients negative for the allele (Figure 3). Similar differences in birth seasonality were found between juvenile IIM patients with and without the DRB1*0301-linked risk factor allele, DQA1*0501.
Figure 3.
HLA DRB1*0301 positive juvenile IIM patients differ in birth distribution from HLA DRB1*0301 negative juvenile IIM patients.*
There was a borderline statistically significant difference (p = 0.050) between adult IIM patients positive and negative for HLA DQA1*0501, but neither group showed significant birth seasonality by the Rayleigh test (Rayleigh p = 0.118 and 0.447, respectively). No significant differences were found between other adult IIM patient subgroups tested: patients with and without the HLA DRB1*0301 allele, or by gender, major clinical subgroups (polymyositis, DM, and inclusion body myositis), patients with and without anti-synthetase autoantibodies, or racial groups (Table 2). Sample sizes were not sufficient for birth date analysis of Hispanic adult IIM patients or p155 autoantibody positive adult DM patients.
Discussion
Several subgroups of juvenile IIM patients demonstrated seasonal birth patterns that differed significantly from other juvenile IIM patients or from controls, while only one subgroup of adult IIM patients demonstrated some evidence of a pattern of birth that differed from that of other adult IIM patients. The lack of a distinct overall seasonal pattern in births of IIM patients may be due to etiologic heterogeneity of the IIM. These findings suggest that some forms of the IIM, particularly in subgroups of patients with childhood–onset disease, are influenced by seasonal environmental exposures during early development, while other forms are not. In the IIM, seasonal influences during gestation or shortly after birth might be more likely to have an impact earlier in life, leading to juvenile-onset disease. However, it is important to note that the sample size was too small to examine the birth distribution for Hispanic adult IIM patients and for adult IIM patients with the anti-p155 autoantibody. Therefore, it is not possible to tell whether the seasonal patterns of birth displayed for these subgroups were unique to juvenile IIM patients, because the birth distributions of adult IIM patients in these subgroups could not be determined.
Studies of the influences of exposures early in life on later disorders such as cardiovascular disease have focused primarily on birth weight and maternal nutrition (25). The seasonal birth distributions observed in certain subgroups of IIM patients might be explained by several potential genetic or environmental factors, including factors that are not related to maternal nutrition. Phillips has recently highlighted the evidence that prenatal toxic exposures, nutritional influences, antigen exposure, and infection may influence the development of autoimmune disease (2)
Parental occupational exposures such as silica (26) or seasonally applied agricultural pesticides (27) might play a role. Cord serum levels of pesticides have been associated with childhood asthma (28). For children fathered by pesticide workers in the Red River Valley of Minnesota, Garry et al. (29) found that the percent of birth defects was highest for those conceived in the spring, the season when herbicide application occurs in this region. A recent study of a primarily Hispanic population in Salinas Valley, an agricultural region of California, found that environmental exposures during the first year of life, including living with an agricultural worker, having a gas stove in the home, owning pets, and being breast-fed, were associated with the Th1/Th2 immune response at age two (30).
Other potential exposures include infectious agents (8). Outbreaks of illness occur in distinct seasonal patterns for many pathogens (31). Early-life infection with seasonal pathogens, such as viruses, has been proposed as a mechanism leading to other autoimmune diseases, particularly Type 1 diabetes (8). It is also possible that the same factor can influence both susceptibility to infection (31) and susceptibility to autoimmune disease. Thus, meteorological factors that vary by season, such as ultraviolet light, might influence the immune system and the development of autoimmune disease (31-33).
Although there is some evidence for immune system modulation during early development, birth patterns may actually reflect the influence of seasonal factors acting at various time points: pre-conception, during gestation, shortly after birth, or during specific developmental windows later in childhood. Birth season is related not only to the timing of environmental exposures, but also to the timing of social exposures during childhood. For example, birth date is a determining factor in whether a child is amongst the oldest, youngest, or in the middle age-range for his or her grade level. Relative age within a school grade has been found to be associated with academic performance (34), with children of younger relative age being at a disadvantage. It is possible that differing levels of stress based on relative age within a school grade might influence immune function in children, thus potentially influencing childhood-onset autoimmune disease. While this is speculative, it demonstrates that birth patterns may be a surrogate for exposures at a variety of time points in life.
The seasonal pattern of birth observed for Hispanic juvenile IIM patients, with a peak in October, strongly differed from that of other juvenile IIM patients and from controls. Similarly, Laron et al. (12) found that birth patterns in patients with Type 1 diabetes can vary by ethnic group so that examining a heterogeneous population as a whole could mask differences between ethnic groups. It is also possible that Hispanics have genetic risk factors that predispose them to susceptibility to certain seasonal environmental risk factors (35).
The finding of a difference in birth pattern between juvenile-onset DM patients with and without the p155 autoantibody supports the hypothesis that a seasonal environmental exposure during gestation or shortly after birth might trigger an immune response that produces this autoantibody (17). It is interesting to note that adult IIM patients with anti-synthetase autoantibodies did not display a seasonal pattern of birth, but a previous study found that adults with anti-synthetase autoantibodies have seasonal patterns in the onset of their IIM (36). Thus, agents that influence the p155 autoantibody response might have a greater effect during early development, while factors that influence the production of anti-synthetase autoantibodies may have a greater influence later in life.
Differences in birth patterns were found between juvenile IIM patients with and without the HLA allele DRB1*0301 (16), with similar results for the linked risk factor allele, HLA DQA1*0501. The birth distributions of juvenile and adult IIM patients positive and negative for DQA1*0501, respectively, had similar mean birth dates, indicating that this allele might interact with environmental factors to influence both juvenile-onset and adult-onset IIM. The DQA1*0501 allele binds class II associated invariant chain peptide (CLIP) differently than other HLA alleles, suggesting that this allele has access to peptides earlier in the antigen processing pathway, thus potentially encountering novel peptides that induce autoimmunity (37). Similar to the present findings, HLA alleles have also been associated with birth season of schizophrenia patients (6) and with season of onset of Type 1 diabetes (38;39). Thus, genetic risk factors potentially interact with environmental influences during early development to result in disease onset later in life.
Despite attempts to decrease possible confounders in this study, several limitations remain. One potential limitation is that the IIM patients or controls might not be representative of the general population or that the controls might come from a population with different characteristics than the population of IIM patients. A control group from NIH was used due to the availability of birth dates for this sample, in contrast to broader population-based data.
There is some evidence that seasonal patterns of birth in the general population might be associated with socio-demographic factors such as maternal age, marital status, maternal education, and birth order (40). Information on these variables was unavailable for both the patients and controls so the possibility of confounding by any of these factors cannot be excluded.
A limitation of the study is that the geographical distribution of patients and controls within the U.S. was not the same. The majority of controls were from Maryland, Virginia, or Washington, D.C., while juvenile IIM patients were from throughout the contiguous U.S., and information was not available on the specific geographic location of adult IIM patients. While there is some potential for these geographic differences to bias the comparisons of patients to controls, significant differences were not found in our data when birth distributions of those from Maryland, Virginia, and Washington, D.C. were compared to those from the rest of the contiguous U.S.
It is possible that any seasonal variation in birth patterns might have been diluted by pooling data from across the contiguous U.S. There is some evidence that seasonal patterns of birth in the general population differ between northern and southern states and that this difference might be due to decreased conception during times of extreme heat (41). Although meteorological factors can vary across different regions of the U.S., our analyses found no significant differences in birth distributions between northern and southern states. Therefore, while the pooling of our data across the U.S. is a potential limitation, the effect on the results is likely to be minimal.
It is also possible that any seasonal variation in birth patterns might have been diluted by pooling data over a somewhat wide time period because the seasonal distribution of births can vary over time (42). However, many seasonal occurrences, such as infectious disease outbreaks, occur at relatively constant time points from year to year (31), and consequently the effect of pooling data across years should be minimal. No significant seasonal birth patterns were found when patients' birth dates were analyzed by decade. Some decade-matched analyses were performed in an attempt to correct for differences in birth years between patients and controls, and these results were reassuringly similar to those of the entire study group (data not shown).
Another limitation of this study is that several subgroup analyses were conducted, and some subgroups had small sample sizes. Independent studies of birth season in Hispanic, p155, and HLA subgroups of IIM are needed to verify the seasonal birth pattern findings in these patient groups. This is an exploratory analysis with small sample sizes and therefore all results with uncorrected p-values of less than 0.05 are considered to be of interest as they highlight potential associations that can be further assessed in future studies.
This work provides evidence that seasonal birth distributions of Hispanic juvenile IIM patients, juvenile IIM patients with the p155 autoantibody, and juvenile IIM patients with certain HLA alleles differ from the birth distributions of patients in other subgroups or from the birth distribution of a population of individuals not known to have an autoimmune disease. These subgroups of myositis patients may have been affected by specific seasonally varying environmental agents around the time of conception, during a certain period of gestation, or during early childhood. Our data suggest that early environmental influences have a greater influence on childhood-onset myositis than on adult-onset myositis. The finding of seasonal patterns of birth in certain subgroups of IIM patients, but not in the overall patient population, is consistent with the notion that different IIM subgroups are distinct entities that have different underlying causes. Further investigations, including studies of interactions among factors, are needed to elucidate the role of the environment in the development of the IIM.
Acknowledgements
We thank Dr. Gulnara Mamyrova and Ms. Bhanu Koneru for assistance with portions of the myositis databases. We thank members of the Childhood Myositis Collaborative Study Group, Dr. Paul Plotz, and other physicians for referral of patients to the study. We thank Drs. Michael Ward and Christine Parks for valuable feedback on the manuscript, and Drs. Patrick N. Breysse, Ellen K. Silbergeld and Michael T. Heaney for helpful suggestions on earlier drafts of the manuscript.
Grant Support: Leora Vegosen received funding from the NIEHS Summers of Discovery Program for this research. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences and National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Conflicts of Interest: We declare that we have no conflicts of interest.
Footnotes
Contributors: L Vegosen, C Weinberg, F Miller, and L Rider designed the study; L Rider and T O'Hanlon created the patient database; L Vegosen managed the database and performed the analysis and C Weinberg and L Rider supervised the analysis; T O'Hanlon performed HLA typing and I Targoff performed autoantibody testing of patients; F Miller and L Rider secured funding; L Vegosen and L Rider wrote the report; all authors corrected drafts of the manuscript.
A similar difference (rank-based p = 0.039) was observed between the birth distribution of juvenile IIM patients positive for the linked allele HLA DQA1*0501 (mean August 7, 95% CI > 6 months, Rayleigh p = 0.553) and HLA DQA1*0501 negative juvenile IIM patients (mean April 14, 95% CI March 2 - May 27, Rayleigh p = 0.018).
Reference List
- 1.Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips DI. External influences on the fetus and their long-term consequences. Lupus. 2006;15(11):794–800. doi: 10.1177/0961203306069354. [DOI] [PubMed] [Google Scholar]
- 3.Gazala E, Ron-Feldman V, Alterman M, Kama S, Novack L. The association between birth season and future development of childhood asthma. Pediatr Pulmonol. 2006;41(12):1125–1128. doi: 10.1002/ppul.20442. [DOI] [PubMed] [Google Scholar]
- 4.Dauvilliers Y, Carlander B, Molinari N, Desautels A, Okun M, Tafti M, et al. Month of birth as a risk factor for narcolepsy. Sleep. 2003;26(6):663–665. doi: 10.1093/sleep/26.6.663. [DOI] [PubMed] [Google Scholar]
- 5.Jones AL, Mowry BJ, Pender MP, Greer JM. Immune dysregulation and self-reactivity in schizophrenia: do some cases of schizophrenia have an autoimmune basis? Immunol Cell Biol. 2005;83(1):9–17. doi: 10.1111/j.1440-1711.2005.01305.x. [DOI] [PubMed] [Google Scholar]
- 6.Narita K, Sasaki T, Akaho R, Okazaki Y, Kusumi I, Kato T, et al. Human leukocyte antigen and season of birth in Japanese patients with schizophrenia. Am J Psychiatry. 2000;157:1173–1175. doi: 10.1176/appi.ajp.157.7.1173. [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM, Gutnikov SA, McKinney PA, Schober E, Ionescu-Tirgoviste C, Neu A. Seasonality of birth in children with diabetes in Europe: multicentre cohort study. European Diabetes Study Group. BMJ. 1999;319(7214):887–888. doi: 10.1136/bmj.319.7214.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ursic-Bratina N, Battelino T, Krzisnik C, Laron-Kenet T, Ashkenazi I, Laron Z. Seasonality of birth in children (0−14 years) with type 1 diabetes mellitus in Slovenia. J Pediatr Endocrinol Metab. 2001;14:47–52. doi: 10.1515/jpem.2001.14.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC. Timing of birth and risk of multiple sclerosis: population based study. BMJ. 2005;330:120. doi: 10.1136/bmj.38301.686030.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowers Y, Odes S, Bujanover Y, Eliakim R, Bar MS, Avidan B. The month of birth is linked to the risk of Crohn's disease in the Israeli population. Am J Gastroenterol. 2004;99:1974–1976. doi: 10.1111/j.1572-0241.2004.40058.x. [DOI] [PubMed] [Google Scholar]
- 11.Card TR, Sawczenko A, Sandhu BK, Logan RF. No seasonality in month of birth of inflammatory bowel disease cases: a prospective population based study of British under 20 year olds. Gut. 2002;51:814–815. doi: 10.1136/gut.51.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laron Z, Lewy H, Wilderman I, Casu A, Willis J, Redondo MJ, et al. Seasonality of month of birth of children and adolescents with type 1 diabetes mellitus in homogenous and heterogeneous populations. Isr Med Assoc J. 2005;7:381–384. [PubMed] [Google Scholar]
- 13.Le CT, Liu P, Lindgren BR, Daly KA, Scott GG. Some statistical methods for investigating the date of birth as a disease indicator. Stat Med. 2003;22:2127–2135. doi: 10.1002/sim.1343. [DOI] [PubMed] [Google Scholar]
- 14.Mardia K, Jupp P. Directional Statistics. John Wiley & Sons, Ltd; Chichester: 2000. [Google Scholar]
- 15.Dorph C, Lundberg IE. Idiopathic inflammatory myopathies - myositis. Best Pract Res Clin Rheumatol. 2002;16(5):817–832. doi: 10.1053/berh.2002.0261. [DOI] [PubMed] [Google Scholar]
- 16.O'Hanlon TP, Carrick DM, Arnett FC, Reveille JD, Carrington M, Gao X, et al. Immunogenetic risk and protective factors for the idiopathic inflammatory myopathies: distinct HLA-A, -B, -Cw, -DRB1 and -DQA1 allelic profiles and motifs define clinicopathologic groups in Caucasians. Medicine (Baltimore) 2005;84:338–349. doi: 10.1097/01.md.0000189818.63141.8c. [DOI] [PubMed] [Google Scholar]
- 17.Targoff IN, Mamyrova G, Trieu EP, Perurena O, Koneru B, O'Hanlon TP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54(11):3682–3689. doi: 10.1002/art.22164. [DOI] [PubMed] [Google Scholar]
- 18.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 19.Miller FW, Leitman SF, Cronin ME, Hicks JE, Leff RL, Wesley R, et al. Controlled trial of plasma exchange and leukapheresis in polymyositis and dermatomyositis. N Engl J Med. 1992;326(21):1380–1384. doi: 10.1056/NEJM199205213262102. [DOI] [PubMed] [Google Scholar]
- 20.Cronin ME, Miller FW, Hicks JE, Dalakas M, Plotz PH. The failure of intravenous cyclophosphamide therapy in refractory idiopathic inflammatory myopathy. J Rheumatol. 1989;16(9):1225–1228. [PubMed] [Google Scholar]
- 21.Villalba L, Hicks JE, Adams EM, Sherman JB, Gourley MF, Leff RL, et al. Treatment of refractory myositis: a randomized crossover study of two new cytotoxic regimens. Arthritis Rheum. 1998;41(3):392–399. doi: 10.1002/1529-0131(199803)41:3<392::AID-ART3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 22.Adams EM, Pucino F, Yarboro C, Hicks JE, Thornton B, McGarvey C, et al. A pilot study: use of fludarabine for refractory dermatomyositis and polymyositis, and examination of endpoint measures. J Rheumatol. 1999;26(2):352–360. [PubMed] [Google Scholar]
- 23.Targoff IN. Autoantibodies to aminoacyl-transfer RNA synthetases for isoleucine and glycine. Two additional synthetases are antigenic in myositis. J Immunol. 1990;144:1737–1743. [PubMed] [Google Scholar]
- 24.Rojansky N, Brzezinski A, Schenker JG. Seasonality in human reproduction: an update. Hum Reprod. 1992;7:735–745. doi: 10.1093/oxfordjournals.humrep.a137729. [DOI] [PubMed] [Google Scholar]
- 25.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 26.Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999;107(Suppl 5):793–802. doi: 10.1289/ehp.99107s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradman A, Eskenazi B, Barr DB, Bravo R, Castorina R, Chevrier J, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunyer J, Torrent M, Munoz-Ortiz L, Ribas-Fito N, Carrizo D, Grimalt J, et al. Prenatal dichlorodiphenyldichloroethylene (DDE) and asthma in children. Environ Health Perspect. 2005;113:1787–1790. doi: 10.1289/ehp.8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garry VF, Harkins ME, Erickson LL, Long-Simpson LK, Holland SE, Burroughs BL. Birth defects, season of conception, and sex of children born to pesticide applicators living in the Red River Valley of Minnesota, USA. Environ Health Perspect. 2002;110(Suppl 3):441–449. doi: 10.1289/ehp.02110s3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duramad P, Harley K, Lipsett M, Bradman A, Eskenazi B, Holland NT, et al. Early Environmental Exposures and Intracellular Th1/Th2 Cytokine Profiles in 24MonthOld Children Living in an Agricultural Area. Environ Health Perspect. 2006;114(12):1916–1922. doi: 10.1289/ehp.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis. 2001;7(3):369–374. doi: 10.3201/eid0703.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada S, Weatherhead E, Targoff IN, Wesley R, Miller FW. Global surface ultraviolet radiation intensity may modulate the clinical and immunologic expression of autoimmune muscle disease. Arthritis Rheum. 2003;48:2285–2293. doi: 10.1002/art.11090. [DOI] [PubMed] [Google Scholar]
- 33.Haus E, Smolensky MH. Biologic rhythms in the immune system. Chronobiol Int. 1999;16(5):581–622. doi: 10.3109/07420529908998730. [DOI] [PubMed] [Google Scholar]
- 34.Lien L, Tambs K, Oppedal B, Heyerdahl S, Bjertness E. Is relatively young age within a school year a risk factor for mental health problems and poor school performance? A population-based cross-sectional study of adolescents in Oslo, Norway. BMC Public Health. 2005;5:102. doi: 10.1186/1471-2458-5-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shamim EA, Rider LG, Pandey JP, O'Hanlon TP, Jara LJ, Samayoa EA, et al. Differences in idiopathic inflammatory myopathy phenotypes and genotypes between Mesoamerican Mestizos and North American Caucasians: ethnogeographic influences in the genetics and clinical expression of myositis. Arthritis Rheum. 2002;46:1885–1893. doi: 10.1002/art.10358. [DOI] [PubMed] [Google Scholar]
- 36.Sarkar K, Weinberg CR, Oddis CV, Medsger TA, Jr., Plotz PH, Reveille JD, et al. Seasonal influence on the onset of idiopathic inflammatory myopathies in serologically defined groups. Arthritis Rheum. 2005;52:2433–2438. doi: 10.1002/art.21198. [DOI] [PubMed] [Google Scholar]
- 37.Reed AM, Collins EJ, Shock LP, Klapper DG, Frelinger JA. Diminished class II-associated Ii peptide binding to the juvenile dermatomyositis HLA-DQ alpha 1*0501/DQ beta 1*0301 molecule. J Immunol. 1997;159(12):6260–6265. [PubMed] [Google Scholar]
- 38.Weets I, Kaufman L, Van der AB, Crenier L, Rooman RP, De BC, et al. Seasonality in clinical onset of type 1 diabetes in Belgian patients above the age of 10 is restricted to HLA-DQ2/DQ8-negative males, which explains the male to female excess in incidence. Diabetologia. 2004;47:614–621. doi: 10.1007/s00125-004-1369-8. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg CR, Dornan TL, Hansen JA, Raghu PK, Palmer JP. HLA-related heterogeneity in seasonal patterns of diagnosis in Type 1 (insulin-dependent) diabetes. Diabetologia. 1984;26:199–202. doi: 10.1007/BF00252407. [DOI] [PubMed] [Google Scholar]
- 40.Bobak M, Gjonca A. The seasonality of live birth is strongly influenced by socio-demographic factors. Hum Reprod. 2001;16(7):1512–1517. doi: 10.1093/humrep/16.7.1512. [DOI] [PubMed] [Google Scholar]
- 41.Lam DA, Miron JA. The effects of temperature on human fertility. Demography. 1996;33(3):291–305. [PubMed] [Google Scholar]
- 42.Sellar C, Goldacre MJ. Season of birth, year of birth, and arthritic disease in later life. Lancet. 1988;1(8600):1461. doi: 10.1016/s0140-6736(88)92271-4. [DOI] [PubMed] [Google Scholar]