Abstract
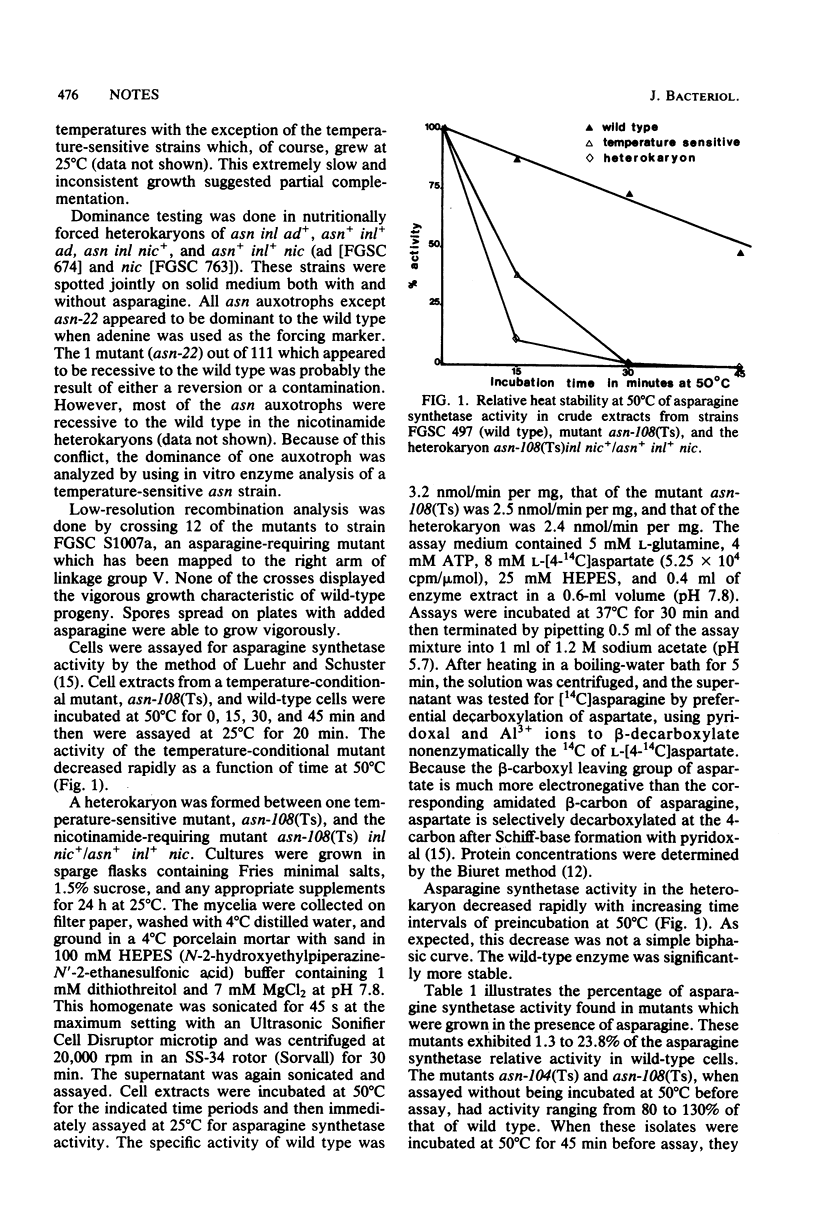
Neurospora crassa mutants deficient in asparagine synthetase were selected by using the procedure of inositol-less death. Complementation tests among the 100 mutants isolated suggested that their alterations were genetically allelic. Recombination analysis with strain S1007t, an asparagine auxotroph, indicated that the mutations were located near or within the asn gene on linkage group V. In vitro assays with a heterokaryon indicated that the mutation was dominant. Thermal instability of cell extracts from temperature-sensitive strains in an in vitro asparagine synthetase assay determined that the mutations were in the structural gene(s) for asparagine synthetase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURCHALL J. J., REICHELT E. C., WOLIN M. J. PURIFICATION AND PROPERTIES OF THE ASPARAGINE SYNTHETASE OF STREPTOCOCCUS BOVIS. J Biol Chem. 1964 Jun;239:1794–1798. [PubMed] [Google Scholar]
- CODDINGTON A., FINCHAM J. R. PROOF OF HYBRID ENZYME FORMATION IN A CASE OF INTER-ALLELIC COMPLEMENTATION IN NEUROSPORA CRASSA. J Mol Biol. 1965 May;12:152–161. doi: 10.1016/s0022-2836(65)80289-3. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. The asparagine synthetase of Escherhic coli. I. Biosynthetic role of the enzyme, purification, and characterization of the reaction products. J Biol Chem. 1969 Aug 10;244(15):4112–4121. [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. The asparagine synthetase of Escherichia coli. II. Studies on mechanism. J Biol Chem. 1969 Aug 10;244(15):4122–4127. [PubMed] [Google Scholar]
- Coddington A., Fincham J. R., Sundaram T. K. Multiple active varieties of Neurospora glutamate dehydrogenase formed by hybridization between two inactive mutant proteins in vivo and in vitro. J Mol Biol. 1966 Jun;17(2):503–512. doi: 10.1016/s0022-2836(66)80160-2. [DOI] [PubMed] [Google Scholar]
- Felton J., Michaelis S., Wright A. Mutations in two unlinked genes are required to produce asparagine auxotrophy in Escherichia coli. J Bacteriol. 1980 Apr;142(1):221–228. doi: 10.1128/jb.142.1.221-228.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb P. S., Carritt B., Hooper M. L., Slack C. The isolation and characterization of asparagine-requiring mutants of Chinese hamster cells. Exp Cell Res. 1977 Feb;104(2):357–367. doi: 10.1016/0014-4827(77)90101-x. [DOI] [PubMed] [Google Scholar]
- Horowitz B., Meister A. Glutamine-dependent asparagine synthetase from leukemia cells. Chloride dependence, mechanism of action, and inhibition. J Biol Chem. 1972 Oct 25;247(20):6708–6719. [PubMed] [Google Scholar]
- Jones G. E. L-Asparagine auxotrophs of Saccharomyces cerevisiae: genetic and phenotypic characterization. J Bacteriol. 1978 Apr;134(1):200–207. doi: 10.1128/jb.134.1.200-207.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER H. E., GROSS S. R. Efficient method for selection of auxotrophic mutants of Neurospora. Science. 1959 Feb 27;129(3348):572–572. doi: 10.1126/science.129.3348.572. [DOI] [PubMed] [Google Scholar]
- LEVINTOW L. Evidence that glutamine is a precursor of asparagine in a human cell in tissue culture. Science. 1957 Sep 27;126(3274):611–612. doi: 10.1126/science.126.3274.611. [DOI] [PubMed] [Google Scholar]
- Luehr C. A., Schuster S. M. A new assay for L-asparagine synthetase. J Biochem Biophys Methods. 1980 Sep;3(3):151–161. doi: 10.1016/0165-022x(80)90014-7. [DOI] [PubMed] [Google Scholar]
- Markin R. S., Schuster S. M. Multiple forms of rat liver L-asparagine synthetase. Biochem Biophys Res Commun. 1979 May 28;88(2):583–588. doi: 10.1016/0006-291x(79)92088-6. [DOI] [PubMed] [Google Scholar]
- RAVEL J. M., NORTON S. J., HUMPHREYS J. S., SHIVE W. Asparagine biosynthesis in Lactobacillus arabinosus and its control by asparagine through enzyme inhibition and repression. J Biol Chem. 1962 Sep;237:2845–2849. [PubMed] [Google Scholar]
- Ramos F., Wiame J. M. Synthesis and activation of asparagine in asparagine auxotrophs of Saccharomyces cerevisiae. Eur J Biochem. 1979 Mar;94(2):409–417. doi: 10.1111/j.1432-1033.1979.tb12908.x. [DOI] [PubMed] [Google Scholar]
- Ramos F., Wiame J. M. Two asparagine synthetases in Saccharomyces cerevisiae. Eur J Biochem. 1980 Jul;108(2):373–377. doi: 10.1111/j.1432-1033.1980.tb04732.x. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Asparagine synthetases of Klebsiella aerogenes: properties and regulation of synthesis. J Bacteriol. 1982 Sep;151(3):1299–1313. doi: 10.1128/jb.151.3.1299-1313.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLESINGER M. J., LEVINTHAL C. Hybrid protein formation of E. coli alkaline phosphatase leading to in vitro complementation. J Mol Biol. 1963 Jul;7:1–12. doi: 10.1016/s0022-2836(63)80014-5. [DOI] [PubMed] [Google Scholar]


